Abstract
Clostridioides difficile is a frequent cause of hospital-acquired diarrhea associated with antibiotics. Hypervirulent strains of Clostridioides difficile are associated with a severe course of Clostridioides difficile infection and a higher mortality. We have confirmed 322 non-duplicate Clostridioides difficile isolates in stool samples of patients hospitalized in three Slovak hospitals providing primary health care between January and December 2021 and determined genes encoding toxins, ribotypes and susceptibility to antimicrobials. 93.7% of them were toxigenic and co-occurrence of genes tcdA, tcdB, cdtA, cdtB, which encode toxins, was detected in 71.4% isolates. Nosocomially associated ribotypes 176, 001, and 027 were confirmed in 61.1%, 10.2%, and 5.2% of isolates, respectively, and found to be the most frequent. Although ribotype 176 predominated in all three hospitals, the highest incidence was recorded in Košice (73.5%). Results of antimicrobial susceptibility testing revealed 65.8% resistance rate for rifampicin, 5.2% for vancomycin, 1.5% for metronidazole, 1.2% for teicoplanin and 0.6% for doxycycline. The correlation of frequent ribotypes and results of antimicrobial susceptibility testing points to the predominance of rifampicin resistance in isolates belonging to ribotypes 176 and 027. The above results reflect the problematic situation in our hospitals during the COVID-19 pandemic, that was related to the misuse of broad-spectrum antibiotics and impossibility to follow epidemiological measures to prevent the spread of hypervilulent isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clostridioides difficile, also known as C. difficile, is a Gram-positive, anaerobic, spore forming bacterium and the etiologic agent of Clostridioides difficile infection (CDI). The infection has a wide spectrum of clinical manifestations from mild diarrhea, through to severe courses of disease such as pseudomembranous colitis or toxic megacolon, perforation of colon, and finally, death (Isidro et al. 2017). Spores of C. difficile are characterized by viability for months and resistance to heat and desinfectants, which contributes significantly to the spread of CDI in healthcare facilities through direct or indirect contact with infected patients and asymptomatic carriers (Rodriguez-Palacios et al. 2011, Czepiel et al. 2019). CDI is currently considered one of the most common causes of nosocomial diarrhea and represents a serious problem on a global scale due to high morbidity, mortality and negative consequences for healthcare system and overall public health (Balsells et al. 2019, Banawass 2018).
In addition to primary virulence factors of C. difficile, toxin A (TcdA, enterotoxin) and toxin B (TcdB, cytotoxin), some strains may also produce the additional, binary toxin (CDT, C. difficile transferase). These strains are referred to as hypervirulent as they are associated with a severe course of CDI and higher mortality (Isidro et al. 2017; Sachsenheimer et al. 2018). Since 2003, increasing rates of CDI caused by fluoroquinolone-resistant strains of C. difficile belonging to the PCR-ribotype 027 have been reported in Canada and in the USA. In Europe, this hypervirulent ribotype was first reported in 2005 in England and subsequently in many European countries (Kuijper et al. 2008). Ribotypes 181, 014, 176, 078 are also associated with more severe disease and outbreaks in Europe (Eyre et al. 2018; Herbert et al. 2019).
There are two antimicrobial agents currently recommended for therapy of CDI, vancomycin and fidaxomicin. Metronidazole is currently recommended for supportive treatment or if other options are no longer available. For cases where unacceptable adverse effects have been associated with standard therapy, rifaximin, nitazoxanide, ramoplanin, teicoplanin, and tigecycline have been used (McDonald et al. 2018; Oksi et al. 2020). Results of meta-analysis focused on antimicrobial resistance of human clinical C. difficile isolates point to weighted pooled resistance of 1% for metronidazole, 1% for vancomycin, and 0.08% for fidaxomicin (Sholeh et al. 2020). According to a more recent study (Dilnessa et al. 2022), weighted pooled resistance for metronidazole and vancomycin reaches even 5% and 3%, respectively.
