Abstract
Diabetes mellitus (DM) is a set of metabolic diseases defined by a persistently high blood sugar level. Mesenchymal stem cells (MSCs) are a novel potential therapeutic intervention in treatments of various diseases, which is also referred to as regenerative medicine. We aimed to compare the pro-inflammatory cytokines’ levels during bone marrow mesenchymal stem cells (BM-MSCs) transplantation in rats with induced type I (T1D) and type II diabetes (T2D). Thirty-five male Sprague dawley rats were divided into: Group I: the healthy control group, group II: untreated rats with streptozotocin (STZ)-induced T1D (65 mg/kg), group III: BM-MSCs treated rats with STZ-induced T1D, group IV: untreated rats with high-fat diet (HFD)/STZ-induced T2D (40 mg/kg), group V: BM-MSCs-treated rats with HFD/STZ-induced T2D. Biochemical, histopathological and immunohistochemical studies were applied. Our results showed that transplantation reduced hyperglycemia and increased insulin levels in both induced T1D and T2D. Also, reductions in the levels of inflammatory markers were noticed after transplantation that was coincided with nuclear factor-kappa B (NF-кB) immunohistochemical results; which showed negative or moderate cytoplasmic reactivity in treated groups III and V. These results indicated the ability of BM-MSCs transplantation to modulate the pro-inflammatory cytokine profile during treatment of both T1D and T2D.
Key points
-
BM-MSCs transplantation, in both T1D and T2D, led to the reduction in blood glucose levels.
-
The biochemical and histological findings indicated an improvement in pancreatic islets.
-
The immunomodulatory effect of BM-MSCs allowed the islets to neogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM), a non-communicable disease, is the seventh greatest cause of mortality and one of the major worldwide health problems. The global prevalence of DM is suggested to be 7.7% (more than 425 million people) (IDF 2017). According to the World Health Organization (WHO), DM was directly responsible for 1.5 million deaths worldwide (WHO 2014). These numbers for diabetes-related mortality, however, are probably an underestimate because they did not completely account for how DM affects other causes of death. WHO has reported an expected increase in the prevalence of DM in many regions of the world in 2030 compared to 2011 (North America and the Caribbean: from 10.8 to 11.8%, Middle East and North Africa: from 8.8 to 10.6%, low and middle-income countries: from 7.5 to 9% and in African countries: from 3.5 to 4.1%) (Wou et al. 2019). DM is a set of metabolic disorders characterized by high blood glucose levels caused by issues with insulin synthesis, insulin usage, or both which cause serious problems like blindness, kidney failure, cardiovascular disease, stroke, neuropathy, and vascular dysfunction (Miniawy et al. 2017). Type 1 diabetes (T1D) is most commonly diagnosed in children and young adults, and it is caused by the autoimmune destruction of pancreatic islet cells, which results in the loss of insulin production (Patlak 2002). Globally, about 8 million people worldwide had T1D in 2021; of these, 1 million (18%) were under 20 years old, 5 million (64%) were 20–59, and 1 million (19%) were 60 years or older, A modelling study has predicted a rise in prevalent cases to 13.5–17.4 million is expected in 2040 (Gregory et al. 2022). Insulin is still the most effective T1D treatment at this time. Type 2 diabetes (T2D) affects about 86–91% of the diabetic population, mostly adults. However, its incidence in children is on the rise as a result of childhood obesity (Alberti et al. 2007). T2D is defined as an increase in insulin resistance combined with a failure of the pancreatic β-cells to produce enough insulin (Colberg et al. 2010). Pro and anti-inflammatory cytokines have been linked to the development of DM (Banerjee and Saxena 2014). Previous research has shown that certain pro- and anti-inflammatory cytokines can inhibit insulin-responsive, glucose absorption and increase insulin resistance (Saxena and Modi 2014). Researches, studying the involvement of inflammatory pathways in DM and its complications are expected to shed light on the processes that support the disease’s genesis and progression. With a better knowledge of the inflammatory basis for DM, innovative therapeutic methods, in addition to presently utilized non-pharmacologic and pharmacologic treatments, might be introduced.
The use of living cells to maintain, restore, or enhance the functionality of organs and tissues is known as cell therapy (Motawea et al. 2020). Stem cells (SCs) are essential for living creatures because they perform functions like maintaining homeostatic tissue and replacing defective and senescent cells. SCs are being used in therapies for a variety of disorders as a unique possible therapeutic intervention, often known as regenerative medicine, due to their extraordinary healing capacities (Gomez-Lopez et al. 2014). The ability of SCs to regenerate and differentiate allows researchers to investigate their therapeutic potential for treating and controlling diabetic complications. Multipotent SCs can only develop into specialized cell types of their tissue of origin, such as mesenchymal stem cells (MSCs) and hematopoietic stem cells, and they may be found throughout the body in various areas. The therapeutic potential of MSCs-based therapy of DM pathology and related consequences has been intensively investigated in recent years. Nevertheless, a number of questions remain unanswered. In the present work, we compared the effects of BM-MSCs transplantation on the levels of pancreatic pro-inflammatory cytokines during T1D and T2D as a chronic inflammation model. The pancreatic inflammatory cytokines levels during BM-MSCs therapy were evaluated together with biochemical analysis, histopathological and immunohistochemical parameters.
Materials and methods
Preparation of bone marrow (BM)-MSCs
A total of twelve Sprague dawley rats (3–4 weeks) were sacrificed after administration of sodium pentobarbital intraperitoneally at a dose of 30 mg/kg. The femora and tibiae of the rats were harvested aseptically. BM-MSCs were flushed using phosphate-buffered saline (PBS), centrifuged at 300 × g for 5 min then the BM-MSCs were resuspended at a density of 107cells/ml in RPMI-164 medium. The cell suspension was plated into 50 cm2 culture plates (3 ml/plate) (Sigma-Aldrich, St. Louis, MO, USA). Isolated BM-MSCs were cultured, propagated, and supplemented with 10% FBS, 0.5% penicillin/ streptomycin and incubated at 37 °C and 5% CO2 until reaching 80–90% confluence within 7 days. The medium was renewed every two to three days. Labeling of BM-MSCs was performed according to Li et al. (2012); briefly, BM-MSCs were incubated in a staining cocktail consisting of Dulbecco’s modified Eagles medium (DMEM) complemented with 1.1% of PKH26 solution in diluent ad 100%, and incubated for 1 h in a humidified incubator (37 °C, 5% CO2). Then the staining was stopped as described by the supplier (Sigma-Aldrich, St. Louis, MO, USA).
