Abstract
Invasive alien plants are one of the most serious threats to agriculture. The growth traits of Solanum elaeagnifolium Cav. in crops and their demographics in invaded vs non-invaded communities were examined. The majority of S. elaeagnifolium germination was observed in the spring compared to the summer. Five stages were distinguished, which started with a short time of seedling and juvenile stages, extended flowering, and fruiting stages, and seed dispersion in the winter season. An increase in shoots/roots ratio, leaf area ratio and leaf mass fraction during growth with the varied rate was proved. The accumulation coefficient of dry mass exceeded 0.93 and was significant (P > 0.001) with great variability within plant parts, and stage intervals. While the high growth rate is influenced by the stages and habitats. The recipient communities are affected negatively by S. elaeagnifolium invasion which is associated with lower diversity, richness, and evenness vs non-invaded communities. High similarities were found in the invaded area and communities. Finally, high and varied growth and plasticity of S. elaeagnifolium characterized their invasion behavior via different habitats. There were suitable determinants indices of diversity that can be used in the comparison between invaded and non-invaded communities. This knowledge may be useful for use in agro-environment protection and to improve the management methods of invasive alien species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are worldwide threats causing ecological, environmental, and economic problems (Weber and Li 2008). Invasive alien plant species pose a serious threat to native ecosystem structure and function (Li et al. 2014). Ecosystem properties can be affected by invasive alien plants (Teixeira et al. 2020) and displace crop species (Wardle et al. 1994). On the other hand, the invasion is controlled by the characteristics of the invaded habitat (Sakai et al. 2001). Invasive weeds in pastures reduce forage quality and yield, impair animal performance, and increase management costs (DiTomaso 2000). While ecologists and land managers have difficulty predicting which species will become invasive (Mack et al. 2000; Kolar and Lodge 2001), the knowledge of invasive plant species biology is necessary to facilitate their management (Chauhan and Johnson 2010).
Silverleaf nightshade (Solanum elaeagnifolium) is an invasive perennial species that originated from North, Central, and South American origin (Henderson 2001), invaded virtually all continents over the past century (Boyd et al. 1984), the Mediterranean basin and Europe (Mekki 2007). It invaded some cultivated areas on the North coast of Egypt (Täckholm 1974; Boulos 2009; Balah 2011) and caused in cotton, grain sorghum, wheat, and lucerne high economic losses (Boyd and Murray 1982; Lemerle and Leys 1991). It is an alternative host for phytophagous insects and plant diseases (Heap and Carter 1999; Qasem 2014). This weed injured crops, causing up to 75% yield loss, as well as an indirect impact by harboring plant pests and diseases (Uludag et al. 2016).
Invasiveness can be related to several traits: copious production of sexual and asexual propagules, the facility for long-distance dispersion, the capacity to tolerate considerable drought, and resistance to herbivory, specifically, toxicity to cattle (Mekki 2007; Scaldaferro et al. 2012). Solanum elaeagnifolium reproduces sexually (Cooley and Smith 1972), which has a massive seed production and different phenotypic plasticity, which could explain their different adaptation and invasiveness in habitats with different water availability (Travlos 2013). Seeds of this weed are dispersed by animal faces, water, machinery, and agricultural products (Boyd et al. 1984), which are viable for at least six years (Stanton et al. 2011). Furthermore, it reproduces by root fragments and creeping lateral roots (Fernandez and Brevedan 1972; Cuthbertson et al. 1976). Whereas, vegetative reproduction was one of the best indicators of invasiveness among woody plants (Reichard 1994). Silverleaf nightshade growth and reproduction were affected significantly by the time of emergence (Zhu et al. 2013a), while, their distribution pattern was affected by soil moisture and rainfall (De Aparicio and Difrieri 1958). It grows on a range of soils although there is some preference for lighter, sandy soils low in organic matter (Heap and Carter 1999). This weed thrives in disturbed areas, specially cultivated and denuded areas (Wassermann et al. 1988). Generally in human-disturbed environments with no or lose tree canopy (Ganatsas et al. 2012; Formozis et al. 2021). Silverleaf nightshade can tolerate relatively high summer temperatures (20–34 °C), low annual rainfall (250–600 mm), saline soil conditions and drought-tolerant (Brunel 2011; Garcia-Fortea et al. 2019; Balah et al. 2021), which explains largely the success of its development in semi-arid bioclimatic zones (Adjim and Tani 2018). Morphological plasticity, adaptability, and a high level of genetic diversity within and between populations are partly explain the establishment and continuing expansion of S. elaeagnifolium (Dekker 1997; Hawker et al. 2006; Zhu et al. 2013b). The abilities of competitive and adaptations as well as spread quickly are traits correlated with invasiveness which makes invasive species very difficult to control (Willis et al. 2010; Wolkovich and Cleland 2014; Tsaballa et al. 2015; Qasem et al. 2019).
Weed surveys are useful for determining the importance of weed species (Thomas 1985). The diversity indices (Simpson and Shannon-Weiner) were useful to assess the shift in the weed population (Cardina et al. 1991). Species entrance into cropped land is mainly governed by the dynamic between habitats that function as a source of species (Williamson 1996). Therefore, the ecological characteristics within plant taxa relate to invasiveness in a particular geographic region (Richardson et al. 1989). Plant invasions often cause reductions in native species richness and overall biodiversity (Hansen et al. 2020). Species abundances are more likely to interact with newly introduced species (Thuiller et al. 2010). The richness decreased in invaded compared to non-invaded localities (Hejda et al. 2009). Whereas, resident plant evenness may be important in suppressing weed invasion into grassland communities (Wilsey and Polley 2002; Tracy and Sanderson 2003).
The study hypothesized that the growth rate of S. elaeagnifolium differed among the crops, localities and seasons due to their plasticity. Also, their ecological impacts can differentiate clearly from the diversity indices via a comparison of invaded vs. non-invaded resident communities, whereas, there were more suitable diversity indices than others for studying this task. The objectives were to study the phenological traits and developmental growth of S. elaeagnifolium in invaded crops and non-invaded communities. Surveillance data about the species relative abundance, diversity and evenness, as well as the coefficient of similarity among localities were provided. This knowledge is relevant for promoting prevention methods for invasive weed agro-ecological problems and control efforts.
