Abstract
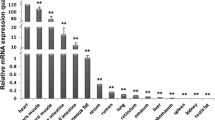
Angiopoietin-like protein 8 (Angptl8) is a new constituent of angiopoietin-related protein family. In the present study, Landrace × Yorkshire pigs, C57BL/6 J mice, and Wistar rat were used as experimental animals. Using normal PCR, T-A cloning, RACE, and other experimental techniques, full length of porcine Angptl8 gene was cloned. Through real-time PCR, Angptl8 expression was detected in assorted tissues (kidney, heart, spleen, liver, lung, abdominal fat, and back longest muscle) of different animals. The present result showed that the full-length of porcine Angptl8 was 875 bp. Its ORF region contained 597 bp, from 21 bp to 618 bp, it had high conservation. The protein of Angptl8 had 198 amino acids. Its relative molecular mass was 21,988.3 Da. Its theoretical isoelectric point was 6.35. There was a highly hydrophobic region near the 10th amino acid site of the Angptl8 gene, while 177th and 187th were highly hydrophilic. Angptl8 protein may be not a transmembrane protein. The first to the 19th amino acids on the N-terminal were signal peptides, and the first 18th–19th amino acid sites may be the signal peptide shear site. In addition, Angptl8 in healthy pigs was mainly expressed in the liver. Its expression in heart and adipose tissue was minimum. Angptl8 of both mouse and rat was enriched in the fat and liver; mouse and rat minimum Angptl8 expression was in muscle and spleen, respectively. The present results suggest that there may exist species difference in tissue-specific expression of Angptl8.




Similar content being viewed by others
References
Abu-Farha M, Abubaker J, Al-Khairi I, Cherian P, Noronha F, Kavalakatt S, Khadir A, Behbehani K, Alarouj M, Bennakhi A, Elkum N (2016) Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc Diabetol 15:25. https://doi.org/10.1186/s12933-016-0346-0
Cahová M, Habart D, Olejár T, Berková Z, Papáčková Z, Daňková H, Lodererova A, Heczková M, Saudek F (2017) Lipasin/betatrophin is differentially expressed in liver and white adipose tissue without association with insulin resistance in Wistar and Goto-Kakizaki rats. Physiol Res 66(2):273–281. https://doi.org/10.33549/physiolres.933339
Chen NB, Ma Y, Yang T, Lin F, Fu WW, Xu YJ, Li F, Li JY, Gao SX (2015) Tissue expression and predicted protein structures of the bovine ANGPTL3 and association of novel SNPs with growth and meat quality traits. Animal 9:1285–1297. https://doi.org/10.1017/S1751731115000658
Chen S, Chen J, Meng XL, Shen JS, Huang J, Huang P, Pu Z, McNeill NH, Grayburn PA (2016) ANGPTL8 reverses established adriamycin cardiomyopathy by stimulating adult cardiac progenitor cells. Oncotarget 7:80391-80403. https://doi.org/10.18632/oncotarget.13061
Dijk W, Kersten S (2016) Regulation of lipid metabolism by angiopoietin like proteins. Curr Opin Lipidol 27:249–256. https://doi.org/10.1097/MOL.0000000000000290
Ding Y, Young CN, Li J, Luan X, McAllister JP 2nd, Clark JD, Diaz FG (2003) Reduced inflammatory mediator expression by pre-reperfusion infusion into ischemic territory in rats: a real-time polymerase chain reaction analysis. Neurosci Lett 353:173–176. https://doi.org/10.1016/j.neulet.2003.09.055
Espes D, Lau J, Carlsson PO (2014) Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes. Diabetologia 57:50–53. https://doi.org/10.1007/s00125-013-3071-1