Characterization and fluorescence-activated cell sorting (FACS) analysis
BM-MSCs were cultured onto 6-well culture plates (Corning; Lowell, MA, USA). Following 14 days of culture, the cells were detached using 0.25% trypsin. Cells were washed extensively with PBS. Rat BM-MSCs specific cell surface markers, a cluster of differentiation (CD) 90 and CD105 were characterized for purity of cultured population and negative markers for hematopoietic lineage (CD34 and CD45) by flow cytometry (BD Biosciences, Franklin Lanes, NJ, USA). Briefly, the cells were detached and incubated for 20 min in the dark at 4 °C with rat monoclonal antibodies labeled with fluorescein isothiocyanate (FITC) and phycoerythrin (PE) (Farid et al. 2022a). The samples were centrifuged at room temperature for 5 min at 300 × g, resuspended in 500 µl PBS and evaluated using flow cytometry.
BM-MSCs homing
BM-MSCs were labeled for in vivo tracing using PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The labeled BM-MSCs were examined in unstained pancreatic tissues by Leica DM 5500B fluorescence microscope (Leica, Wetzlar, Germany).
Animals and experimental design
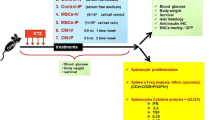
Male Sprague dawley rats, one-month-old (180–200 g), were purchased from National Organization for Drug Control and Research (NODCAR, Cairo, Egypt). Rats were maintained on a standard diet and tap water ad libitum for 1 week for acclimatization. Animals were housed in conventional cages at a temperature (22 ± 1 °C) and humidity (40–60%) with 12 h of dark/light cycle. The study was approved by the Institutional Animal Care and Use Committee (CU-IACUC), Cairo University, Egypt (CUIF 1719). A normal diet was composed of 11.4% fat, 62.8% carbohydrate, and 25.8% protein (12.6 kJ/g) (The central animal house of the National Research Center, Dokki, Giza, Egypt). Thirty-five male rats were divided into five groups (7 rats each); Group I: the healthy control group, group II: untreated rats with T1 STZ-induced diabetes, group III: BM-MSCs-treated rats with T1 STZ-induced diabetes, group IV: untreated rats with T2 HFD/STZ-induced diabetes, group V: BM-MSCs-treated rats with T2 HFD/SZ-induced diabetes. Rats received (2 × 106 cells/rat) BM-MSCs in 0.2 ml DMEM by intravenous (i.v.) injection.
T1D (STZ-induced)
at day one of the experiment, all rats fasted for 6–8 h prior to STZ (Sigma-Aldrich, St. Louis, MO, USA) administration (water was provided ad libitum). 32.5 mg of STZ was dissolved in 50 mM sodium citrate buffer (enzyme grade; Fisher) (pH 4.5) to a final concentration of 32.5 mg/ml. Rats were intravenously (i.v.) injected by the prepared STZ solution at 65 mg/kg (2.0 ml/kg) according to Furman (2015). Rats were administrated normal food and 10% sucrose water, only, on the first day of STZ administration.
T2D (HFD/STZ-induced)
Animals were weighed accurately before HFD administration. Rats were placed on HFD (D12492 diet), 60 kcal% Fat (Research Diets, Inc. NJ, USA) for two weeks. On the 15th day, rats fasted for 6–8 h prior to STZ treatment (water was provided ad libitum). STZ was prepared as mentioned previously, and rats were i.v. injected at 40 mg/kg according to Furman (2015).
The control group was injected with citrate buffer only. On the 7th day after STZ administration, all rats fasted for 6–8 h and a blood sample from the tail vein was collected for measuring fasting blood glucose (FBG) levels. Rats with FBG levels > 150 mg/dl in comparison to the control group were considered diabetic.
Biochemical parameters in serum
FBG was measured using a glucose assay Colorimetric/Fluorometric kit (Abcam, MA, USA, cat lot.: ab65333) (El-Hadad et al. 2022; Farid et al. 2022a, b). Insulin was assessed using a rat insulin ELISA kit (CUSABIO, Texas, USA, cat lot: CSB-E05070r) (Farid et al. 2022a, b). C- reactive protein (CRP) was evaluated with the Rat CRP ELISA kits (Abcam, UK, cat lot: ab256398).
Pancreatic homogenate preparation and cytokine assay
Dissected pancreatic segments (a half gram) were homogenized in 4.5 ml of cold Tris-HCl buffer (10 mmol, pH = 7.4); followed by centrifugation for 10 min at 4 oC at 3000 rpm. Protein content was determined, in the supernatant, using standard Lowry’s protocol (Waterborg and Matthews 1984). Tissue interleukin (IL)-1 beta (β), tumor necrosis factor (TNF) alpha (α) and IL-6 were determined using rat IL-1β (E-EL-R0012), TNF-α (Biolegend 438,206) and IL-6 (Biovision K4145-100) ELISA Kits (Hegazy et al. 2015; El-Hadad et al. 2022; Shatter et al. 2022; Farid et al. 2023).
Histological study
Pancreatic segments from the different experimental groups were fixed in 10% buffered formalin. The specimens were carefully cleaned in tap water before being dehydrated in gradient ethanol solutions. Following that, the specimens were cleared in xylene and embedded in paraffin wax at 55 °C. Five sections of 4 μm thickness were cut from each segment. Sections were mounted for haematoxylin and eosin (H&E) staining for routine histopathological examination. Steps for sections’ preparation for histopathological examination were performed according to Madbouly et al. (2021a, b) and Farid et al. (2022c).
Immunohistochemical parameters
For immunohistochemical localization of insulin and NF-кB, 4 μm paraffin pancreatic sections were deparaffinized in xylene, then rehydrated by graded alcohols and washed using PBS. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol followed by washing with PBS. Sections were blocked in 5% bovine serum albumin (BSA) for an hour. Primary antibody anti- NF-кB p65 antibody (phospho S536) (1:500, Abcam, MA, USA, cat lot.: ab86299) or monoclonal anti-insulin antibody (Sigma-Aldrich, cat lot.: I2018) were applied to pancreatic sections. Sections were incubated for 30 min and then washed with PBS. After washing, the sections were incubated with secondary antibody (HRP conjugated rabbit anti-rat IgG, ab6734) for an hour. The reaction was visualized by chromogen (3, 3-diaminobenzidine, DAB) until brown color appears. Sections were washed and counterstained with Mayer’s haematoxylin.
Statistical analysis
Data were evaluated with the One Way ANOVA test using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, N.Y., USA). Results were expressed as mean ± SD. Values were considered significant at p < 0.05.