The aim of this study was to confirm C. difficile in stool samples of patients hospitalized in three Slovak hospitals between January and December 2021 and to determine ribotypes, genes encoding toxins and susceptibility to antimicrobials for all C. difficile isolates.
Materials and methods
Patient research sample
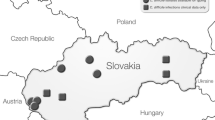
The study included 322 non-duplicate C. difficile isolates obtained from stool samples of patients suspected for CDI between January and December 2021. Patients were hospitalized in three of the Slovak hospitals, namely: Louis Pasteur University Hospital in Košice (LPUH KE), Faculty Hospital in Nitra (FH NR), University Hospital in Bratislava (UH BA).
Cultivation and identification
Stool samples were first analyzed for glutamate dehydrogenase (GDH) and toxins A/B by immunochromatographic test (Intermedical CLOSTRIDIUM TRIO TOXIN A/B/GDH). GDH-positive and toxins A/B-positive/negative stool samples were cultured anaerobically (Whitley A35 Anaerobic workstation, Don Whitley Scientific) on selective agar for C. difficile (Brazier, Oxoid) at 36 °C for 48 h. The MALDI TOF mass spectrometry (Bruker Daltonics) was used to identify bacterial colonies.
Multiplex PCR
Genomic DNA was isolated using the GRISP isolation kit (GRS Genomic DNA kit). Genes encoding toxins A and B (tcdA and tcdB), binary toxin (cdtA and cdtB) and the 16 S rDNA, which serves as a positive control for DNA isolation, were detected by multiplex PCR reaction (Persson et al. 2008). PCR products were separated in 1.5% agarose gel with ECO Safe fluorescent dye (Uniscience), visualized by UV light, captured by Gel DocTMEZ System (BIO-RAD) and analyzed by Image Lab Software (BIO-RAD).
Ribotyping
PCR ribotyping was performed according to the standardized protocol for capillary electrophoresis PCR ribotyping with primers as described by Bidet et al. (2000). Fragmentation analysis was performed on an ABI3130 genetic analyzer (Thermo Fisher Scientific) using POP7 polymer (Thermo Fisher Scientific) and LIZ1200 size standard (Thermo Fisher Scientific). All samples were evaluated with GeneMapper v4.1 software (Thermo Fisher Scientific) and electrophoretic profiles were compared with the Webribo database (https://webribo.ages.at/).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) of C. difficile isolates to metronidazole (MTZ), vancomycine (VA), tigecycline (TGC), doxycycline (DO), teicoplanin (TEC) and rifampicin (RD) was performed by disc diffusion test. Bacterial suspension with a density of 0.5 McFarland and antibiotic discs were plated on Wilkins-Chalgren agar and cultured anaerobically at 36 °C for 48 h. The BACMED 6iG2 automated antimicrobial susceptibility testing reader and analyzer was used to measure the inhibition zone diameters and calculate the minimum inhibitory concentrations (MIC) for all tested antimicrobials by an expert system. Based on the breakpoints for interpretation of MIC (MTZ > 2 mg/L, VA > 2 mg/L, TGC > 0.25 mg/L, DO > 16 mg/L, TEC > 0.12 mg/L, RD > 0.004 mg/L) issued by the European Committee on Antimicrobial Susceptibility Testing (EUCAST, breakpoints of MTZ, VA, and TGC) and the Clinical and Laboratory Standards Institute (CLSI, breakpoints of DO, TEC, and RD), C. difficile isolates were classified as susceptible or resistant to antimicrobials.
Statistical analysis
Statistical assessments were performed using IBM SPSS Statistics (version 19). Comparisons of proportions for a particular characteristic between different populations were studied using the Pearson χ2 test and the two-tailed Fisher’s exact test. All p-values lower than 0.05 were considered statistically significant.
Results
In the period of January - December 2021, a total of 322 non-duplicate C. difficile isolates were collected from stool samples of patients hospitalized in three of the Slovak hospitals and further tested. 189 isolates were from LPUH KE, 90 isolates from FH NR, and 43 isolates from UH BA. From the total number of patients, 150 (46.6%) were men and 172 (53.4%) were women. The average age of patients was 67 years.