Feng SQ, Chen XD, Xia T, Gan L, Qiu H, Dai MH, Zhou L, Peng Y, Yang ZQ (2006) Cloning, chromosome mapping and expression characteristics of porcine ANGPTL3 and −4. Cytogenet Genome Res 114:44–49. https://doi.org/10.1159/000091927
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788. https://doi.org/10.1093/nar/gkg563
Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, Wang D, Yang L, Wang J, Mao C, Zhou L, Yuan G (2014) Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 7:2718–2722. https://doi.org/10.2337/dc14-0602
Huang S, Wang M, Rehman MU, Zhang L, Tong X, Shen Y, Li J (2018) Role of Angiopoietin-like 4 on bone vascularization in chickens exposed to high-altitude hypoxia. J Comp Pathol 161:25–33. https://doi.org/10.1016/j.jcpa.2018.04.007
Huang Y, Fang C, Guo H, Hu J (2016) Increased angiopoietin-like protein 8 levels in patients with type 2 diabetes and cardiovascular disease. Diabetes Res Clin Pract 220:229–231. https://doi.org/10.1016/j.diabres.2016.08.017
Hung JH, Weng Z (2016) Sequence alignment and homology search with BLAST and ClustalW. Cold Spring Harb Protoc 2016:pdb.prot093088. https://doi.org/10.1101/pdb.prot093088
Ikeda M, Arai M, Lao DM, Shimizu T (2002) Transmembrane topology predicttion methods: a re-assessment and improvement by a consensus method using a dataset of experimentally-characterized transmembrane topologies. In Silico Biol 2:19–33
Issa YA, Abd ElHafeez SS, Amin NG (2019) The potential role of angiopoietin-like protein-8 in type 2 diabetes mellitus: a possibility for predictive diagnosis and targeted preventive measures? EPMA J 10:239–248. https://doi.org/10.1007/s13167-019-00180-3
Jiao X, Yang Y, Li L, Yu H, Yang Y, Li J, Du Y, Zhang J, Hu C, Qin Y (2020) Angiopoietin-like protein 8 accelerates atherosclerosis in ApoE(−/)(−) mice. Atherosclerosis 307:63–71. https://doi.org/10.1016/j.atherosclerosis.2020.06.014
Koltes DA, Spurlock DM (2012) Adipose tissue angiopoietin-like protein 4 messenger RNA changes with altered energy balance in lactating Holstein cows. Domest Anim Endocrinol 43:307–316. https://doi.org/10.1016/j.domaniend.2012.05.004
Krahel JA, Baran A, Kamiński TW, Flisiak I (2020) Proprotein convertase Subtilisin/Kexin Type 9, Angiopoietin-like protein 8, Sortilin, and cholesteryl ester transfer protein-friends of foes for psoriatic patients at the risk of developing cardiometabolic syndrome? Int J Mol Sci 21:3682. https://doi.org/10.3390/ijms21103682
Lee G, Han D, Song JY, Lee YS, Kang KS, Yoon S (2010) Genomic expression profiling in lymph nodes with lymphoid depletion from porcine circovirus 2-infected pigs. J Gen Virol 91:2585–2591. https://doi.org/10.1099/vir.0.022608-0
Lee YH, Lee SG, Lee CJ, Kim SH, Song YM, Yoon MR, Jeon BH, Lee JH, Lee BW, Kang ES, Lee HC, Cha BS (2016) Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci Rep 6:24013. https://doi.org/10.1038/srep24013
Li AM, Lan XY, Sun XM, Gao Y, Ma W, Ma Y, Chen H (2012a) Genetic variations of ANGPTL6 gene and their associations with growth traits and slaughter traits in Qinchuan cattle. Mol Biol Rep 39:9223–9232. https://doi.org/10.1007/s11033-012-1795-5
Li MM, Geng J, Guo YJ, Jiao XQ, Lu WF, Zhu HS, Wang YY, Yang GY (2015) Cloning and prokaryotic expression of the porcine lipasin gene. Genet Mol Res 14:14698–14705. https://doi.org/10.4238/2015.November.18.34