Results
Characterization and pancreatic homing of BM-MSCs
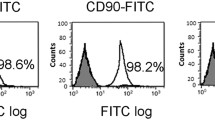
After in vitro culture, for 14 days, BM-MSCs demonstrated fibroblast-like morphology and were adhered to the flask surface (Fig. 1a). FACs analysis showed a high expression of CD90 and CD105 and negative staining for CD34 and CD45 (Fig. 1b) markers indicating that the cultured cells were of mesenchymal origin as well as of high purity without hematopoietic lineage cell contamination. Immunofluorescence staining showed labeled BM-MSCs in pancreatic islets of group III (Fig. 1c) and group V (Fig. 1d).
Characterization and pancreatic homing of bone marrow mesenchymal stem cells (BM-MSCs). a Photomicrographs of cultured BM-MSCs showing fibroblast-like stem cells, b fluorescence-activated cell sorting (FACS) of BM-MSCs: the cells demonstrated the expected phenotype for rat MSCs (positive expression of CD105 and CD90 and negative expression of CD34 and CD45), c and d fluorescent microscopic image of sections in pancreas of rat showing the red fluorescence of BM-MSCs labeled with PKH26 two weeks after implantation in BM-MSCs treated group III with T1 Streptozotocin (STZ)-induced diabetes and group IV with T2 high-fat diet (HFD)/STZ-induced diabetes, respectively (X200), e body weights of the different experimental groups with respect to experimental time; results were expressed as mean ± standard deviation (SD). Group I: healthy control group, group II: untreated T1 STZ-induced diabetic rats, group III: MSCs treated T1 STZ-induced diabetic rats, group IV: untreated T2 HFD-STZ induced diabetic rats, group V: MSCs treated T2 HFD-STZ induced diabetic rats
Body weight
Body weights of different experimental groups were presented in Fig. 1e. Body weight of the healthy control group I was significantly elevated, directly, with experimental time (190.25, 201.43 and 415.43 g on the 1st, 7th and 42nd day, respectively). Groups IV and V showed a significant increase in body weights after two weeks of HFD administration (211.53 and 209.53 g, respectively). On the 7th day, either in groups II or IV, diabetes induction significantly decreased animals’ body weights (177.54 and 201.61 g, respectively). No significant difference was noticed between body weights of group V on the 1st and 42nd day (203.43 and 201.63 g, respectively). On the other hand, the body weight of group V (201.63 g) was significantly higher than that of group IV (187.64 g) on the 42nd day. No significant difference was noticed between body weights of groups II and III on the 42nd day (168.24 and 172.44 g, respectively).
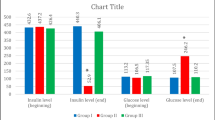
Effect of BM-MSCs transplantation on FBG levels after diabetes induction
Significantly elevated FBG levels were developed in groups II and IV (490.68 ± 0.53 and 420.45 ± 0.76 mg/dl, respectively) when compared to control group I (99.04 ± 0.11 mg/dl). Group II showed no significant differences in FBG levels on the 7th and 42nd day (490.68 ± 0.53 and 500.63 ± 0.13 mg/dl, respectively). While group IV showed an increase in FBG levels on the 42nd day (504.36 ± 0.74) when compared to that on the 7th day (420.45 ± 0.76 mg/dl). A significant reduction in FBG levels were recorded in BM-MSCs treated groups III and V (142.87 and 136.47 mg/dl, respectively) on the 42nd day when compared to that of the 7th day (Table 1).
Effect of BM-MSCs transplantation on insulin levels after diabetes induction
During T1D induction in group II, a significant reduction of the insulin levels (1.9 ± 0.02 ng/ml) was recorded on the 7th day, this reduction continued to reach 1.3 ng/ml on the 42nd day of the experiment (Table 2). On the other hand, T2D in group IV showed a non-significant difference in insulin level during all the experimental periods (4.5 ± 0.41 and 4.6 ± 0.56 ng/ml) at 7th to 42nd day, respectively. A significant elevation in insulin level (3.6 ± 1.10 ng/ml) was recorded in group III after BM-MSCs transplantation on the 42nd day when compared to that of the 7th day (1.50 ± 0.21 ng/ml). On day 42, group V showed a significant upregulation of insulin to a level comparable to that of the healthy control group (group V: 5.1 ± 1.44 Vs group I: 5.6 ± 0.16 ng/m) (Table 2).
Effect of BM-MSCs transplantation on pancreatic pro-inflammatory cytokines and serum CRP during treatment of T1 and T2D
Induction of T1D and T2D in groups II and IV developed a significant elevation of pancreatic pro-inflammatory cytokines (IL-1β, TNF-α and IL-6) when compared to healthy control group I. IL-1β serum level was highly elevated in group II (981.63 pg/mg) more than in group IV (567.69 pg/mg). On the contrary, levels of TNF-α and IL-6 (883.47 and 756.39 pg/mg, respectively) were significantly elevated in untreated T2D group IV than those of untreated T1D group II (548.26 and 579.21 pg/mg) for TNF-α and IL-6, respectively). A significant reduction in cytokine levels was recorded in BM-MSCs treated groups III and V. In group V Level of IL-1β, was non-significantly different (245.23 ± 0.99 pg/mg) from that of healthy control group I (205.87 ± 0.56 pg/mg) while the levels of TNF-α and IL-6 were significantly reduced (648.66 ± 0.68 and 440.01 ± 0.21 pg/mg, respectively) when compared to T2D group IV (883.47 ± 0.47 and 756.39 ± 0.67 pg/mg, respectively). However, their levels remained significantly elevated when compared to healthy control group I. In group III, the IL-6 level was significantly decreased (220.45 ± 0.56 pg/mg) when compared to group II (579.21 ± 1.45 pg/mg), to reach a comparable level with the group I (199.56 ± 2.10 pg/mg). Also, we have noticed the same trend of CRP level, in T1D and T2D, groups II and IV (34.11 ± 0.43 and 24.77 ± 0.36 mg/ml, respectively) was significantly elevated in comparison to healthy control group I (4.21 ± 0.11 mg/ml). BM-MSCs transplantation significantly decreased the CRP levels in groups III and V (5.40 ± 0.77 mg/ml and 4.32 ± 0.41 mg/ml, respectively) (Table 2).