Materials and methods
Growth trials of S. elaeagnifolium
The study sites were conducted in the infested area at Borg al-arab in south-west Alexandria and El-Hammam in an eastern area in Matruoh governorates on the north coast of Egypt from 2017 to 2020. These areas are characterized by a moderate climate with average temperatures between 18–30 °C, rains above 50 mm in winter, and average relative humidity of approximately 65% per year. The soil was a sandy loam to loamy sand according to FAO – UNESCO methods and Soil Taxonomy (FAO – UNESCO 1971–1981), with, pH 8.2 and 8.4 and electrical conductivity of 0.819 and 0.854 ds /m in Borg al-arab and El-Hammam respectively. The soil contains the following anions; (1.912 meq/l l) sodium, (1.23 meq/l) potassium, (2.55 meq/l) calcium and cations by (2.39 meq/l) HCO3, (1.98 meq/l), chloride, (2.18 meq/l) sulfate, respectively. The seedling growth of S. elaeagnifolium was observed regularly during the visits to wheat, faba bean, and Egyptian clove crops in winter and sesame, maize in summer, gapes, fig and uncultivated lands over the year. Some plants were marked to determine their age. The studied weed was collected and sorted at different stages, whereas, the numerical phenology ratings were given to phenophases; 1 = seedling, 2 = juvenile, 3 = flowering, 4 = fruiting, and 5 = seed dispersal according to the phenological index technique of West et al. (1971).
Another experiment was conducted in pots to investigate the germination and growth during the year under different environmental factors than their current spreading environment. The plastic pots filled with the same sandy loam soil and placed outdoors (outside the laboratory). The seeds were sown every month at a depth of 0.5 cm after soaking in running water for 24 h to remove the mucilage. The pots were watered at regular intervals. The average temperature during experiments was ranging from 20 to 35 °C. Five pots per harvest regularly after 15, 30, 60, and 90 days to determine the growth rates. To analyze the change in germination rate or germinability during the year, 5 pots were sowed every month, each pot containing 5 seeds. The number of germinated seeds was counted once at the end of the month (four weeks), then the germination percentage was calculated.
Ten plants were collected per life stage from each tested crop and sectioned into roots, shoots, leaves, fruits, and whole plants. These tissues were dried for 24 h at 80 °C and weighed. Numbers of leaves, flowers and branches were recorded. Leaf area per plant was measured with a leaf area meter (C1-203 handheld laser area meter CID, Inc. made in USA).The data of dry mass weight via the time [days from emergence (DFE)] were transferred to natural logarithms for fitting to polynomial regression equations. Plant traits such as leaf area ratio (LAR = plant leaf area/plant mass), root/shoot ratio (R/S ratio, the ratio of root biomass to shoot), leaf mass fraction (LMF = Leaf dry mass ⁄ Total plant dry mass), root mass fraction (RMF = Root dry mass ⁄ Total plant dry mass), stem mass fraction (SMF = Stem dry mass ⁄ Total plant dry mass), specific leaf area (SLA = Leaf area ⁄ Leaf dry mass), specific stem length (SSL = Stem length/ Stem dry mass) were determined. Relative growth rate (RGR) is calculated as RGR = (ln W2 – ln W1) / (t2 – t1), where W1 and W2 are dry weights at times t1 and t2 as described in Cornelissen et al. (1996). Net assimilation rate is defined as the rate of increase of dry weight (W) per unit of leaf area (L) for each time-interval (t2–t1) according to (Gregory 1926) from the formula;
The values for growth were derived from the regression coefficients using the general formula for a second-order polynomial equation f = (y0 + a * x + b * x^2 + c * x^3) derived from the relationship between; F is the phenotypic trait value, y0 are fixed effects of the overall intercept, (a), (b), and (c) are fixed components of the model, x is the weights variable. The phenotypic plasticity index is according to the following formula: IPF = (value of maximum mean – value of minimum mean) / (value of maximum mean) for each trait (Valladares et al. 2000).
Floristic analysis
The flora surveys were conducted between the years 2017 to 2020 in Borg al-arab and El-Hammam areas of Egypt. These studies have monitored a total of 320 samples (160 and 160) of invaded and non-invaded sites respectively (including 20 samples from each crop or rangelands) that were chosen adjacent to each other in each crop and season to represent (maize and sesame) summer and (wheat, faba bean and Egyptian clover) winter crops as well as (fig, grape) fruits (to represent most invaded crops) and rangelands. The number of species or individuals and the total weeds in m2 were counted in a random pattern according to (Thomas et al. 1997). Weeds were counted within quadrates of 100 lengths X 1 m2 wide and taken randomly to assess the individual species densities. Data were summarized by relative abundance (RA) value; the sum of the individual relative frequency (frequencies % divided by the total number of all frequencies), relative field uniformity (the percentage infestation of the field by the species of interest to the surface area occupied by the weed) and relative field density percentages (main field density of k species divided by sum to main field density of all species multiplied by100) according to (Thomas 1985).
-
1
Relative frequency for species k
$$\mathrm{RFk}=\frac{\mathrm{Frequency\ value\ of\ species\ k}}{\mathrm{Sum\ of\ frequency\ value\ for\ all\ species}}\times 100$$ -
2
Relative field uniformity for species k
$$\mathrm{RFU\ k}=\frac{\mathrm{Field\ uniformity\ value\ of\ species\ k}}{\mathrm{Sum\ of\ field\ uniformity\ value\ for\ all\ species}}\times 100$$ -
3
Relative mean field density value of species k
$$\mathrm{RMFDk}=\frac{\mathrm{Main\ Field\ Density\ value\ of\ species\ k}}{\mathrm{Sum\ of\ mean\ field\ density\ value\ for\ all\ species}}\times 100$$ -
4
Relative abundance RA value for species k
$${\mathrm{RA}}_{\mathrm{k}}={\mathrm{RF}}_{\mathrm{k}}+{\mathrm{RFU}}_{\mathrm{k}}+{\mathrm{RMDF}}_{\mathrm{k}}$$
Importance value (IV) = Relative cover (RCi) + Relative density (RDi) + Relative frequency (RFi). Sorenson coefficient of similarity was used to measure the similarity of species compositions between the invaded and non-invaded areas within the localities and seasons (Newsome and Dix 1968) obtained from = [2C / (A + B)] × 100 where C = number of species in common, A = total number of species in the first area and B = total number of species in the second area. The diversity was measured by the Simpson dominance index to characterize species diversity in a community through uniform of species distribution, Shannon-Wiener diversity index to describe the disorder and uncertainty of individual species according to (Smith and Wilson 1996; Shannon and Weaver 1949), richness index (Margalef 1958) and measures species evenness (J') which related to individual distribution according to (Pielous 1975) by Pielou's index (E1), Sheldon’s index (E2), Heip's index (E3), Hill's index (E4), and Modified hill's ratio (E5) to give more information about these communities (Simpson 1949).