Li Q, Yan Q, Chen J, He Y, Wang J, Zhang H, Yu Z, Li L (2012b) Molecular characterization of an ice nucleation protein variant (inaQ) from Pseudomonas syringae and the analysis of its transmembrane transport activity in Escherichia coli. Int J Biol Sci 8:1097–1108. https://doi.org/10.7150/ijbs.4524
Liao Z, Wu X, Song Y, Luo R, Yin H, Zhan S, Li S, Wang K, Zhang Y, Yang C (2019) Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. J Cell Mol Med 23:5737–5750. https://doi.org/10.1111/jcmm.14488
Lightbourne M, Wolska A, Abel BS, Rother KI, Walter M, Kushchayeva Y, Auh S, Shamburek RD, Remaley AT, Muniyappa R, Brown RJ (2020) Apolipoprotein CIII and Angiopoietin-like Protein 8 are elevated in lipodystrophy and decrease after Metreleptin. J Endocr Soc 5:bvaa191. https://doi.org/10.1210/jendso/bvaa191
Lord E, Murphy BD, Desmarais JA, Ledoux S, Beaudry D, Palin MF (2006) Modulation of peroxisome proliferator-activated receptor delta and gamma transcripts in swine endometrial tissue during early gestation. Reproduction 131:929–942. https://doi.org/10.1530/rep.1.00657
Lu Q, Lu L, Chen W, Lu P (2017) Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255:1515–1523. https://doi.org/10.1007/s00417-017-3676-z
Mamedova LK, Robbins K, Johnson BJ, Bradford BJ (2010) Tissue expression of angiopoietin-like protein 4 in cattle. J Anim Sci 88:124–130. https://doi.org/10.2527/jas.2009-2258
Martinez-Perez B, Ejarque M, Gutierrez C, Nuñez-Roa C, Roche K, Vila-Bedmar R, Ballesteros M, Redondo-Angulo I, Planavila A, Villarroya F, Vendrell J, Fernández-Veledo S, Megía A (2016) Angiopoietin-like protein 8 (ANGPTL8) in pregnancy: a brown adipose tissue-derived endocrine factor with a potential role in fetal growth. Transl Res 178:1–12. https://doi.org/10.1016/j.trsl.2016.06.012
Mishra S (2009) Function prediction of Rv0079, a hypothetical Mycobacterium tuberculosis DosR regulon protein. J Biomol Struct Dyn 27:283–292. https://doi.org/10.1080/07391102.2009.10507316
Massahi A, Çalık P (2015) In-silico determination of Pichia pastoris signal peptides for extracellular recombinant protein production. J Theor Biol 364:179–188. https://doi.org/10.1016/j.jtbi.2014.08.048
Monzavi N, Zargar SJ, Gheibi N, Azad M, Rahmani B (2019) Angiopoietin-like protein 8 (betatrophin) may inhibit hepatocellular carcinoma through suppressing of the Wnt signaling pathway. Iran J Basic Med Sci 22:1166-1171. https://doi.org/10.22038/ijbms.2019.36612.8764
Niki D, Katsu K, Yokouchi Y (2009) Ontogeny of angiopoietin-like protein 1, 2, 3, 4, 5, and 7 genes during chick embryonic development. Develop Growth Differ 51:821–832. https://doi.org/10.1111/j.1440-169X.2009.01145.x
Oike Y, Akao M, Yasunaga K, Yamauchi T, Morisada T, Ito Y, Urano T, Kimura Y, Kubota Y, Maekawa H, Miyamoto T, Miyata K, Matsumoto S, Sakai J, Nakagata N, Takeya M, Koseki H, Ogawa Y, Kadowaki T, Suda T (2005) Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med 11:400–408. https://doi.org/10.1038/nm1214
Pankovics P, Boros Á, Reuter G (2015) Novel 5'/3'RACE method for amplification and determination of single-stranded RNAs through double-stranded RNA (dsRNA) intermediates. Mol Biotechnol 57:974–981. https://doi.org/10.1007/s12033-015-9889-7
Peng Y, Park JS, Melton DA (2013) Betatrophin: a hormone that controls pancreastic β cell proliferation. Cell 153:747–758. https://doi.org/10.1016/j.cell.2013.04.008