BM-MSCs transplantation alleviated histopathological parameters of the pancreas in T1 and T2D
Healthy control (group I) showed average-sized pale-staining islets of Langerhans with predominating β-cells with pale blue cytoplasm in the center and less frequently alpha cells with pink cytoplasm in the periphery with average intervening blood capillaries, average ducts, and average exocrine areas (Fig. 2a, b). Pancreatic section of T1 STZ-induced diabetes (group II) recorded few small-sized hypocellular pale-staining islets of Langerhans with scattered apoptotic β-cells and markedly edematous cytoplasm. Few alpha cells in the periphery dilated intervening blood capillaries, dilated ducts, and average exocrine areas were noticed in group II (Fig. 2c, d). Pancreatic sections in BM-MSCs treated T1D (Group III), showed average-sized normocellular islets of Langerhans with few scattered apoptotic β-cells, few alpha cells in the periphery, mildly dilated intervening blood capillaries, and average exocrine areas (Fig. 2e, f). In STZ/HFD-induced T2D (group IV), a hypocellular edematous islet with scattered apoptotic β-cells and dilated intervening blood capillaries, and average exocrine areas were observed (Fig. 2g, h). STZ/HFD diabetic group V, BM-MSCs treated rats, showed average-sized normocellular islets of Langerhans with scattered apoptotic β-cells and others with vacuolated cytoplasm, mildly dilated intervening blood capillaries, average ducts, and average exocrine areas (Fig. 2i, j).
Rat pancreatic sections stained with H&E showing a average-sized pale-staining islets of Langerhans (thick arrow), average ducts (thin arrows), and average exocrine areas (dashed arrow) (healthy control group I, X 200), b high power view predominating beta cells with pale blue cytoplasm (thick arrows) and less frequently alpha cells with pink cytoplasm in the periphery (thin arrow), average intervening blood capillaries (arrowhead), and average exocrine areas (dashed arrow) (healthy control group I, X 400), c few small-sized hypocellular pale staining islets of Langerhans (thick arrow), average ducts (thin arrow), and average exocrine areas (dashed arrow) (untreated group II with T1 Streptozotocin (STZ)-induced diabetes, X200), d small-sized hypocellular islets with scattered apoptotic beta cells (thick arrow) and markedly edematous cytoplasm (dashed arrow), and mildly dilated intervening blood capillaries (thin arrow) (untreated group II with T1 STZ-induced diabetes, X400), e average-sized normocellular islets (thick arrow), average ducts (dashed arrow), and average exocrine areas (thin arrow) (bone marrow mesenchymal stem cells (BM-MSCs)-treated group III with T1 STZ-induced diabetes, X200), f average-sized normocellular islets with average beta cells (black arrow), scattered large cells with bright eosinophilic cytoplasm (dashed arrow), and average intervening blood capillaries (thin arrow) (BM-MSCs-treated group III with T1 STZ-induced diabetes, X400), g small-sized islets of Langerhans (thick arrow), average ducts (thin arrow), and average exocrine areas (dashed arrow) (untreated group IV with T2 high-fat diet (HFD)/STZ-induced diabetes, X200), h small-sized hypocellular islets with few beta cells with vacuolated cytoplasm (thick arrow), and mildly dilated intervening blood capillaries (dashed arrow) (untreated group IV with T2 HFD/STZ-induced diabetes, X400), i average-sized normocellular islets of Langerhans (thick arrow), average ducts (dashed arrow), and average exocrine areas (thin arrow) (BM-MSCs-treated group V with T2 HFD/STZ-induced diabetes, X200), j average-sized normocellular islet with scattered beta cells with vacuolated cytoplasm (thick arrow), and average intervening blood capillaries (thin arrow) (BM-MSCs-treated group V with T2 HFD/STZ-induced diabetes, X400)
Effect of BM-MSCs transplantation on pancreatic insulin and NF-кB in T1 and T2D
Marked cytoplasmic reactivity (+++) to insulin antibodies in more than 90% of the islet cells was observed in pancreatic sections of control group I (Fig. 3a) and BM-MSCs treated groups III (T1D, Fig. 3e) and V (T2D, Fig. 3i). On the other hand, mild cytoplasmic reactivity (+) to insulin antibodies in less than 20% of Islet cells was noticed in T1 and T2-induced diabetic groups II and IV (Fig. 3c and g, respectively). Negative cytoplasmic reactivity for NF-кB was observed in pancreatic sections of control group I and BM-MSCs treated groups III and V (Fig. 3b, f and i). Marked cytoplasmic reactivity for NF-кB was observed in pancreatic islet cells of diabetic groups II and IV (Fig. 3d and h).
Immunohistochemical staining of rat pancreatic sections showing a marked cytoplasmic reactivity (+++) (thin arrow) to insulin antibodies (in more than 90% of Islet cells) with few peripheral negative cells (thick arrow) (healthy control group I, X400), b negative cytoplasmic reactivity for nuclear factor-kappa B (NF-кB) (0%) in Islet cells (arrows) (healthy control group I; X400), c mild cytoplasmic reactivity (+) to insulin antibodies (in less than 20% of Islet cells) (arrows) (untreated group II with T1 Streptozotocin (STZ)-induced diabetes, X400), d cytoplasmic reactivity for NF-кB (++) in Islet cells (arrow) (untreated group II with T1 STZ-induced diabetes, X400), e marked cytoplasmic reactivity (+++) (arrows) to insulin antibodies (in more than 90% of Islet cells) with few peripheral negative cells (thin arrow) (bone marrow mesenchymal stem cells (BM-MSCs)-treated group III with T1 STZ-induced diabetes, X400), f negative cytoplasmic reactivity for NF-кB (0) in Islet cells (arrow) (BM-MSCs-treated group III with T1 STZ-induced diabetes, X200), g mild cytoplasmic reactivity (+) to insulin antibodies (in less than 20% of Islet cells) (arrows) (untreated group IV with T2 high-fat diet (HFD)/STZ-induced diabetes, X400), h positive cytoplasmic reactivity for NF-кB (+) in Islet cells (arrow) (untreated group IV with T2 HFD/STZ-induced diabetes, X400), i moderate cytoplasmic reactivity (++) (thick arrows) to insulin antibodies (in more than 90% of Islet cells) with few peripheral negative cells (thin arrow) (BM-MSCs-treated group V with T2 HFD/STZ-induced diabetes, X400), j mild cytoplasmic reactivity for NF-кB (+) in Islet cells (arrows) (BM-MSCs-treated group V with T2 HFD/STZ-induced diabetes, X400)
Discussion
Traditional insulin therapy helps to manage blood glucose levels in diabetic patients. However, it has been shown to be ineffective over time causing loss of glycaemic control (Pasquel et al. 2021). In addition, due to a lack of pancreatic cells, cell rejection, the use of immunosuppressive medications, and other factors; islet transplantation therapy is limited (Hussain and Theise 2004). Because of their low immunogenic potential, immune-privileged, and immunomodulating features, SCs therapies could help overcome these restrictions (Petrus-Reurer et al. 2021). SCs can also be genetically modified, allowing the desired MHC complex to be injected to reduce the risk of immunological rejection (Kumar et al. 2008). MSCs have also been linked to the inhibition of T-cell proliferation, the formation of dendritic cells, and the proliferation of B-cells (Weiss and Dahlke 2019).