Multiplex PCR was used to detect genes tcdA, tcdB, cdtA, cdtB and 16 S rDNA. Gene 16 S rDNA was confirmed in all tested isolates. In our set of C. difficile isolates, 302 (93.7%) isolates were toxigenic and 20 (6.2%) non-toxigenic. The representation of toxigenic isolates in hospitals was as follows: 185 in LPUH KE, 80 in FH NR, and 37 in UH BA. Co-occurrence of tcdA and tcdB genes was found out in 71 (22%) isolates, 36 of them (19%) were from LPUH KE, 26 (28.8%) from FH NR and 9 (20.9%) from UH BA. Co-occurrence of tcdA, tcdB, cdtA and cdtB genes was detected in 230 (71.4%) isolates, 148 of them (78.3%) came from LPUH KE, 54 (60%) from FH NR and 28 (65.1%) from UH BA. We have noted statistical significance between LPUH KE and FH Nitra (p < 0.001). Gene tcdB alone was recorded in one isolate, being from LPUH KE (0.53%). For non-toxinogenic isolates, statistical significance between LPUH KE and FH NR (p < 0.0012), LPUH KE and UH BA (p < 0.0005) was detected.
Ribotypes (RT) were determined by capillary electrophoresis. The most frequent ribotype in our set was hypervirulent RT 176, confirmed in 197 (61.1%) isolates. 139 of them (73.5%) were from LPUH KE, 42 (46.6%) from FH NR and 16 (37.2%) from UH BA. We have found significant differences in the incidence of RT 176 between LPUH KE and FH NR (p < 0.001), LPUH KE and UH BA (p < 0.001). Ribotype RT 001 was the second most common ribotype, detected in 33 (10.2%) isolates, of which 28 (14.8%) were from LPUH KE, three (3.3%) from FH NR and two (11.6%) from UH BA. Statistical significance was noted between LPUH KE and FH NR (p < 0.0043), LPUH KE and UH BA (p < 0.001). RT 027 was identified in 17 (5.2%) isolates, six of them (3.1%) came from LPUH KE, six (6.6%) from FH NR and five (11.6%) from UH BA. Less frequent ribotypes were RT 005 (n = 4, 1.2%), RT 018 (n = 4, 1.2%), RT 070 (n = 3, 0.9%), RT 010 (n = 3, 0.9%), RT 081 (n = 3, 0.9%). Other ribotypes (RT 011, RT 014, RT 106, RT 009 and others) were confirmed in 43 CD isolates (13.4%) and their occurrence in the total set of isolates was lower than 3. Among the listed ribotypes, RT010 and RT 009 are non-toxigenic. Ribotyping failed in 15 (4.7%) isolates. Results of multiplex PCR and ribotyping are shown in Table 1.
Antimicrobial resistance of C. difficile isolates to 6 antimicrobials was determined using the BACMED 6iG2 automatic zone analyzer. The highest rate of resistance was found for rifampicin, the number of resistant isolates was 212 (65.8%), 133 of them (70.3%) were from LPUH KE, 53 (58.8%) from FH NR and 26 (60.4%) from UH BA. Vancomycin resistance was detected in 17 (5.2%) isolates, of which 4 isolates (2.1%) were from LPUH KE, 8 (8.8%) from FH NR, 5 (11.6%) from UH BA and statistical significance was demonstrated between LPUH KE and FH NR (p < 0.0091). The MIC for all these C. difficile isolates was 4 mg/L. Metronidazole resistance was recorded in 5 isolates (1.5%) of which three (1.5%) came from LPUH KE and two (2.2%) from FH NR. Four isolates (1.2%) were resistant to teicoplanin, two isolates (0.6%) were resistant to doxycycline, none of the isolates exhibited tigecycline resistance. An overview of the antimicrobial resistance of the tested isolates is shown in Table 1.