Ren ZQ, Wu WJ, Liu WH, Zheng R, Li JL, Zuo B, Xu DQ, Li FE, Lei MG, Ni DB, Xiong YZ (2014) Differential expression and effect of the porcine ANGPTL4 gene on intramuscular fat. Genet Mol Res 13:2949–2958. https://doi.org/10.4238/2014.April.16.3
Santulli G (2014) Angiopoietin-like proteins: a comprehensive look. Front Endocrinol 5:4. https://doi.org/10.3389/fendo.2014.00004
Stephens JM (2012) RIFL aims to be a new player in lipid metabolism. Am J Physiol Endocrinol Metab 303:E332–E333. https://doi.org/10.1152/ajpendo.00169.2012
Wang XF, Liu QH, Wu Y, Huang J (2016) Litopenaeus vannamei clathrin coat AP17 involved in white spot syndrome virus infection. Fish Shellfish Immunol 52:309–316. https://doi.org/10.1016/j.fsi.2016.03.007
Xiao HB, Fang J, Lu XY, Sun ZL (2012a) Kaempferol improves carcase characteristics in broiler chickens by regulating ANGPTL3 gene expression. Br Poult Sci 53:836–842. https://doi.org/10.1080/00071668.2012.751486
Xiao HB, Niu XW, Sun ZL (2012b) Kaempferol reduces angiopoietin-like protein 4 expression to improve carcass characteristics and meat quality traits in Holstein steers. Lives Sci 145:219–222. https://doi.org/10.1016/j.livsci.2012.02.006
Yamada H, Kusaka I, Saikawa R, Hara K, Kakei M, Ishikawa SE (2018) Relationship between Angiopoietin-like protein 8 and fasting serum triglyceride level. J Clin Med Res 10:134–136. https://doi.org/10.14740/jocmr3286w
Yang Y, Jiao X, Li L, Hu C, Zhang X, Pan L, Yu H, Li J, Chen D, Du J, Qin Y (2020) Increased circulating Angiopoietin-like protein 8 levels are associated with thoracic aortic dissection and higher inflammatory conditions. Cardiovasc Drugs Ther 34:65–77. https://doi.org/10.1007/s10557-019-06924-7
Zeng L, Dai J, Ying K, Zhao E, Jin W, Ye Y, Xu J, Xie Y, Mao Y (2003) Identification of a novel human angiopoietin-like gene expressed mainly in heart. J Hum Genet 48:159–162. https://doi.org/10.1007/s100380300025
Zhang R (2012) Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun 424:786–792. https://doi.org/10.1016/j.bbrc.2012.07.038
Zhang R, Abou-Samra AB (2013) Emerging roles of Lipasin as a critical lipid regulator. Biochem Biophys Res Commun 432:401–405. https://doi.org/10.1016/j.bbrc.2013.01.129
Zhang R, Abou-Samra AB (2014) A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc Diabetol 13:133. https://doi.org/10.1186/s12933-014-0133-8
Wang X, Xu L, Wang H, Young PR, Gaestel M, Feuerstein GZ (2002) Mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 deficiency protects brain from ischemic injury in mice. J Biol Chem 277:43968–43972. https://doi.org/10.1074/jbc.M206837200
Acknowledgments
Project supported by Hunan Provincial Natural Science Foundation of China (14JJ2079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable national Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Animal Studies Subcommittee of the Hunan Agricultural University at which the studies were conducted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1.03 MB)
Rights and permissions
About this article
Cite this article
Wang, CR., Xiao, HB., Lu, XY. et al. Molecular cloning and characterization of angiopoietin-like protein-8 gene in pigs and its tissue-specific expression in different animals. Biologia 76, 3345–3353 (2021). https://doi.org/10.1007/s11756-021-00824-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00824-8