Multipotent MSCs, are self-regenerating cells found in nearly all postnatal organs and tissues (Fitzsimmons et al. 2018). BM-MSCs are multipotent cells that can develop into fully functioning insulin-producing cells; they represent a prospective source of insulin-producing cells (Gabr et al. 2014). Successful SCs therapy can eradicate diabetes etiology and improve the pancreas regeneration (Bhartiya 2016). Various immunomodulatory effects, with various cytokine secretion patterns have been observed following the delivery of MSCs in earlier investigations (Yin et al. 2019), but the results are frequently conflicting and a complete picture is still insufficient. Moreover, cytokines are a significant issue of exploration for understanding a lot of diseases (Farid et al. 2013), disorders and types of involved immune cells in these disorders (Farid et al. 2020; Madbouly et al. 2021b). Therefore, the present study aimed to compare the pancreatic pro-inflammatory cytokine profile in T1 and T2D in male Sprague Dawley rats after BM-MSCs transplantation. Also, the effect of BM-MSCs on pancreatic NF-κB was evaluated through immunohistochemical methods. The MSCs localization in the pancreas was demonstrated through histological methods.
In this study, BM-MSCs were isolated and characterized by FACs analysis, where cells have fibroblast-like morphology and were adhered to the flask surface. FACs analysis showed a high expression of CD90 and CD105 and negative staining for CD34 and CD45 markers indicating that the cultured cells were of mesenchymal origin as well as of high purity without haematopoietic lineage cell contamination. After DM induction, rats received (2 × 106 cells/rat) MSCs in 0.2 ml DMEM by i.v. injection. The dose of MSCs is an important determinant of glucose-insulin homeostasis outcome. The doses vary from 2 to 10 million cells either as a single injection (Azab et al. 2011) or as multiple injections (Banerjee et al. 2005) have been used in animal experiments.
In this study, we observed transplanted labeled BM-MSCs in the pancreas of the recipient rats which indicated the selective homing of BM-MSCs to the pancreas. The homing ability of BM-MSCs means their ability to migrate into injured sites, and their capacity to differentiate into local components of the injured sites and the ability to secrete chemokines, cytokines, and growth factors that help in tissue regeneration (Fu et al. 2019; Kang et al. 2012) suggested the role of inflammatory cytokines such as transforming growth factor (TGF)-1β, IL-1β, and TNF-α in elevation of the production of matrix metalloproteinases in MSCs, resulting in a substantial stimulation of chemotactic migration across the extracellular environment to reach the damaged tissue. The role of chemokines and growth factors as migratory cues in MSC trafficking to the damaged region have been proven (Karp and Teo 2009). This ability of implanted MSCs to seek out the site of tissue damage has been demonstrated in bone or cartilage fractures (Murphy et al. 2003), myocardial infarction (Barbash et al. 2003), and ischemic cerebral injury (Ji et al. 2004; Sordi et al. 2005) provided evidence that BM-MSCs are attracted by pancreatic islets in vitro and in vivo, and confirmed that CXCL12 and its ligand CXCR4 play an important role in homing. Intravenous delivery of BM-MSCs, which was applied in the present work, was found to be efficient for their specific migration to the site of injury (Ji et al. 2004; Leibacher and Henschler 2016).
Several studies have observed FBG and serum insulin levels as biomarkers of STZ’s diabetogenic action (Ahangarpour et al. 2018). In this study, DM induction significantly increased FBG levels and reduced insulin levels in the serum of both T1 and T2 diabetic rats. The elevated blood glucose level was suggested to stimulate free radical production, oxidative stress and lipid peroxidation (Tangvarasittichai 2015). In the present data, BM-MSCs transplantation significantly dropped FBG levels and upgraded insulin levels in both T1 and T2 diabetic rats on the 42nd day compared to their levels on the 7th day of the experimental period. The improvement of the β-cells mass and/or functions include fusion of BM-MSCs with islet cells or trans-differentiation of MSCs into β-cells. Yanai et al. (2013) showed that co-transplantation of electrofused MSCs and islet cells in rats improved blood glucose profile due to bi-directional reprogramming of both β-cells and MSCs nuclei, thereby allowing the insulin gene expression. Moreover, Khatri et al. (2020) suggested the proliferation of pancreatic β-cells and provided evidence for the effect of MSCs on the endocrine part of the pancreas.
In this study, the histopathological sections of the pancreas revealed a few small-sized hypocellular pale-staining islets of Langerhans with scattered apoptotic β-cells and markedly edematous cytoplasm in T1 induced diabetic rats. In addition, T2 diabetic induction showed hypocellular edematous islet with scattered apoptotic β-cells and dilated intervening blood capillaries, and average exocrine areas. Upon BM-MSCs transplantation, the pancreas of diabetic rats treated with BM-MSCs showed histopathological improvement in parallel with the biochemical enhancement where average-sized normocellular islets of Langerhans with few scattered apoptotic β-cells were noticed. Furthermore, a marked cytoplasmic reactivity to anti-insulin antibodies was evidenced in both MSCs treated T1 and T2 diabetic rats; with negative or moderate cytoplasmic reactivity for NF-кB. The mechanism of BM-MSCs in repairing islets of Langerhans and regeneration of resident β-cells was discussed in some studies as it was suggested that these cells are able to migrate and settle in the damaged tissues after the systemic intravenous transfer (Anzalone et al. 2010).