Data analysis
The difference among the mean germination values and months were determined by ANOVA, as well as other traits (lengths, leaf numbers, leaf area, leaf weight, main stem weight, root weight and total dry weight) and phenology stages, while the interspecific comparison between growth stages and hast crops were performed using two way A nova using statistical package of SPSS IPM 19 (IBM Corp. Armonk, NY, USA) by Tukey’s test to examine the differences between growth in different stages. Polynomial regression equations were prepared using SigmaPlot software v.12.5 (Systat Software Inc., CA, and USA) of dry weight accumulation per stage.
Results
Phenology of S. elaeagnifolium
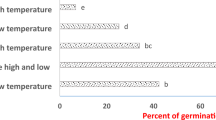
The differences in main germination values and months were significant (F = 117.345, p ≤ 0.00) (Fig. 1). The seed germination was recorded in the spring and summer seasons and the maximum germinability was recorded mainly in March (63.5%) and April (63.8%), respectively. However, there was a remarkable decrease from October (20.05%), November (16.67%), to reach the minimum germination in December (6.33%) and January (6.37%), respectively.
The phenological of S. elaeagnifolium was distinguished by five life stages as follows: seedlings emerge quickly after germination, followed by a juvenile stage that occurs in the winter, spring, and early summer, and then a lengthy flowering stage that occurs in the spring, summer, or autumn seasons. The majority of flowering occurred from May to September and is prolonged to November. While, the matured fruits started with few numbers from April to July, the majority of the matured fruits were found from August to November. Lastly, in the winter season, most of the seed dispersion occurred from December to next February or later if the plant did not remove. S. elaeagnifolium life cycle has a wide range of 40 to 90 days with an average of 65 days to transform from emergence to produce matured fruits depending on the condition and habitats (Fig. 2).
In general, S. elaeagnifolium growth is very rapid in spring and summer as compared with the growth in autumn seasons and there was a gradual increase of phytomass dry weight from the seedling to the final stage at different rates. The plant growth and development were significantly increased over the times (F = 66.91, p ≤ 0.00) lengths, (F = 19.94, p ≤ 0.00) leaf numbers. (F = 891.182, p ≤ 0.00) leaf area, (F = 59.09, p ≤ 0.000) leaf weight, (F = 110.77, p ≤ 0.000) root weight, (F = 117.73, p ≤ 0.000) stem weight, (F = 93.45, p ≤ 0.000) of total dry weight, respectively. Likewise, the ratio of shoots/ roots increased. The ratio of leaf area and leaf mass fraction has highly increased from the seedling stages until the flowering stage and slightly to the end (dispersal stages). Root mass fraction, stem mass fraction, specific leaf area and specific stem length have been steadily reduced. High growth plasticity was observed in the measured traits which reached the optimum (0.985) in total dry mass and specific leaf mass fraction (0.899) respectively. However, minimum plasticity values were detected in stem mass fraction, shoot to root ratio and leaf area ratio (Table 1).
Relative growth rate and net assimilation
The relative growth rate (RGR) of S. elaeagnifolium was important as a key for growth measurement and competition with other plant species. The maximum RGR observed in the seedling–juvenile interval and develops slowly during the juvenile-flowering interval, then develops with a small rate at the remaining flowering and fruiting growth intervals. The relative growth rate of S. elaeagnifolium was higher in summer than in winter and in rangelands than in fruit fields. However, it was lower in winter crops than in other tested crops (Fig. 3). The differences in S. elaeagnifolium growth of total dry weight were significant (F = 108.56, p ≤ 0.00) among crop types, (F = 47.69, p ≤ 0.00) life stages, and (F = 2.69, p ≤ 0.03) seasonal crops respectively. The interaction effect in S. elaeagnifolium of total dry weight was significant (F = 3.082, p ≤ 0.00) between crop types and life stages, however, this interaction was insignificant (F = 1.03, p ≤ 0 0.47) between seasonal crops and crop types and (F = 0.76, p ≤ 0.065) between seasonal crops and stages of total dry weight.
The net assimilation rate (NAR) is associated with photosynthetic and respiration rates. It increased significantly over the stages (F = 1417.8, p ≤ 0.00) seedling, (F = 2,356,717.7, p ≤ 0.00) juvenile, (F = 4,385,821.8, p ≤ 0.00) flowering, (F = 1,908,451.9, p ≤ 0.000) fruiting respectively. The NAR was observed greatest in the flowering stages and reaches the maximum in grapes followed by Figs. However, it was low in wheat and Egyptian clover crops. In general, NAR of S. elaeagnifolium appeared high in the summer season, fruit crops and rangelands (Fig. 4).
The relationship of S. elaeagnifolium biomass accumulation and times from days of emergence to the final stage has been described by polynomial regression equations (Table 2). This accumulation indicated the plant performance from stage to stage and from time to time under various conditions. The coefficients of determination exceeded 0.93 and the cubic term was significant (P > 0.001). Plant dry matter exhibited great variability within plant parts and between the harvest intervals. While the overall accumulation of biomass has varied between crops, it was higher in sesame and figs than in other crops. The interaction effects of S. elaeagnifolium growth traits among different crops and stages were significantly (F = 262.7, p ≤ 0.00) root, (F = 392.1, p ≤ 0.00) stem, (F = 346.3, p ≤ 0.00) leaves, (F = 787.2, p ≤ 0.000) fruits and (F = 3135, p ≤ 0 0.00) total plant of dry weight respectively.