The correlation of toxigenic ribotypes RT 176, RT 001, RT 027, RT 005, RT 018, RT 070, RT 081 and results of AST points to predominance of rifampicin resistance in isolates belonging to RT 176 and RT 027. This correlation is presented in Table 2. For the RT 176 (n = 197), we have found 7 different antimicrobial resistance profiles where 24 isolates were fully susceptible to all tested antimicrobials, 162 isolates were resistant to RD only. For the RT 027 (17), three different profiles were detected. Resistance to RD only was confirmed in 14 isolates, susceptibility to all antimicrobials in two isolates. In the case of RT 001 (n = 33), 29 isolates were fully susceptible and four isolates resistant to RD only. All isolates belonging to RT 018, RT 070 and RT 081 and three isolates RT 005 were fully susceptible. One isolate of RT 005 was resistant to RD and VA.
Discussion
Clostridioides difficile isolates producing toxins are the main causative agents of hospital-acquired diarrhea in humans. Among the 322 C. difficile isolates analyzed, the co-occurrence of tcdA, tcdB genes was confirmed in 71 (22%) isolates, while the co-occurrence of tcdA, tcdB, cdtA, cdtB genes in 230 (71.4%) of isolates. Different ribotypes were identified in our set of toxigenic isolates, namely RT 176, RT 027, RT 001, RT 005, RT 018, RT 070, RT 081, and some others. Ribotypes RT 176 and RT 027 belong to the same multilocus sequence type and express the same proteomic profile, suggesting their relatedness (Dresler et al. 2017). While RT 027 is distributed worldwide, including European countries, RT 176 is typically found in Central and Eastern European countries (Couturier et al. 2018). Both ribotypes are hypervirulent due to their ability to spread epidemically in hospitals and produce three toxins. Among the 302 toxigenic C. difficile isolates, RT 176 and RT 027 were identified in 197 (61.1%) and 17 (5.2%) isolates, respectively. RT 176 was the most frequent ribotype in all three hospitals with the highest incidence in LPUH KE at the level of 73.5%. Such percentage frequency points to the rapid spread and local epidemics in hospitals. Similar findings were obtained in Croatia with 18 of 19 isolates belonging to RT 176 (Rupnik et al. 2016) and in Poland with 48% and 82.4% incidence of this ribotype (Pituch et al. 2018; Aptekorz et al. 2017). In the Czech Republic, a decrease in the incidence of RT 176 was recorded, from 26.7 to 11.6% (Krutova et al. 2020). However, in some regions or more precisely in some hospitals, a higher prevalence of RT 176 was detected (60.9%, Beran et al. 2017). RT 027 was predominant in Bosnia and Herzegovina, as well as in Serbia (Rupnik et al. 2016). The second most common ribotype was RT 001, identified in 10.2% of isolates. Several studies point to the prevalence of this ribotype in clinical isolates, e.g. 33.5% in the Czech Republic (Krutova et al. 2020), or 12.9% in Iran (Azimirad et al. 2020). The results of the latest research concerning molecular C. difficile spidemiology show a decrease in the occurrence of classical hypervirulent ribotypes (RT 027, RT 176, RT 078 and others) in many countries due to the implementation of antibiotic stewardship measures as well as local surveillance and infection control measures in healthcare settings (Tschudin-Sutter et al. 2018, Krutova et al. 2020, Azimirad et al. 2020, Abdrabou et al. 2021, Abdrabou et al. 2022).
Vancomycin and fidaxomicin are the first-line antibiotics recommended for the treatment of CDI while metronidazole is currently recommended for supportive treatment. From this point of view, continuous antimicrobial susceptibility testing plays an important role in the management of CDI. In recent years, C. difficile exhibiting vancomycin resistance have emerged, which is considered seriously concerning (Wickramage et al. 2021). Weighted poooled resistance for vancomycin at the level of 1% was reported by Sholeh (Sholeh et al. 2020) and 3% by Dilnessa (Dilnessa et al. 2022). In a Slovak study (Novakova et al. 2021), a reduced VA susceptibility was recorded in one isolate belonging to RT 017. In our sample, MIC value 4 mg/L was detected in 17 (5.2%) isolates. Based on the current EUCAST breakpoints for interpretation of MIC, these isolates were classified resistant. However, it is known that if MICs are in the range of 3–15 mg/L, the concentration of VA in the stool is still high, reaching values higher than 1024 mg/l. No association with a specific ribotype was confirmed in our isolates.
