Abstract
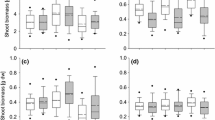
Phenotypic plasticity is often considered important for invasive plant success, yet relatively few studies have assessed plasticity in both native and invasive populations of the same species. We examined the plastic response to temperature for Bromus tectorum populations collected from similar shrub-steppe environments in the Republics of Armenia and Georgia, where it is native, and along an invasive front in California and Nevada. Plants were grown in growth chambers in either ‘warm’ (30/20 °C, day/night) or ‘cold’ (10/5 °C) conditions. Invasive populations exhibited greater adaptive plasticity than natives for freezing tolerance (as measured by chlorophyll a fluorescence), such that invasive populations grown in the cold treatment exhibited the highest tolerance. Invasive populations also exhibited more rapid seedling emergence in response to warm temperatures compared to native populations. The climatic conditions of population source locations were related to emergence timing for invasive populations and to freezing tolerance across all populations combined. Plasticity in growth-related traits such as biomass, allocation, leaf length, and photosynthesis did not differ between native and invasive populations. Rather, some growth-related traits were very plastic across all populations, which may help to dampen differences in biomass in contrasting environments. Thus, invasive populations were found to be particularly plastic for some important traits such as seedling emergence and freezing tolerance, but plasticity at the species level may also be an important factor in the invasive ability of B. tectorum.




Similar content being viewed by others
References
Andonian K, Hierro J (2011) Species interactions contribute to the success of a global plant invader. Biol Invasions 13:2957–2965
Baker HG (1974) The evolution of weeds. Annu Rev Ecol Evol Syst 5:1–24
Beckstead J, Meyer SE, Allen PS (1996) Bromus tectorum seed germination: between-population and between-year variation. Can J Bot 74:875–882
Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Cano L, Escarre J, Fleck I, Blanco-Moreno JM, Sans FX (2008) Increased fitness and plasticity of an invasive species in its introduced range: a study using Senecio pterophorus. J Ecol 96:468–476
Chun YJ (2011) Phenotypic plasticity of introduced versus native purple loosestrife: univariate and multivariate reaction norm approaches. Biol Invasions 13:819–829
Chun YJ, Collyer ML, Moloney KA, Nason JD (2007) Phenotypic plasticity of native vs. invasive purple loosestrife: a two-state multivariate approach. Ecology 88(6):1499–1512
Concilio AL, Loik ME, Belnap J (2013) Global change effects on Bromus tectorum L. (Poaceae) at its high-elevation range margin. Glob Change Biol 19:161–172
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431
Fenesi A, Redei T, Botta-Dukat Z (2011) Hard traits of three Bromus species in their source area explain their current invasive success. Acta Oecol Int J Ecol 37:441–448
Friedman JM, Roelle JE, Gaskin JF, Pepper AE, Manhart JR (2008) Latitudinal variation in cold hardiness in introduced Tamarix and native Populus. Evol Appl 1:598–607
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Goergen E, Daehler CC (2001) Reproductive ecology of a native Hawaiian grass (Heteropogon contortus; Poaceae) versus its invasive alien competitor (Pennisetum setaceum; Poaceae). Int J Plant Sci 162:317–326
Griffith AB (2010) Positive effects of native shrubs on Bromus tectorum demography. Ecology 91:141–154
Griffith AB, Loik ME (2010) Effects of climate and snow depth on Bromus tectorum population dynamics at high elevation. Oecologia 164:821–832
Kao RH, Brown CS, Hufbauer RA (2008) High phenotypic and molecular variation in downy brome (Bromus tectorum). Invasive Plant Sci Manag 1:216–225
Knapp PA (1996) Cheatgrass (Bromus tectorum L) dominance in the Great Basin Desert—history, persistence, and influences to human activities. Glob Environ Change 6:37–52
Lambers H, Chapin FS, Pons T (1998) Plant physiological ecology. Springer, New York
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA 104:3883–3888