Floristic analysis of areas and seasons
The floristic analysis was conducted in the adjacent areas of S. elaeagnifolium invaded and non-invaded sites. The mean percent of S. elaeagnifolium invaded lands ranged from 31 to 37% (Borg al-arab) and 25 to 28% (El-hammam) of the cultivated lands respectively. A total of 33 species were found belonging to 30 genera within 14 families in these areas. Regarding non-invaded sites, the number of species was 33 species (Borg Al-arab) and 30 (El-hammam), 21 (winter) and 19 (summer) respectively. While in the invaded areas, the number of species including 30 species (Borg al-arab) and 27 (El-hammam), 18 (winter) and 14 (summer) respectively. The highest number of species was found in the family Poaceae, followed by Brassicaceae. The recorded species in the studied communities were Sonchus oleraceus L., Xanthium stramonium L., Conyza linifolia (Willd.) Täckh., Amaranthus retroflexus L., Sisymbrium irio L., Silybum marianum (L.) Gaertn., Sonchus arvensis L., Brassica tournefortii Gouan., Capsella bursa-pastoris (L.) Medik., Beta vulgris L., Chenopodium album L., Chenopodium murale L., Cichorium endivia L., Lathyrus hirsutus L., Urtica urens L., Amaranthus ascendens Loisel., Amaranthus viridis L., Conyza bonariensis L., Convolvulus arvensis L., Cyperus rotundus L., Euphorbia peplus L., Melilotus indica (L.) All., Medicago polymorpha L., Vicia sativa L., Malva parviflora L., Oxalis corniculata L., Rumex dentatus L., Emex spinosum L., Cynodon dactylon (L.) Pers., Imperta cylindrica (L.) P.Beauv., Poa annua L., Avena fatua L., Lolium multiflorum Lam., Bromus tectorum L., Phalaris minor Retz., Hordeum marinum Huds., Dactyloctenium aegyptium (L.) Willd., Setaria viridis (L.) P. Beauv., Cenchrus ciliaris L., Agropyron repens L., Digitaria sanguinalis (L.) Scop., Dichanthium aristatum (Poir.) C.E.Hubb., Lolium perenne L., Phragmites communis (Cav.) Trin. ex Steud., Echinochloa colona (L.) Link, Solanum nigrum L., Solanum eleaegnifolium Cav. and Kochia indica Wight.. These species were belonging to Asteraceae, Amaranthceae, Brassicaceae, Chenopodiaceae, Convolvulaceae, Cyperaceae, Euphorbiaceae, Fabaceae, Malvaceae, Oxalidaceae, Polygonaceae, Poaceae, Solanaceae and Urticaceae.
In the invaded areas, S. elaeagniflium species had relative abundances (RA) reached 19.97 (Borg el-arab) and 30.81 (El-hammam), respectively. Followed by Silybum marianum, Cynodon dactylon and Malva parviflora species with RA reached 18.14, 18.56 and 15.97 (Borg el-arab) and Hordeum marinum, Convolvulus arvensis and Beta vulgris by 17.67, 17.45 and 16.54 (El-hammam), respectively. Regarding seasonal infestation, S. eleaegniflium had RA of 25.37 and 59.04 in the winter and the summer respectively. The highest RA species were Chenopodium sp. by 22.40 (winter) and Dactyloctenium aegyptium by 23.84 (summer) respectively. Among the non-invaded, regarding areas, the higher relative abundance was S. marianum and C. dactylon by 18.14 and 18.56 (Borg al- arab) and Convolvulus arvensis, Hordeum marinum and Imperata cylindrica by 17.45, 17.65 and 15.02 (El-hammam), respectively. Regarding seasonal infestation, the high RA was achieved from Phalaris sp. by 30.51 in winter and C. dactylon by 20.61 in summer respectively (Fig. 5).
In the present research, S. eleaegniflium was the most common weed in invaded areas, which had slightly lower richness than in non-invaded areas. The diversity indices of Simpson (λ), Shannon (H') gave the highest values for non-invaded as compared with invaded localities. Evenness indices of hill's index (E4) and modified hill's ratio (E5) were more adaptive and have the lowest value in the invaded area relative to non-invaded localities. However, Pielou's index (E1), Shannons’s index (E2), Heip's index (E3) did not appear any differences between the invaded and non-invaded localities. While Shannons’s indices and Simpson indices allowed better comparison between various areas, we found Evenness indices E4 and E5 have a great sensitivity for comparison between the invaded and non-invade areas, these indices refer to the greatest importance to S. eleaegniflium invasion (Table 3).
The coefficient of similarity (CS) of species was calculated between various areas and seasons of non-invaded and invaded sites. The CS reached 75.80% and 82.61% in non-invaded and invaded areas respectively. Whereas, it was calculated by 24.13% and 25.33% winter and summer seasons respectively. Regarding the region, it reached 92.0% (Borg al-arab : Borg al-arab) and 80.4% (El-hammam : El-hammam) respectively. As for the CS within the same season, it reached 79.4% (winter : winter) and 65.3% (summer : summer) respectively (Table 3).
In the present research, the importance value index (IV) in the invaded area pointed to S. elaeagnifolium as a high IV species reached 108.14 (Borg al-arab), 115.21 (El-hammam), 109.88 (winter), 129.46 (summer), respectively in the concerning communities. It has shown the second set of IVI achieved from C. dactylon by 90.78 (Borg arl- arab) and 71.09 (El-hammam), Silybum marianum by 91.87 (winter), Portulaca oleraceae by 77.05 (summer) respectively. Regarding the non-invaded area, the greater IV was Cynodon dactylon by 93.86, followed by Convolvulus arvensis by 75.25 (Borg al-arab), C. arvensis and Cyperus rotundus reached 73.49 and 70.06 (El-hammam area), respectively. As for seasons in non-invaded, the highest IV was Malva parviflora by 80.27 (winter), Dactyloctenium aegyptium and C. arvensis by 81.41, 83.39 (summer) respectively (Fig. 6).
Discussion
The growth traits of S. elaeagnifolium and the impacts in different habitats were illustrated which could be important for understanding its invasion behavior and helps to highlight the importance of S. elaeagnifolium management effectively.