The highest rate of resistance in our sample was found for rifampicin and reached the level of 65.8%. In Greece and Poland, RD resistance was detected in 31.8% and 40.5% of isolates, respectively (Chatedaki et al. 2019; Aptekorz et al. 2022). In a five-year pan-European study provided during the period of 2011–2016, in which the Czech Republic, France, Germany, Ireland, Spain and the United Kingdom participated, RD resistance ranged from 10.2 to 13.5%. It is worth to mention that hypervirulent ribotypes RT 027, RT 198, RT 018, RT 356, RT 017 and RT 176 had a significantly high proportion of rifampicin resistance (Freeman et al. 2020). This was proven by typing of C. difficile isolates among 26 patients with osteoarticular infections, where 9 of 15 isolates belonging to RT 027 exhibited resistance to rifampicin and other antimicrobials. The incidence of CDI was reduced after implementation of an intensified antibiotic stewardship program (reduction in the use of flouroquinolones, third generation cephalosporins, and clindamycin) focused on patients treated for osteoarticular infections (Färber et al. 2017). We also noted the highest RD resistance in hypervirulent ribotypes RT 176 (87.3%) and 027 (88.2%).
Low levels of MTZ resistance in clinical isolates have been reported in many countries (Wickramage et al. 2021). The results of several studies point to a rate of MTZ resistance in the range of 0-18.3% (Freeman et al. 2018). According to study of Sholeh (Sholeh et al. 2020), an increase in MTZ resistance rate from 0 to 2% was noted between isolates from the period 1992–2014 and 2015–2019. In the study of Dilnessa (Dilnessa et al. 2022), weighted pooled resistance for MTZ was reported at the level of 5%. We have found MTZ resistance in 5 (1.5%) of the isolates, of which three belonged to RT 176. In a Slovak study conducted in 2016, only reduced susceptibility to MTZ was noted, namely for 13 of 78 isolates (16.7%), belonging to RT 001 (11.5%), RT 017 (1.28), RT 020 (1.28%), RT 027 (1.28%), RT 176 (1.28%) (Novakova et al. 2020).
The year 2021 was marked by the COVID-19 pandemic, which led to a collapse of the healthcare system in Slovakia. All hospitals, including LPUH KE, FH NR and UH BA were overcrowded and unable to select and isolate patients suffering from infectious diseases such as CDI and COVID-19. Compared to 2020, we observed an increase in the occurence of hypervirulent ribotypes RT 176 and RT 027 in LPUH in Košice from 47.6% and 1.2% to 73.5% and 3.1% (Curova et al. 2023). These ribotypes spread both within one hospital department, and among other hospital departments. The situation regarding the use of broad-spectrum antibiotics during the COVID-19 pandemic was also problematic. Our national and regional antibiotic stewardship programs were not respected and cephalosporins, fluoroquinolones, marcolides, which are associated with a higher risk of developing CDI (Spigaglia 2015), were among the most frequently prescribed ATBs in 2020 (Curova et al. 2023) and also in 2021 in this set of isolates (data not shown). From our point of view, these two factors significantly contributed to the spread and increase of hypervirulent C. difficile ribotypes. Since the aim of this study was to determine C. difficile ribotypes in three hospitals and we found a high incidence of hypervirulent ribotypes, it would be appropriate to subtype them. This could help us understand the molecular epidemiology of C. difficile in outbreaks. Presence of a very closely related strain in the hospital points to a common source being revealed. We see this as a limitation of our study.