Leffler AJ, Monaco TA, James JJ (2011) Nitrogen acquisition by annual and perennial grass seedlings: testing the roles of performance and plasticity to explain plant invasion. Plant Ecol 212:1601–1611
Leger EA, Espeland EK, Merrill KR, Meyer SE (2009) Genetic variation and local adaptation at a cheatgrass (Bromus tectorum) invasion edge in western Nevada. Mol Ecol 18:4366–4379
Lowe PN, Lauenroth WK, Burke IC (2003) Effects of nitrogen availability on competition between Bromus tectorum and Bouteloua gracilis. Plant Ecol 167:247–254
Mack RN, Pyke DA (1983) The demography of Bromus tectorum—variation in time and space. J Ecol 71:69–93
Maron JL, Elmendorf SC, Vila M (2007) Contrasting plant physiological adaptation to climate in the native and introduced range of Hypericum perforatum. Evolution 61:1912–1924
McEwan RW, Birchfield MK, Schoergendorfer A, Arthur MA (2009) Leaf phenology and freeze tolerance of the invasive shrub Amur honeysuckle and potential native competitors. J Torrey Bot Soc 136:212–220
Meimberg H, Milan NF, Karatassiou M, Espeland EK, McKay JK, Rice KJ (2010) Patterns of introduction and adaptation during the invasion of Aegilops triuncialis (Poaceae) into Californian serpentine soils. Mol Ecol 19:5308–5319
Meyer SE, Allen PS, Beckstead J (1997) Seed germination regulation in Bromus tectorum (Poaceae) and its ecological significance. Oikos 78:475–485
Novak SJ, Mack RN (1993) Genetic variation in Bromus tectorum (Poaceae)—comparison between native and introduced populations. Heredity 71:167–176
Novak SJ, Mack RN (2001) Tracing plant introduction and spread: genetic evidence from Bromus tectorum (Cheatgrass). Bioscience 51(2):114–122
O’Donnell KL, Pigliucci M (2010) Selection dynamics in native and introduced Persicaria species. Int J Plant Sci 171:519–528
Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, D’Amico JA, Itoua I, Strand HE, Morrison JC, Loucks CJ, Allnutt TF, Ricketts TH, Kura Y, Lamoreux JF, Wettengel WW, Hedao P, Kassem KR (2001) Terrestrial ecoregions of the worlds: a new map of life on Earth. Bioscience 51:933–938
Parker IM, Rodriguez J, Loik ME (2003) An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv Biol 17:59–72
Potvin C (2001) ANOVA: experimental layout and analysis. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 63–76
Ramakrishnan AP, Meyer SE, Fairbanks DJ, Coleman CE (2006) Ecological significance of microsatellite variation in western North American populations of Bromus tectorum. Plant Species Biol 21:61–73
Rice KJ, Mack RN (1991) Ecological genetics of Bromus tectorum 2: intraspecific variation in phenotypic plasticity. Oecologia 88:84–90
Rice KJ, Black RA, Radamaker G, Evans RD (1992) Photosynthesis, growth, and biomass allocation in habitat ecotypes of cheatgrass (Bromus tectorum). Funct Ecol 6:32–40
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993
Roundy BA, Hardegree SP, Chambers JC, Whittaker A (2007) Prediction of cheatgrass field germination potential using wet thermal accumulation. Rangel Ecol Manag 60:613–623
Schlaepfer DR, Glattli M, Fischer M, van Kleunen M (2010) A multi-species experiment in their native range indicates pre-adaptation of invasive alien plant species. New Phytol 185:1087–1099
Sultan SE (2003) Phenotypic plasticity in plants: a case study in ecological development. Evol Dev 5:25–33
Upadhyaya MK, Turkington R, Mcilvride D (1986) The biology of Canadian weeds 75: Bromus tectorum L. Can J Plant Sci 66:689–709
Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
Acknowledgments
Many thanks to Jim Velzy, Denise Polk, Murdoch McDonald, Devon Arscott, Robert Finer, Katie Griffith, Holly Alpert, Amy Concilio, Ingrid Parker, Katrina Dlugosch, Sarah Swope, Kristina Jones, Grigor Janoyan, Levan Janoyan, Jason Sexton, and the staff of the Valentine Eastern Sierra Reserve. This research was funded by the Environmental Studies Department at the University of California, Santa Cruz and the Wellesley College Botanic Gardens (A.G.), and by a Fulbright Student Fellowship (K.A.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Griffith, A.B., Andonian, K., Weiss, C.P. et al. Variation in phenotypic plasticity for native and invasive populations of Bromus tectorum . Biol Invasions 16, 2627–2638 (2014). https://doi.org/10.1007/s10530-014-0692-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0692-3