Previous studies hypothesized the activation of a final common pathway involving IL-1β, NF-κB, and Fas in T1 and T2D, in spite of different origins. As in T1D, invading immune cells release cytokines such as IL-1β, TNF-α, and IFN-γ. IL-1β and/or TNF-α with IFN-γ cause apoptosis in β-cells by activating the transcription factors NF-кB and STAT-1, which induce NO production and β-cells apoptosis (Ortis et al. 2010). While in T2D, β-cells themselves release IL-1β in response to exposure to high glucose levels, which attracts macrophages that serve as an additional source of IL-1β and other cytokines, contributing to β-cell failure (Zhao et al. 2014). IL-6 was found to play a role in the inflammatory response linked to insulin disturbance. Hundhausen et al. (2016) demonstrated a link between IL-6 and T cell migration; increasing the probability that T cell responses to IL-6, in T1D, contributed to disease pathogenesis by influencing T cell homing to foci of islet inflammation. The authors also suggested that IL-6 reactivity may be used to predict chronic progression. Ellingsgaard et al. (2008) indicated that, in addition to a systemic elevation in IL-6, local islet IL-6 levels were also enhanced in T2D, implying that IL-6 may have a function in the pancreatic islets in this illness. Furthermore, the elevation of local islet IL-6, found to accelerate cell apoptosis. In the present work, DM induction significantly elevated the inflammatory cytokines (IL-1 β, TNF-α and IL-6) in both T1 and T2 diabetic groups when compared to the control group. Many studies have discussed the role of inflammatory markers in the progression of both types of DM. Where an elevation in CRP and IL-1β levels in patients with T1 induction showed hypocellular edematous islet with scattered apoptotic β-cells diabetes has been reported. Daryabor (2020) reported that T2 diabetic patients have higher levels of IL-6 and TNF-α than healthy controls. Our results were in agreement with these previous studies; where the levels of CRP and pancreatic IL-1β were higher in T1 rather than those of T2 diabetic rats. On the contrary, pancreatic TNF-α and IL-6 levels exhibited an opposite pattern such that their levels were higher in T2 rather than in T1 diabetic rats.
One of the predominant components of MSCs is their immunomodulatory functions in response to the local environment (Petryk and Shevchenko 2020). Numerous mediators, including TGF- β, indolamine 2, 3-dioxygenase, inducible nitric oxide synthase (iNOS), and prostaglandin E2 are thought to be responsible for MSCs’ immunomodulatory function. BM-MSCs produce numerous regulatory cytokines that have immunomodulatory activities on T cells, B cells, dendritic cells, and natural killer cells (Hao et al. 2013). In the current work, BM-MSCs transplantation in T1 diabetic rats, decreased pancreatic IL-1β level compared to that of the diabetic untreated group; while its level remained higher than that of the healthy control group. BM-MSCs treated T2 diabetic group, also, showed a significant downregulation of IL-1β level, compared to untreated diabetic group IV but with non-significant difference from that of healthy control group I. At the same time, the levels of TNF-α, IL-6 and CRP in BM-MSCs treated T1 diabetic group III dropped significantly to be comparable to those of normal control group I. While, in BM-MSCs treated T2 diabetic group V, the levels of TNF-α and IL-6 were reduced but still significantly higher than those of healthy control group I.
The anti-inflammatory efficacy of MSCs was found to be via different mechanisms involving both innate and adaptive immune components; one mechanism involves blocking the complement-induced proliferation of peripheral blood mononuclear cells. Another mechanism involves the regulation of macrophage activity in the healing tissue area. When MSCs interact with macrophages, they boost their polarization from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype cells, the issue which explains the inhibited production of pro-inflammatory cytokines like TNF-α, IL-1β, IL-6 and IFN-γ and increases the secretion of anti-inflammatory cytokines like IL-10 (Trzyna and Banaś-Ząbczyk 2021; Monguió-Tortajada et al. 2017) demonstrated that MSCs also increase the expression of CD39 and CD73 in monocytes, causing the environment to shift from pro-inflammatory to anti-inflammatory.
Cytokine-mediated NF-κB activation stimulates the production of a variety of pro-apoptotic and pro-inflammatory gene (Farid et al. 2021). The present histochemical studies revealed a concurrent activation of NF-κB with the elevated levels of pancreatic IL-1β and TNF-α, this was in harmony with recent suggestions that IL-1β and/or soluble TNF- α are capable of activating the NF-κB non-canonical pathway in β-cells (Meyerovich et al. 2016). In parallel to the reduction in the levels of inflammatory markers after BM-MSCs transplantation, the present data revealed inhibition of the cytoplasmic reactivity of NF-κB (negative or moderate) in treated T1D and T2D groups. Salem et al. (2014) also reported that mice with continuous NF-κB activation in β-cells spontaneously develop insulitis and immune-mediated diabetes. Eldor et al. (2006) found that, in vivo inhibition of NF-κB signaling in mouse β-cells protects STZ-induced diabetes. While Kim et al. (2007) reported that, NF-κB inactivation in β-cells from non-obese diabetic mice promotes disease development.
This study had some limitations. First, we only compared the effects of BM-MSCs on the two types of DM. In future research, MSCs from other origins should be compared. Second, the origin of the tissues is just one of the vital factors influencing MSC heterogeneity. Other relevant aspects which influence the therapeutic efficacy of MSC such as the dose, isolation and preparation methods need to be extensively explored. Third, the lack of adequate evidences about the impact of the diabetes duration on BM-MSCs therapy. Hence, based on our findings we suggest that future studies take into consideration the disease duration factor.
In conclusion, BM-MSCs transplantation, in T1 and T2D, has led to a reduction in blood glucose levels; which was accompanied by an increase in insulin level. Furthermore, the biochemical and histological findings indicated a significant improvement in pancreatic islets of Langerhans, as well as increased insulin release from the islets-cells. Where the immunohistochemical examination revealed moderate cytoplasmic reactivity for insulin in pancreatic sections of BM-MSCs-treated groups III and V which indicated the islets neogenesis. This can be explained by the immunomodulatory effect of BM-MSCs and their downregulation of NF-κB and the pro-inflammatory cytokines; that in turn restored the homeostasis of the immune microenvironment and led to the neogenesis of β-cells function.
Data availability
All data generated or analysed during this study are included in this published article.