Conclusion
The results of our study point to the high incidence of toxigenic C. difficile isolates in three Slovak hospitals during the year 2021with the highest prevalence in Košice. Nosocomially associated ribotypes RT 176, RT 001, and RT 027 dominated in the monitored hospitals. Furthermore, hypervirulent isolates belonging to RT 176 and RT 027 showed high level of resistance to rifampicin. The above results reflect the critical situation in Slovak hospitals during the COVID-19 pandemic, especially in 2021, that was characterized by failure to comply with antibiotic stewardship programs and follow epidemiological measures focused on preventing the spread of hypervirulent isolates.
Abbreviations
- AST:
-
antimicrobial susceptibility testing
- ATBs:
-
antibiotics
- °C:
-
celsius degree
- CDI:
-
Clostridioides difficile infection
- cdtA, cdtB :
-
genes enconding binary toxin
- CDT:
-
binary toxin
- CLSI:
-
Clinical and Laboratory Standards Institute
- COVID-19:
-
coronavirus disease caused by the SARS-CoV-2 virus
- DNA:
-
deoxyribonucleic acid
- DO:
-
doxycycline
- EUCAST:
-
European Committee on Antimicrobial Susceptibility Testing
- FH NR:
-
Faculty hospital in Nitra
- GDH:
-
glutamate dehydrogenase
- LPUH KE:
-
Louis Pasteur University hospital in Košice
- MALDI TOF:
-
matrix assisted desorption/ionization, time of flight
- MIC:
-
minimum inhibitory concentration
- MTZ:
-
metronidazole
- PCR:
-
polymerase chain reaction
- RD:
-
rifampicin
- RT:
-
ribotype
- TcdA:
-
toxin A
- TcdB:
-
toxin B
- tcdA :
-
gene enconding toxin A
- tcdB :
-
gene enconding toxin B
- TGC:
-
tigecycline
- TEC:
-
teicoplanin
- UH BA:
-
University hospital in Bratislava
- VA:
-
vancomycin
References
Abdrabou AMM, Bajwa HU, Z, Halfmann A et al (2021) Molecular epidemiology and antimicrobial resistance of Clostridioides difficile in Germany 2014–2019. Int J Med Microbiol 311(4):151507. https://doi.org/10.1016/j.ijmm.2021.151507
Abdrabou AMM, Bischoff M, Mellmann A et al (2022) German C. difficile surveillance group. Implementation of a Clostridioides difficile sentinel surveillance system in Germany: first insights for 2019–2021. Anaerobe 77:102548. https://doi.org/10.1016/j.anaerobe.2022.102548
Aptekorz M, Szczegielniak A, Wiechula B et al (2017) Occurrence of Clostridium difficile ribotype 027 in hospitals of Silesia. Pol Anaerobe 45:106–113. https://doi.org/10.1016/j.anaerobe.2017.02.002
Aptekorz M, Sacha K, Gofron Z et al (2022) Antibiotic Resistance Profile of RT 027/176 Versus other Clostridioides difficile isolates in Silesia, Southern Poland. Pathogens 11(8):949. https://doi.org/10.3390/pathogens11080949
Azimirad M, Krutova M, Yadegar A et al (2020) Clostridioides difficile ribotypes 001 and 126 were predominant in Tehran healthcare settings from 2004 to 2018: a 14-year-long cross-sectional study. Emerg Microbes Infect 9(1):1432–1443. https://doi.org/10.1080/22221751.2020.1780949
Balsells E, Shi T, Leese C et al (2019) Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health 9(1):010407. https://doi.org/10.7189/jogh.09.010407
Banawas SS (2018) Clostridium difficile infections: A Global Overview of Drug Sensitivity and Resistance Mechanisms. Biomed Res Int. https://doi.org/10.1155/2018/8414257
Beran V, Kuijper EJ, Harmanus C et al (2017) Molecular typing and antimicrobial susceptibility testing to six antimicrobials of Clostridium difficile isolates from three czech hospitals in Eastern Bohemia in 2011–2012. Folia Microbiol (Praha) 62(5):445–451. https://doi.org/10.1007/s12223-017-0515-x
Bidet P, Lalande V, Salauze B, Burghoffer B et al (2000) Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J Clin Microbiol 38(7):2484–2487. https://doi.org/10.1128/JCM.38.7.2484-2487.2000