The role of growth characteristics in S. elaeagnifolium behavior
A high growth rate of S. elaeagnifolium had recorded in different habitats with variation in dry matter accumulation based on the life stages. Which could be accelerated their maturity from the seedling to the fruiting stage. There was an increase in shoots/ roots ratio, leaf area ratio and leaf mass fraction across the time that reflects a better colonizing in this area. However, root mass fraction, stem mass fraction, specific leaf area, and specific stem length were reduced via time. S. elaeagnifolium has a wide time for germination extending from the spring to summer with the optimum in March and April. It has a rapid and wide life cycle duration (40 to 90 days) influenced by habitat environmental conditions. The short seedling and juvenile period indicated that the time available for control is very short. Perennial weeds at the 5 to 7 leaf stage of seedling or juvenile are recommended for herbicide applications (Aulakh et al. 2014). It is exhibited from a wide range of life cycles, with a high ratio of leaf area and rapid and high germination are excellent seed traits for many invasive species under stress conditions (Ye and Wen 2017). Plant-plant interaction via competitive indices, plant morphological traits, soil nutrient contents, enzyme activities, and microbial biomass are essential to weed invasion success (Xu et al. 2020). Regarding the growth analysis by regression and relative growth rate, S. elaeagnifolium has a higher growth rate in rangelands than in cultivated crops (Table 2). Likewise, a high relative growth rate and net assimilation were perceived in summer than in the winter season. The highest NAR was detected during the flowering stages and in the summer (Fig. 3). The potentiality of S. elaeagnifolium growth has an ascending order; rangelands > sesame > fig > faba bean > maize > grape > wheat > Egyptian clover. These rankings are functional to strong growth and respectable traits of S. elaeagnifolium which can be facilitated their establishment. Accordingly, the obtained results supported the hypothesis that S. elaeagnifolium high growth rate and plasticity are affected by the growth stage and habitat conditions. Phenology can be a key determinant of species distribution and range dynamics (Chapman et al. 2014). Phenology evolution may be a potential process during range expansion for many invasive plant species (Novy et al. 2013). Phenotypic plasticity is one of the mechanisms enabling exotics species to colonize large, environmentally diverse areas (Davidson et al. 2011; Griffith et al. 2014). The high relative growth ability should be an important characteristic of invasive plant species (Baker 1974). Fast growth results in the rapid occupation of a large space, advantageous in competitive situations (Grime and Hunt 1975). Invasive African grasses have higher relative growth rates than native, and noninvasive grasses (Baruch et al. 1989). The fast grower species may have a more opportunistic approach to pulses in resources and translates into more growth or regeneration (Miao and Bazzaz 1990). Relative growth rate (RGR) is a significant determinant of the competitive ability of exotic plant species after disturbance (Sattin and Sartorato 1997). High RGR associated with opportunistic resource acquisition (high specific leaf areas) and increased root allocation to survive summer drought may be critical for the success of plant invaders in regions with Mediterranean climates (Grotkopp and Rejmanek 2007). Phenotypic plasticity in growth may contribute to the invasion success of Erigeron canadensis (Mojzes et al. 2020).
Characterization of the community’s vegetation in invaded and non-invaded sites and seasons
The community’s characteristics of species relative abundance and diversity metrics between the invaded vs non-invaded communities are crucial to determine the importance of S. elaeagnifolium invasive species. Whereas, these communities are not only affected by invasion but also by many factors including the soil type, climate conditions and the used agricultural management systems. Therefore, we chose the adjacent invaded and non-invaded localities whereas, only mechanical methods are used during land preparation for weed control and no herbicides are used. The composition of weeds is related to the regional climate and soil characteristics and management methods (Dale et al. 1992; Marshall et al. 2003).whereas, the distribution from place to place over time is dependent upon soil factors and the regional climatic condition (Andreasen and Skovgaard 2009). In this research, weed population characteristics were concluded according to Thomas (1985), Relative abundance (RA) is more effective in determining and comparing the abundance of each species in the different communities. The findings showed that the invasive S. elaeagnifolium species affected negatively the new recipient communities. Whereas, there was a lower relative abundance of species in invaded than non-invaded areas. Abundance is an important ecological quantity necessary for monitoring invasive species (Veldtman et al. 2010). Abundance and distribution in space are positively correlated (Gaston and Blackburn 2000). Nevertheless, the effect of altitude on invasive weed distribution was more important than other environmental factors (Hassannejad and Ghafarbi 2014). The differences in the structure and composition of native community population dynamics appeared from the lower weed diversity and richness as well as evenness indexes in the invaded sites as compared with the non-invaded community, especially in the summer season. According to the results, there were some ecological indices are suitable to assess the impact of S. elaeagnifolium invasions such as the diversity index of Simpson index1 (λ), Shannon index (H'), Hill’s index (E4) and Modified hill's ratio (E5) and Coefficient of similarity (CS) due to their high sensitivity. However, the evenness parameters of Pielou's index (E1), Shannons’s index (E2), Heip's index (E3) were not appropriate due to their lowest response and sensitivity (Table 3). Additionally invaded communities have high similarity based on the coefficient of similarity as compared with uninvaded communities. The relative importance value was used to describe the impacts of invasive species on a community (Bradford et al. 2006). Invasive plants primarily had negative effects on plant diversity when they became abundant at a much lower cover level (less than 35%), compared with the native plants (over 60%) (Qi et al. 2014). Species richness, diversity and evenness were found to be significantly reduced as the density of invasive parthenium weed increased (Nguyen et al. 2017). Lantana invasion greatly reduces the density and diversity of the vegetation in the invaded area (Singh et al. 2014). Evenness influences the level of invasion that the introduced species can promote functionality under stress (De Roy et al. 2013). Invasive species reduced local species richness (Crystal-Ornelas and Lockwood 2020). The impact of invasive plant species on resident species, communities and ecosystems is reducing species richness and abundance of native biota and decreasing their local species diversity (Olden and Poff 2003; Sax and Gaines 2003). Invasive alien species break down biogeographic realms and affect native species richness and abundance (Pyšek et al. 2020).
We can conclude that S. elaeagnifolium has a wide range of typical germinability in the spring and summer. It has five life developmental stages with short seedling and juvenile stages which are proper for herbicide application and control purposes, an extended flowering period (May to November) and fruiting period (April to November) and finally dispersion time in the winter season (December to February). The specific growth traits of S. elaeagnifolium might be important for surviving in water-limited environments. Based on growth analysis, the ascending order of crop competition was Egyptian clover > wheat > grape > maize > broad bean > fig > sesame with S. elaeagnifolium. Whereas, S. elaeagnifolium growth traits were influenced by habitats and conditions. This strong and rapid growth, as well as the highest plasticity, may have been facilitated their establishment by increasing the influence upon the recipient community characteristics and their distribution, which expected to expand in several directions in the future. These results are supported by Formozis et al. (2021), who found that the different microclimatic conditions (i.e., wind speed and air temperature, humidity, light conditions, soil moisture, and levels of photosynthetically active radiation, the height of vegetation) affect S. eleagnifolium invasion. The vegetation analysis exhibited the effects of invasive S. elaeagnifolium on species diversity and evenness in invaded vs. non-invaded resident communities. A lower value in diversity metrics and higher similarity were associated with species invasion. Finally, the specific growth traits, worthy plasticity, and abilities to impact the recipient communities might be important keys for their successful spreading in massive ranges. It is noteworthy to use this information in the prediction of its spreading and S. elaeagnifolium impacts in the future. This overview can minimize social and economic disadvantages, raising awareness and understanding the invasion behavior of invasive alien species to preserve the balance in the agroecosystem.