References
Ahangarpour A, Shabani R, Farbood Y (2018) The effect of betulinic acid on leptin, adiponectin, hepatic enzyme levels and lipid profiles in streptozotocin-nicotinamide-induced diabetic mice. Res Pharm Sci 13(2):142–148. https://doi.org/10.4103%2F1735-5362.223796
Alberti K, George MM, Zimmet P, Shaw J (2007) International Diabetes Federation: A consensus on type 2 diabetes prevention. Diabet Med 24(5):451–463. https://doi.org/10.1111/j.1464-5491.2007.02157.x
Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G (2010) New emerging potentials for human Wharton’s Jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev 19:423–438. https://doi.org/10.1089/scd.2009.0299
Azab NI, Al Kholy AF, Salem RF, Gabr H, Abd EI AM (2011) Comparison between bone marrow derived mesenchymal stem cells and hematopoietic stem cells in Β-islet transdifferentiation. Stem Cell 2(1):1–10. https://doi.org/10.7537/marsscj020111.01
Banerjee M, Saxena M (2014) Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes 5:493–504. https://doi.org/10.4239/wjd.v5.i4.493
Banerjee M, Kumar A, Bhonde RR (2005) Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun 328:318–325. https://doi.org/10.1016/j.bbrc.2004.12.176
Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J (2003) Systemic delivery of bone marrowderived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108:863–868. https://doi.org/10.1161/01.cir.0000084828.50310.6a
Bhartiya D (2016) Stem cells to replace or regenerate the diabetic the pancreas: huge potential & existing hurdles. Indian J Med Res 143(3):267–274. https://doi.org/10.4103/0971-5916.182615
Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, American College of Sports Medicine, American Diabetes Association (2010) Exercise and type 2 diabetes: American College of Sports Medicine and the american Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc 42(12):2282–2303. https://doi.org/10.1249/mss.0b013e3181eeb61c
Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K (2020) The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol 11:1582. https://doi.org/10.3389/fimmu.2020.01582
El-Hadad G, Farid AA, El Amir AM, Madbouly NA (2022) Hazard effects of chronic consumption of sucralose and saccharin-sodium cyclamate mixture in murine model. Egypt J Chem 65(5):279–289. https://doi.org/10.21608/ejchem.2021.99319.4622
Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D (2006) Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci USA 103:5072–5077. https://doi.org/10.1073/pnas.0508166103
Ellingsgaard H, Ehses JA, Hammar EB, Lommel LV, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, Halban PA, Schuit FC, Donath MY (2008) Interleukin-6 regulates pancreatic α-cell mass expansion. Proc Natl Acad Sci USA 105:13163–13168. https://doi.org/10.1073/pnas.0801059105
Farid A (2023) Preparation of polyclonal anti-Schistosoma mansoni cysteine protease antibodies for early diagnosis. Appl Microbiol Biotechnol 107(5–6):1609–1619.
Farid A, Malek AA, Rabie I, Helmy A, El Amir AM (2013) Overview on cysteine protease inhibitors as chemotherapy for Schistosomiasis mansoni in mice and also its effect on the parasitological and immunological profile. Pak J Biol Sci 16(24):1849–1861. https://doi.org/10.3923/pjbs.2013.1849.1861
Farid A, Tawfik A, Elsioufy B, Safwat G (2020) Narrow band ultraviolet B therapy deactivates Th1/Th17 pathway and activates Th2 cytokines secretion in egyptian psoriatic arthritis patients. J Radiation Res Appl Sci 13(1):356–361. https://doi.org/10.1080/16878507.2020.1742443
Farid A, El-Dewak M, Diab A (2021) Anti-apoptotic and antioxidant effects of melatonin protect spleen of whole body γ-irradiated male Sprague-dawley rats. Int J Radiat Res 19(4):861–872. https://doi.org/10.52547/ijrr.19.4.12
Farid A, Haridyy H, Ashraf S, Ahmed S, Safwat G (2022a) Co-treatment with grape seed extract and mesenchymal stem cells in vivo regenerated beta cells of islets of Langerhans in pancreas of type I-induced diabetic rats. Stem Cell Res Ther 13(1):528. https://doi.org/10.1186/s13287-022-03218-y
Farid A, Moussa P, Youssef M, Haytham M, Shamy A, Safwat G (2022b) Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J Biol Sci 29(7):103313. https://doi.org/10.1016/j.sjbs.2022.103313
Farid A, Yousry M, Safwat G (2022c) Garlic (Allium sativum Linnaeus) improved inflammation and reduced cryptosporidiosis burden in immunocompromised mice. J Ethnopharmacol 292:115174. https://doi.org/10.1016/j.jep.2022.115174
Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA (2018) Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int 2018:8031718. https://doi.org/10.1155/2018/8031718
Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG (2019) Mesenchymal stem cell migration and tissue repair. Cells 8(8):784. https://doi.org/10.3390/cells8080784
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70:5471–54720. https://doi.org/10.1002/0471141755.ph0547s70
Gabr M, Zakaria MM, Refaie AF, Khater SM, Ashamallah SA, Ismail AM, El-Badri N, Ghoneim MA (2014) Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int 2014:ID832736. https://doi.org/10.1155/2014/832736
Gomez-Lopez S, Lerner RG, Petritsch C (2014) Asymmetric cell division of stem and progenitor cells during homeostasis and cancer. Cell Mol Life Sci 71(4):575–597. https://doi.org/10.1007/s00018-013-1386-1
Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, Donaghue KC, International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group, Magliano DJ, Maniam J, Orchard TJ, Rai P, Ogle GD (2022) Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol 10(10):741–760. https://doi.org/10.1016/s2213-8587(22)00218-2
Hao H, Liu J, Shen J, Zhao Y, Liu H, Hou Q, Tong C, Ti D, Dong L, Cheng Y, Mu Y, Liu J, Fu X, Han W (2013) Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. J Biochem Biophys Res Commun 436:418–423. https://doi.org/10.1016/j.bbrc.2013.05.117
Hegazy S, Farid A, Rabae I, El-Amir A, (2015) Novel IMB-ELISA assay for rapid diagnosis of human toxoplasmosis using SAG1 antigen. Jpn J Infect Dis 68(6):474–480. https://doi.org/10.7883/yoken.JJID.2014.444
Hundhausen C, Roth A, Whalen E, Chen J, Schneider A, Long SA, Wei S, Rawlings R, Kinsman M, Evanko SP, Wight TN, Greenbaum CJ, Cerosaletti K, Buckner JH (2016) Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Sci Transl Med 8(356):356ra119. https://doi.org/10.1126/scitranslmed.aad9943
Hussain MA, Theise ND (2004) Stem-cell therapy for diabetes mellitus. Lancet 364:203–205. https://doi.org/10.1016/s0140-6736(04)16635-x
Ji JF, He BP, Dheen ST, Tay SS (2004) Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 22:415–427. https://doi.org/10.1634/stemcells.22-3-415
Kang SK, Shin IS, Ko MS, Jo JY, Ra JC (2012) Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells International 2012:342968. https://doi.org/10.1155/2012/342968
Karp JM, Teo GSL (2009) Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4(3):206–216. https://doi.org/10.1016/j.stem.2009.02.001