Chatedaki C, Voulgardi I, Kachrimanidou et al (2019) Antimicrobial susceptibility and mechanisms of resistance of greek Clostridium difficile clinical isolates. J Glob Antimicrob Resist 16:53–58. https://doi.org/10.5812/ircmj.5189
Couturier J, Davies K, Gateau C et al (2018) Ribotypes and new virulent strains across Europe. Adv Exp Med Biol 1050:45–58. https://doi.org/10.1007/978-3-319-72799-8_15
Curova K, Novotny M, Ambro L et al (2023) High prevalence of Clostridioides difficile ribotype 176 in the University Hospital in Kosice. Pathogens 12(3):430. https://doi.org/10.3390/pathogens12030430
Czepiel J, Dróżdż M, Pituch H et al (2019) Clostridium difficile infection: review. Eur J Clin Microbiol Infect Dis J 38(7):1211–1221. https://doi.org/10.1007/s10096-019-03539-6
Dilnessa T, Getaneh A, Hailu W et al (2022) Prevalence and antimicrobial resistance pattern of Clostridium difficile among hospitalized diarrheal patients: a systematic review and meta-analysis. PLoS ONE 17(1):e0262597. https://doi.org/10.1371/journal.pone.0262597
Dresler J, Krutova M, Fucikova A et al (2017) Analysis of proteomes released from in vitro cultured eight Clostridium difficile PCR ribotypes revealed specific expression in PCR ribotypes 027 and 176 confirming their genetic relatedness and clinical importance at the proteomic level. Gut Pathog 9:45. https://doi.org/10.1186/s13099-017-0194-9
Eyre DW, Davies KA, Davis G et al (2018) Two distinct patterns of Clostridium difficile Diversity Across Europe indicating contrasting routes of spread 2018. Clin Infect Dis 67(7):1035–1044. https://doi.org/10.1093/cid/ciy252
Färber J, Illiger S, Berger F et al (2017) Management of a cluster of Clostridium difficile infections among patients with osteoarticular infections. Antimicrob Resist Infect Control 6:22. https://doi.org/10.1186/s13756-017-0181-4
Freeman J, Vernon J, Pilling S et al (2018) The ClosER study: results from a three-year pan-european longitudinal surveillance of antibiotic resistance among prevalent Clostridium difficile ribotypes, 2011–2014. Clin Microbiol Infec 24(7):724–731. https://doi.org/10.1016/j.cmi.2017.10.008
Freeman J, Vernon J, Pilling S et al (2020) Five-year Pan-European, longitudinal surveillance of Clostridium difficile ribotype prevalence and antimicrobial resistance: the extended ClosER study. Eur J Clin Microbiol Infect Dis 39:169–177. https://doi.org/10.1007/s10096-019-03708-7
Herbert R, Hatcher J, Jauneikaite E et al (2019) Two-year analysis of Clostridium difficile ribotypes associated with increased severity. J Hosp Infect 103(4):388–394. https://doi.org/10.1016/j.jhin.2019.06.003
Isidro J, Mendes LA, Serrano M et al (2017) Overview of Clostridium difficile infection: life cycle, epidemiology, Antimicrobial Resistance and Treatment. Clostridium Difficile - A Comprehensive Overview.National and University Library in ZagrebCroatiahttps://doi.org/10.5772/intechopen.69053
Krutova M, Capek V, Nycova E et al (2020) The association of a reduced susceptibility to moxifloxacin in causative Clostridium (Clostridioides) difficile strain with the clinical outcome of patients. Antimicrob Resist Infect Control 9:98. https://doi.org/10.1186/s13756-020-00765-y
Kuijper EJ, Barbut F, Brazier JS et al (2008) Update of Clostridium difficile infection due to PCR ribotype 027 in Europe, 2008. Euro Surveill 13(31):18942. https://doi.org/10.2807/ese.13.31.18942-en
McDonald LC, Gerding DN, Johnson S et al (2018) Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious Diseases Society of America (IDSA) and society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66(7):e1–e48. https://doi.org/10.1093/cid/cix1085