References
Adjim Z, Tani CK (2018) L’infestation par Solanum elaeagnifolium menace l’Algéri. Rev Ecol (Terre Vie) 73(4):569–581. https://hal.science/hal-03532871
Andreasen C, Skovgaard IM (2009) Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agric Ecosys Environ 133:61–67. https://doi.org/10.1016/j.agee.2009.05.003
Aulakh JS, Enloe SF, Loewenstein NJ, Price AJ, Wehtje G, Miller JH (2014) Pushing towards cogongrass patch eradication: The influence of herbicide treatment and application timing on cogongrass rhizome elimination. Invasive Plant Sci Mgt 7:398–407. https://doi.org/10.1614/IPSM-D-13-00055.1
Baker HG (1974) The evolution of weeds. Annu Rev Ecol Evol Syst 5:1–24. https://doi.org/10.1146/annurev.es.05.110174.000245
Balah MA (2011) Impact of mixing glyphosate with multi additives on weeds control and soil microorganism. J Plant Prot Pathol 2:791–804. https://doi.org/10.21608/jppp.2011.86598
Balah MA, Hassany WM, Muosa EA (2021) Response of Invasive Solanum elaeagnifolium Cav. seed germination and growth to different conditions and environmental factors. J Biologia 76:1409–1418. https://doi.org/10.1007/s11756-021-00736-7
Baruch Z, Hernandez AB, Montilla MG (1989) Dinamica del crecimiento, fenologıay repaticion de biomassa en gramıneas nativas e introducidas de unasabana. Neotropical. Ecotropicos 2:1–13. http://www.saber.ula.ve/handle/123456789/25695
Boulos L (2009) Flora of Egypt. Checklist Al-Hadara Publishing, Cairo
Boyd JW, Murray DS (1982) Growth and development of silverleaf nightshade (Solanum elaeagnifolium). Weed Sci 30:238–243. https://doi.org/10.1017/S0043174500040455
Boyd JW, Murray DS, Tyrl RJ (1984) Silverleaf Nightshade, Solanum elaeagnifolium, origin, distribution, and relation to man. Econ Bot 38:210–217. https://doi.org/10.1007/BF02858833
Bradford KR, Kegode GO, Messersmith CG, Nalewaja JD, Nord CA (2006) Long-term effects of spring wheat–soybean cropping systems on weed populations. Field Crop Res 97:197–208. https://doi.org/10.1016/j.fcr.2005.09.010
Brunel S (2011) Pest risk analysis for Solanum elaeagnifolium and international management measures proposed. EPPO Bull 41:232–242. https://doi.org/10.1111/j.1365-2338.2011.02457.x
Cardina J, Regnier E, Harrison K (1991) Long-term tillage effects on seed banks in three Ohio soils. Weed Sci 39:186-l 94. https://doi.org/10.1017/S0043174500071459
Chapman DS, Haynes T, Beal S, Essl F, Bullock JM (2014) Phenology predicts the native and invasive range limits of common ragweed. Glob Chang Biol 20(1):192–202. https://doi.org/10.1111/gcb.12380
Chauhan BS, Johnson DE (2010) The role of seed ecology in improving weed management strategies in the tropics. Adv Agron 105:221–262. https://doi.org/10.1016/S0065-2113(10)05006-6
Cooley AW, Smith DT (1972) Seed aspects of two perennials woolly leaf bursage and silverleaf nightshade. Proceedings 25thAnnual Meeting Southern Weed Science Society, pp 443
Cornelissen JHC, Castro-Díez P, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species. J Ecol 84:755–765. https://doi.org/10.2307/2261337
Crystal-Ornelas R, Lockwood JL (2020) Cumulative meta-analysis identifies declining but negative impacts of invasive species on richness after 20 yr. Ecology 101(8):e03082. https://doi.org/10.1002/ecy.3082
Cuthbertson EG, Leys AR, McMaster G (1976) Silverleaf nightshade - a potential threat to agriculture. Agric Gaz N S W 87:11–13
Dale MRT, Thomas AG, John EA (1992) Environmental factors including management practices as correlates of weed community composition in spring seeded crops. Can J Bot 70:1931–1939. https://doi.org/10.1139/b92-240
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431. https://doi.org/10.1111/j.1461-0248.2011.01596.x
De Aparicio F, Difrieri HA (1958) La Argentina, Suma deGeografı´a, Tomo I. Ed. Peuser, Buenos Aires
De Roy K, Marzorati M, Negroni A, Thas O, Balloi A, Fava F, Verstraete W, Daffonchio D, Boon N (2013) Environmental conditions and community evenness determine the outcome of biological invasion. Nat Commun 4:1383. https://doi.org/10.1038/ncomms2392
Dekker J (1997) Weed diversity and weed management. Weed Sci 45(3):357–363
DiTomaso JM (2000) Invasive weeds in rangelands: species, impacts, and management. Weed Sci 48:255–265. https://doi.org/10.1614/0043-1745(2000)048[0255:IWIRSI]2.0.CO;2
FAO‐UNESCO (1971–1981) FAO/UNESCO Soil Map of the World, 1:5 million. Vols. 1–10. FAO/UNESCO, Rome
Fernandez OA, Brevedan, RE (1972) Regeneración de Solanum Eleagnifolium Cav. a Partir de Fragmentos de Sus Raíces. Darwiniana 17:434–442. https://www.jstor.org/stable/23215056
Formozis G, Tsakaldimi M, Ganatsas P (2021) Are Mediterranean forest ecosystems under the threat of invasive species Solanum elaeagnifolium? iForest 14:236–241. https://doi.org/10.3832/ifor3706-014
Ganatsas P, Tsitsoni T, Tsakaldimi M, Zagas T (2012) Reforestation of degraded Kermes oak shrub lands with planted pines: effects on vegetation cover, species diversity and community structure. New For 43:1–11. https://doi.org/10.1007/s11056-011-9262-z
Garcia-Fortea E, Gramazio P, Vilanova S, Fita A, Mangino G, Villanueva G et al (2019) First successful backcrossing towards eggplant (Solanum melongena) of a New World species, the silverleaf nightshade (S. elaeagnifolium), and characterization of interspecific hybrids and backcrosses. Sci Hortic 246:563–573. https://doi.org/10.1016/j.scienta.2018.11.018
Gaston KJ, Blackburn TM (2000) Pattern and Process in Macroecology. Blackwell Science, Oxford
Gregory FG (1926) The effect of climatic conditions on the growth of barley. Ann Bot 40(157):1–26
Griffith AB, Andonian K, Weiss CP, Loik ML (2014) Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum. Biol Invas 16:2627–2638. https://doi.org/10.1007/s10530-014-0692-3
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptive significance in a local flora. J Ecol 63:393–422. https://doi.org/10.2307/2258728
Grotkopp E, Rejmanek M (2007) High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am J Bot 94:526–532. https://doi.org/10.3732/ajb.94.4.526