Khatri R, Mazurek S, Petry SF, Linn T (2020) Mesenchymal stem cells promote pancreatic β-cell regeneration through downregulation of FoxO1 pathway. Stem Cell Res Ther 11:497. https://doi.org/10.1186/s13287-020-02007-9
Kim S, Millet I, Kim HS, Kim JY, Han MS, Lee MK, Kim KW, Sherwin RS, Karin M, Lee MS (2007) NF-kappa B prevents beta cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci USA 104:1913–1918
Kumar S, Chanda D, Ponnazhagan S (2008) Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther 15:711–715. https://doi.org/10.1073/pnas.0610690104
Leibacher J, Henschler R (2016) Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther 7:7. https://doi.org/10.1186/s13287-015-0271-2
Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan Z-R, Roberts AI, Shi S, Le AD, Shi Y (2012) Mesenchymal stem cells: hepatology 26 a double-edged sword in regulating immune responses. Cell Death Differ 19:1505–1513. https://doi.org/10.1038/cdd.2012.26
Madbouly N, El Amir A, Abdel Kader A, Rabee I, Farid A (2021a) The immunomodulatory activity of secnidazole-nitazoxanide in a murine cryptosporidiosis model. J Med Microbiol 70(3):001327. https://doi.org/10.1099/jmm.0.001327
Madbouly N, Azmy A, Salama A, El-Amir A (2021b) The nephroprotective properties of taurine-amikacin treatment in rats are mediated through HSP25 and TLR-4 regulation. J Antibiot (Tokyo) 74(9):580–592. https://doi.org/10.1038/s41429-021-00441-2
Meyerovich K, Fukaya M, Terra LF, Ortis F, Eizirik DL, Cardozo AK (2016) The non-canonical NF-kappaB pathway is induced by cytokines in pancreatic beta cells and contributes to cell death and proinflammatory responses in vitro. Diabetologia 59:512–521. https://doi.org/10.1007/s00125-015-3817-z
Miniawy HME, Ahmed KA, Ibrahem EM, Sabry D, Tahany A, Amer E (2017) Camel’s wharton jelly mesenchymal stem cell is a novel tool for regeneration of induced diabetes mellitus. J Transl Sci 3(5):1–6. https://doi.org/10.15761/JTS.1000196
Monguió-Tortajada M, Roura S, Gálvez-Montón C, Franquesa M, BayesGenis A, Borràs FE (2017) Mesenchymal stem cells induce expression of CD73 in human monocytes in vitro and in a swine model of myocardial infarction in vivo. Front Immunol 8:1577. https://doi.org/10.3389/fimmu.2017.01577
Motawea SM, Noreldin RI, Naguib YM (2020) Potential therapeutic effects of endothelial cells trans-differentiated from Wharton’s Jelly-derived mesenchymal stem cells on altered vascular functions in aged diabetic rat model. Diabetol Metab Syndr 12:40. https://doi.org/10.1186/s13098-020-00546-y
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474. https://doi.org/10.1002/art.11365
Ortis F, Naamane N, Flamez D, Ladrière L, Moore F, Cunha DA, Colli ML, Thykjaer T, Thorsen K, Orntoft TF, Eizirik DL (2010) Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 59(2):358–374. https://doi.org/10.2337/db09-1159
Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE (2021) Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol 9(3):174–188. https://doi.org/10.1016/s2213-8587(20)30381-8
Patlak M (2002) New weapons to combat an ancient disease: treating diabetes. FASEB J 16:1853
Petrus-Reurer S, Romano M, Howlett S, Jones JL, Lombardi G, Saeb-Parsy K (2021) Immunological considerations and challenges for regenerative cellular therapies. Commun Biol 4:798. https://doi.org/10.1038/s42003-021-02237-4
Petryk N, Shevchenko O (2020) Mesenchymal stem cells anti-inflammatory activity in rats: proinflammatory cytokines. J Inflamm Res 13:293–301. https://doi.org/10.2147/jir.s256932
Salem HH, Trojanowski B, Fiedler K, Maier HJ, Schirmbeck R, Wagner M, Boehm BO, Wirth T, Baumann B (2014) Long-term IKK2/NF-kappaB signaling in pancreatic beta-cells induces immune-mediated diabetes. Diabetes 63:960–975. https://doi.org/10.2337/db13-1037
Saxena M, Modi DR (2014) Inflammation and diabetes. Microinflammation 1:110. https://doi.org/10.4172/2381-8727.1000110
Shater H, Fawzy M, Farid A, El-Amir A, Fouad S, Madbouly N (2022) The potential use of serum interleukin-21 as biomarker for lupus nephritis activity compared to cytokines of the tumor necrosis factor (TNF) family. Lupus 31(1):55–64. https://doi.org/10.1177/09612033211063794
Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L (2005) Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 106:419–427. https://doi.org/10.1182/blood-2004-09-3507
Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6(3):456–480. https://doi.org/10.4239/wjd.v6.i3.456
Trzyna A, Banaś-Ząbczyk A (2021) Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy. Biomolecules 11(6):878. https://doi.org/10.3390/biom11060878
Waterborg JH, Matthews HR (1984) The Lowry method for protein quantitation. Meth Mol Biol 1:1–3. https://doi.org/10.1385/0-89603-062-8:1
Weiss ARR, Dahlke MH (2019) Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol 10:1191. https://doi.org/10.3389/fimmu.2019.01191
Wou C, Unwin N, Huang Y, Roglic G (2019) Implications of the growing burden of diabetes for premature cardiovascular disease mortality and the attainment of the sustainable development goal target 3.4. Cardiovasc Diagnosis Therapy 9(2):140–149. https://doi.org/10.21037/cdt.2018.09.04
Yanai G, Hayashi T, Zhi Q, Yang K-C, Shirouzu Y, Shimabukuro T, Hiura A, Inoue K, Sumi S (2013) Electrofusion of mesenchymal stem cells and islet cells for diabetes therapy: a rat model. PLoS One 8:e64499. https://doi.org/10.1371/journal.pone.0064499
Yin JQ, Zhu J, Ankrum JA (2019) Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng 28:90–104. https://doi.org/10.1038/s41551-018-0325-8
Zhao G, Dharmadhikari G, Maedler K, Meyer-Hermann M (2014) Possible role of interleukin-1β in type 2 diabetes onset and implications for anti-inflammatory therapy strategies. PLoS Comput Biol 10(8):e1003798. https://doi.org/10.1371/journal.pcbi.1003798
International Diabetes Federation [IDF] (2017) Diabetes Atlas, 8th Edn. Brussels: International Diabetes Federation. ISBN: 978-2-930229-87-4
World Health Organization (WHO) (2014) Global status report on noncommunicable diseases 2014. ISBN: 9789241564854
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AF: Conceptualization, methodology and writing the manuscript. LA, NM: Data curation, visualization and investigation. NM: Software and validation. All authors have read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All experimental procedure and animal maintenance were approved by the Institutional Animal Care and Use Committee (CU-IACUC), Cairo University, Egypt (CUIF 1719).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farid, A., El-Alfy, L. & Madbouly, N. Bone marrow-derived mesenchymal stem cells transplantation downregulates pancreatic NF-κB and pro-inflammatory cytokine profile in rats with type I and type II-induced diabetes: a comparison study. Biologia 78, 3165–3177 (2023). https://doi.org/10.1007/s11756-023-01436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01436-0