Novakova E, Kotlebova N, Gryndlerova A et al (2020) Outbreak of Clostridium (Clostridioides) difficile infections within an Acute and Long-Term Care wards due to Moxifloxacin-Resistant PCR ribotype 176 genotyped as PCR ribotype 027 by a commercial assay. J Clin Med 9(11):3738. https://doi.org/10.3390/jcm9113738
Novakova E, Stofkova Z, Sadlonova V et al (2021) Diagnostic methods of Clostridioides difficile infection and Clostridioides difficile Ribotypes in Studied Sample. Antibiotics 10(9):1035. https://doi.org/10.3390/antibiotics10091035
Oksi J, Anttila VJ, Mattila E (2020) Treatment of Clostridioides (Clostridium) difficile infection. Ann Med 52(1–2):12–20. https://doi.org/10.1080/07853890.2019.1701703
Persson S, Torpdahl M, Olsen KE (2008) New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a danish strain collection. Clin Microbiol Infect 14(11):1057–1064. https://doi.org/10.1111/j.1469-0691.2008.02092.x
Pituch H, Obuch-Woszczatyński P, Lachowicz D et al (2018) Prevalence of Clostridium difficile infection in hospitalized patients with diarrhoea: results of a polish multicenter, prospective, biannual point-prevalence study. Adv Med Sci 63(2):290–295. https://doi.org/10.1016/j.advms.2018.03.003
Rodriguez-Palacios A, Lejeune JT (2011) Moist-heat resistance, spore aging, and superdormancy in Clostridium difficile. Appl Environ Microbiol 77(9):3085–3091. https://doi.org/10.1128/AEM.01589-10
Rupnik M, Janezic S (2016) An update pm Clostridium difficile Toxinotyping. J Clin Micorbiol 54(1):13–18. https://doi.org/10.1128/jcm.02083-15
Sachsenheimer FE, Yang I, Zimmermann O et al (2018) Genomic and phenotypic diversity of Clostridium difficile during long-term sequential recurrences of infection 2018. Int J Med Microbiol 308(3):364–377. https://doi.org/10.1016/j.ijmm.2018.02.002
Sholeh M, Krutova M, Forouzesh M et al (2020) Antimicrobial resistance in Clostridioides (Clostridium) difficile derived from humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control 9(1):158. https://doi.org/10.1186/s13756-020-00815-5
Spigaglia P (2015) Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis 3(1):23–42. https://doi.org/10.1177/2049936115622891
Tschudin-Sutter S, Kuijper EJ, Durovic A et al (2018) Guidance document for prevention of Clostridium difficile infection in acute healthcare settings. Clin Microbiol Infect 24(10):1051–1054. https://doi.org/10.1016/j.cmi.2018.02.020
Wickramage I, Spigaglia P, Sun X (2021) Mechanisms of antibiotic resistance of Clostridioides difficile. J Antimicrob Chemother 76(12): 3077–3090. https://doi.org/10.1093/jac/dkab231
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This research was funded by the Ministry of Health of the Slovak Republic, grant number 2019/34-UPJŠ-6. In addition, this paper is the result of the project implementation: „Design and implementation of advanced methods of lungs ventilation treatment and diagnosis of viral pneumonia, including Covid-19 with the possibility of their rapid adoption“, ITMS2014+: 313011ASX1, acronyme IPMVDCov, supported by the Operational Programme Integrated Infrastructure, funded by the ERDF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee (2019/EK/11064, November 2019).
Conflicts of interest
The authors declare no conflict of interest.
Financial interests
The authors declare they have no financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toporová, A., Čurová, K., Novotný, M. et al. Characteristics of Clostridioides difficile isolates circulating in the Slovak hospitals. Biologia 78, 3287–3294 (2023). https://doi.org/10.1007/s11756-023-01493-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01493-5