Hansen W, Wollny J, Otte A, Eckstein RL, Ludewig K (2020) Invasive legume affects species and functional composition of mountain meadow plant communities. Biol Invasions 23:281–296. https://doi.org/10.1007/s10530-020-02371-w
Hassannejad S, Ghafarbi SP (2014) Weed flora survey in alfalfa (Medicago sativa L.) fields of Shabestar (northwest of Iran). Arch Agron Soil Sci 60:971–991. https://doi.org/10.1080/03650340.2013.859383
Hawker V, Preston C, Baker J (2006) Genetic variation within and among silverleaf nightshade (Solanum elaeagnifolium Cav.) populations in South Australia. 15th Australian Weeds Conferences. Adelaide Conference Centre, Adelaide, SouthAustralia, pp 176
Heap JW, Carter RJ (1999) The biology of Australian weeds: 35. Solanum elaeagnifolium Cav. Plant Prot q 14:2–12
Hejda M, Pysek P, Jarosík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97:393–403. https://doi.org/10.1111/j.1365-2745.2009.01480.x
Henderson L (2001) Alien weeds and invasive plants. A complete guide to declared weeds and invaders in South Africa. Plant Protection Research Institute, Handbook No. 12
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Lemerle D, Leys AR (1991) Control of silverleaf nightshade (Solanum elaeagnifolium Cav.) increases the grain yield of wheat. Aust J Exp Agric 31:233–236
Li HN, Xiao B, Liu WX, Wan FH (2014) Changes in soil biota resulting from growth of the invasive weed, Ambrosia artemisiifolia L. (Compositae), enhance its success and reduce growth of co-occurring plants. J Integr Agric 13(9):1962–71. https://doi.org/10.1016/S2095-3119(13)60569-9
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Margalef R (1958) Information theory in Ecology. Int J Gen Syst 3:36–71
Marshall EJP, Brown VK, Boatman ND, Lutman PJW, Squire GR, Ward LK (2003) The role of weeds in supporting biological diversity within crop fields. Weed Res 43:77–89. https://doi.org/10.1046/j.1365-3180.2003.00326.x
Mekki M (2007) Biology, distribution and impacts of silverleaf nightshade (Solanum elaeagnifolium Cav.). EPPO Bull 37:114–118. https://doi.org/10.1111/j.1365-2338.2007.01094.x
Miao SL, Bazzaz FA (1990) Responses to nutrient pulses of two colonizers requiring different disturbance frequencies. Ecology 71:2166–2178. https://doi.org/10.2307/1938630
Mojzes A, Ónodi G, Lhotsky B, Kalapos T, Kröel-Dulay G (2020) Experimental drought indirectly enhances the individual performance and the abundance of an invasive annual weed. Oecologia 193:571–581. https://doi.org/10.1007/s00442-020-04711-y
Newsome RD, Dix RL (1968) The forests of the cypress hill, Alberta and Saskatchewan, Canada. Am Midl Nat 80(1):137. https://doi.org/10.2307/2423608
Nguyen T, Bajwa AA, Belgeri A, Navie S, O’Donnell C, Adkins S (2017) Impact of an invasive weed, Parthenium hysterophorus, on a pasture community in south east Queensland, Australia. Environ Sci Pollut Res Int 24(35):27188–27200. https://doi.org/10.1007/s11356-017-0327-1
Novy A, Flory SL, Hartman JM (2013) Evidence for rapid evolution of phenology in an invasive grass. J Evol Biol 26(2):443–450. https://doi.org/10.1111/jeb.12047
Olden JD, Poff NL (2003) Toward a mechanism understanding and prediction of biotic homogenization. Am Nat 162:442–460. https://doi.org/10.1086/378212
Pielou FD (1975) Ecological diversity. Wiley Intescienco, New York
Pyšek P, Hulme PE, Simberloff D, Bacher S, Blackburn TM, Carlton JT, Dawson W, Essl F, Foxcroft LC, Genovesi P, Jeschke JM, Kühn I, Liebhold AM, Mandrak NE, Meyerson LA, Pauchard A, Pergl J, Roy HE, Seebens H, van Kleunen M, Vilà M, Wingfield MJ, Richardson DM (2020) Scientists’ warning on invasive alien species. Biol Rev Camb Philos Soc 95(6):1511–1534. https://doi.org/10.1111/brv.12627
Qasem JR (2014) Silverleaf nightshade (Solanum elaeagnifolium) in the Jordan Valley: Field survey and chemical control. J Hortic Sci Biotechnol 89:639–646. https://doi.org/10.1080/14620316.2014.11513132
Qasem JR, Al Abdallat AM, Hasan SM (2019) Genetic diversity of Solanum elaeagnifolium, an invasive problematic weed in Jordan. Weed Res 59:222–234. https://doi.org/10.1111/wre.12360
Qi SS, Dai ZC, Zhai DL, Chen SC, Si CC, Huang P, Wang RP, Zhong QX, Du DL (2014) Curvilinear effects of invasive plants on plant diversity: plant community invaded by Sphagneticola trilobata. PLoS One 9(11):e113964. https://doi.org/10.1371/journal.pone.0113964
Reichard S (1994) Assessing the potential invasiveness in woody plants introduced to North America. PhD Dissertation, University of Washington, Seattle, 191 p
Richardson DM, Cowling RM, LeMaitre DC (1989) Assessing the risk of invasive success in Pinus and Banksia in South African mountain fynbos. J Veg Sci 1:629–642. https://doi.org/10.2307/3235569
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC (2001) The population biology of invasive species. Annu Rev Ecol Evol Syst 32:305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Sattin M, Sartorato I (1997) Role of seedling growth on weed-crop competition. In: Proceedings 10th EWRS Symposium, Poznan, Poland, pp 3–12
Sax DF, Gaines SD (2003) Species diversity: from global decreases to local increases. Trends Ecol Evol 18:561–566. https://doi.org/10.1016/S0169-5347(03)00224-6
Scaldaferro M, Chiarini F, Santinaque FF, Bernardello G, Moscone EA (2012) Geographical pattern and ploidy levels of the weed Solanum elaeagnifolium (Solanaceae) from Argentina. Genet Resour Crop Evol 59:1833–1847. https://doi.org/10.1007/s10722-012-9807-9
Shannon CE, Weaver W (1949) The Mathematical Theory of Communication, by CE Shannon (and Recent Contributions to the Mathematical Theory of Communication), W. Weaver. University of Illinois Press
Simpson EH (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Singh HP, Batish DR, Dogra KS, Kaur S, Kohli RK, Negi A (2014) Negative effect of litter of invasive weed Lantana camara on structure and composition of vegetation in the lower Siwalik Hills, northern India. Environ Monit Assess 186:3379–3389. https://doi.org/10.1007/s10661-014-3624-x
Smith B, Wilson JB (1996) A consumer’s guide to evenness indices. Oikos 76:70–82. https://doi.org/10.2307/3545749
Stanton R, Wu H, Lemerle D (2011) Root regenerative ability of silverleaf nightshade (Solanum elaeagnifolium Cav.) in the glasshouse. Plant Prot Q 26(2):54–56
Täckholm V (1974) Students’ Flora of Egypt, 2nd edn. University Press, Cairo
Teixeira LH, Yannelli FA, Ganade G, Kollmann J (2020) Functional diversity and invasive species influence soil fertility in experimental Grasslands. Plants (basel) 9(1):53. https://doi.org/10.3390/plants9010053
Thomas AG (1985) Weed survey system used in Saskatchewan for cereal and oilseed crops. Weed Sci 33:34–43. https://doi.org/10.1017/S0043174500083892
Thomas AG, Frick BL, Kelner D, Wise RF (1997) Manitoba weed survey: Comparing zero and conventional tillage crop production systems 1994. Weed Survey Series Publication 97–1. Manitoba Agriculture, Carman. 130 pp
Thuiller W, Gallien L, Boulangeat I, De Bello F, Münkemüller T, Roquet C, Lavergne S (2010) Resolving Darwin’s naturalization conundrum: a quest for evidence. Divers Distrib 16(3):461–475. https://doi.org/10.1111/j.1472-4642.2010.00645.x
Tracy BF, Sanderson MA (2003) Forage productivity, species evenness and weed invasion in pasture communities. Agric Ecosyst Environ 102:175–183. https://doi.org/10.1016/j.agee.2003.08.002
Travlos IS (2013) Responses of invasive silverleaf nightshade (Solanum elaeagnifolium) populations to varying soil water availability. Phytoparasitica 41:41–48. https://doi.org/10.1007/s12600-012-0262-0
Tsaballa A, Nikolaidis A, Trikka F, Ignea C, Kampranis SC, Makris AM, Argiriou A (2015) Use of the de novo transcriptome analysis of silver-leaf nightshade (Solanum elaeagnifolium) to identify gene expression changes associated with wounding and terpene biosynthesis. BMC Genomics 16(1):504. https://doi.org/10.1186/s12864-015-1738-3
Uludag A, Gbehounou G, Kashefi J, Bouhache M, Bon MC, Bell C, Lagopodi AL (2016) Review of the current situation for Solanum elaeagnifolium in the Mediterranean Basin. Bulletin OEPP/EPPO Bulletin 46(1):139–147. https://doi.org/10.1111/epp.12266
Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81:1925–1936. https://doi.org/10.1890/0012-9658(2000)081[1925:PPRTLO]2.0.CO;2
Veldtman R, Chown SL, McGeoch MA (2010) Using scale-area curves to quantify the distribution, abundance and range expansion potential of an invasive species. Divers Distrib 16:159–169. https://doi.org/10.1111/j.1472-4642.2009.00632.x
Wardle DA, Nicholson KS, Ahmed M, Rahman A (1994) Interference effects of the invasive plant Carduus nutans L. against the nitrogen fixation ability of Trifolium repens L. Plant Soil 163:287–297. https://doi.org/10.1007/BF00007978
Wassermann VD, Zimmermann HG, Neser, S (1988) The weed silverleaf bitter apple ('Satansbos') (Solanum elaeagnifolium Cav.) with special reference to its status in South Africa. Plant Protection Research Institute. Dept. Agriculture and Water Supply, Pretoria. Technical Communication No. 214
Weber E, Li B (2008) Plant invasions in China: what is to be expected in the wake of economic development? Bioscience 58:437–444. https://doi.org/10.1641/B580511
West NE, Ross W, Wein A (1971) Plant phenological index technique. Bioscience 21:116–117. https://doi.org/10.2307/1295391
Williamson M (1996) Biological invasions. Chapman and Hall, London
Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Losos JB, Davis CC (2010) Favorable climate change response explains nonnative species’ success in Thoreau’s woods. PloS ONE 5(1):e8878. https://doi.org/10.1371/journal.pone.0008878
Wilsey BJ, Polley HW (2002) Reductions in grassland species evenness increase dicot seeding invasion and spittle bug infestation. Ecol Lett 5:676–684. https://doi.org/10.1046/j.1461-0248.2002.00372.x
Wolkovich EM, Cleland EE (2014) Phenological niches and the future of invaded ecosystems with climate change. AoB Plants 6:plu013. https://doi.org/10.1093/aobpla/plu013
Xu QY, Wang D, Quan GM, Xiang HM, Zhang JE (2020) Invasive Chromolaena odorata species specifically affects growth of its co-occurring weeds. Ann N Y Acad Sci 1470(1):57–66. https://doi.org/10.1111/nyas.14330
Ye J, Wen B (2017) Seed germination in relation to the invasiveness in spiny amaranth and edible amaranth in Xishuangbanna, SW China. PLoS One 12(4):e0175948. https://doi.org/10.1371/journal.pone.0175948
Zhu XC, Wu H, Stanton R, Raman H, Lemerle D, Burrows G (2013a) Time of emergence impact the growth and reproduction of silverleaf nightshade (Solanum elaeagnifolium Cav.). Weed Biol Manag 13:98–103. https://doi.org/10.1111/wbm.12015
Zhu XC, Wu HW, Stanton R, Burrows GE, Lemerle D, Raman H (2013b) Morphological variation of Solanum elaeagnifolium in south-eastern Australia. Weed Res 53:344–354. https://doi.org/10.1111/wre.12032
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research was funded by the Science & Technology Development Fund (STDF) of Egypt, Grant No. 34767.
Author information
Authors and Affiliations
Contributions
Dr Mohamed Balah participate in all experiments of the manuscripts from germination of Solanum elaeagnifolium, phenological development and growth traits in different crops, as well as its effects on species diversity and evenness in invaded vs. non-invaded resident communities. Dr Whaby Hassany help in the demographic surveys in invaded vs. non-invaded communities and conducted the statistical analysis of this manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, I declare that there is no any conflicts of interest about this research paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balah, M.A., Hassany, W.M. The relationship between Invasive Alien Solanum elaeagnifolium Cav. characters and impacts in different habitats. Biologia 78, 1253–1268 (2023). https://doi.org/10.1007/s11756-023-01336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01336-3