Abstract
Objective
We aimed to determine whether intraoperative low-dose infusion of landiolol, an ultra-short-acting beta blocker, can prevent postoperative atrial fibrillation (POAF) after lung resection.
Methods
A double-blind, randomized, controlled, preliminary study was performed in university academic hospital, single center. Fifty lung surgical patients were key-opened before enrollment of the originally planned 100 patients, who were randomized in a 1:1 ratio in each treatment arm. Landiolol was infused with a dosage of 5 μg/kg/min during general anesthesia in the landiolol group, which was compared with the placebo control group with no landiolol. Atrial fibrillation (AF)-free survival curves were generated by means of Kaplan–Meier estimates and differences in survival were compared with the use of the log-rank test. We examined independent predictors of POAF by the multivariate logistic regression analysis using the perioperative parameters detected with the univariate analysis.
Results
The AF events were recorded for 7 days with Holter monitor in 5 of 25 patients in the landiolol group and 4 of 25 patients in the control group. Kaplan–Meier analysis showed that the landiolol group could not avoid the incidence of POAF in comparison with the placebo saline group (P = 0.806). The multivariate logistic regression analysis for prevalence of POAF identified only one statistically significant predictor: interleukin-6 (IL-6) sampled at 6 h after end of surgery.
Conclusions
We failed to demonstrate that low-dose infusion of landiolol during general anesthesia could prevent the incidence of AF after lung resection. Only IL-6 sampled at 6 h after end of surgery significantly predicted POAF among pulmonary surgical patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is a common complication after lung surgery; its incidence has been reported to range from 8 to 42 % [1]. Some studies have found AF to be benign and transient, although it is associated with a prolonged length of hospitalization and high-related costs [2–5]. Other studies have related it to significantly increased morbidity and mortality [6–9]. Moreover, it is reported that AF after pulmonary lobectomy predicts poorer long-term outcomes in 5-year survivors [10].
In patients undergoing coronary artery bypass graft (CABG) surgery, postoperative AF (POAF) has also been reported to be relevant to long-term outcome, with its incidence between 18.5 and 28.5 % [11–13]. Although the American College of Chest Physicians (ACCP) guidelines recommend that strong consideration should be given to the prophylactic administration of Vaughan–Williams class II beta-blocking drugs as a means of lowering the incidence of new-onset post-cardiac surgery AF [14], no preventive protocol has been validated against POAF after lung surgery. In Japan, the ultra-short-acting beta-blocker landiolol hydrochloride is commercially available for treatment against perioperative supraventricular tachycardia, including AF or atrial flutter. To date, some investigations demonstrated that preemptive administration of landiolol could prevent POAF among CABG surgical patients [15–22].
The aim of this pilot study was to determine whether intraoperative low-dose infusion of landiolol hydrochloride can prevent POAF after lung resection. Simultaneously, we explored perioperative predictors associated with POAF among lung surgical patients.
Methods
Patient population
We obtained regulatory approval for this study from the ethics committee of our university hospital. Before any study-related procedure, each patient gave written informed consent to participate, and patients were free to withdraw from the study at any time. Adult female or male patients could be enrolled if they were between 20 and 90 years old, classified as the American Society of Anesthesiologists physical status I or II, and scheduled to undergo elective segmentectomy, lobectomy, or pneumonectomy of the lung under general anesthesia with thoracic epidural analgesia.
Exclusion criteria included patient refusal, inability to communicate in Japanese, contraindications to epidural anesthesia, recent history or evidence of acute myocardial infarction, and supraventricular arrhythmia (under treatment or requiring treatment). Other reasons for exclusion were second- or third-degree atrioventricular block, severe heart failure graded as the New York Heart Association classification III or IV, the evaluation of left ventricular ejection fraction of 35 % or lower, prohibition from administration of beta-blocker, hypotension at 90/60 mmHg or lower, or bradycardia at 50 beats/min or slower.
Drug and study design
Landiolol hydrochloride, purchased from Ono, Inc. (Osaka, Japan), is an injectable ultra-short-acting beta-receptor antagonist. Its half-life of blood concentration is 4 min in humans with intravenous administration [23]. Landiolol hydrochloride has a much higher cardioselectivity (beta1/beta2 = 255) than esmolol hydrochloride (beta1/beta2 = 33), so it seems to have a little effect on the respiratory system [24].
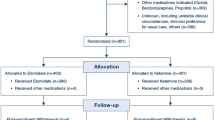
We conducted a randomized, double-blinded controlled preliminary study. After obtaining written consent, the patients were randomized via sealed non-transparent envelope to receive either landiolol or saline placebo from a computer-generated randomization table. We inserted epidural catheters between the Th3-4 and Th6-7 interspace and, after enough oxygenation, induced the patients with intravenous fentanyl (1–2 μg/kg), propofol (2 mg/kg), and vecuronium (0.1 mg/kg), or rocuronium (0.6–0.9 mg/kg). In advance, an investigator otherwise not involved in the study prepared the solutions-containing landiolol or saline placebo. Simultaneously with the start of pre-oxygenation, the study solution prepared by an outsider was intravenously started at a rate of 5 μg/kg/min. Using bag-mask ventilation, we intubated the patient`s trachea with double lumen endotracheal tube afterward connected to ventilator. Ventilation was controlled mechanically to maintain the partial pressure of expiratory carbon dioxide between 30 and 35 mmHg as measured by capnography. The radial artery was cannulated for invasive blood pressure monitor and frequent blood sampling similar to our practical manner in thoracic surgery. General anesthesia was maintained with inhaled sevoflurane, intermittent intravenous non-depolarizing muscle relaxant, and thoracic epidural analgesia with lidocaine or ropivacaine. The anesthesiologists, thoracic surgeons, and intensivists in charge of the patient were blinded throughout the perioperative period as to whether the clear solution was landiolol or physiologic saline. The study drug was continued until the end of general anesthesia. Criteria for cessation of the study drug included intractable bradycardia at 50 beats/min or slower, intractable hypotension at 90/60 mmHg or lower, second- or third-degree atrioventricular block, symptomatic heart failure with audible S3, S4 moist rales over a third of the total lung field, dosing of some medication affecting heart rate (except for atropine, ephedrine and digitalis), operation of electrical or pharmacological defibrillation, and decision of a doctor in charge of the patient. After surgery, thoracic epidural analgesia was maintained with 0.2 % ropivacaine at a rate of 3–8 mL/h with or without 7–37 μg/h Fentanyl for 1–4 days after surgery, using portable disposable infuser in the same way as our clinical practice.
Efficacy evaluation
The primary outcome measure for efficacy was the total incidence of AF occurring during the 7 days after surgery. Five electrodes were put on each patient’s chest just after surgery, and electrocardiographic events were closely watched 24 h per day for 7 days by Holter monitor. The incidence of AF was picked up with onset time and duration from each set of 7-day Holter records. All 24-h electrocardiographic recordings were reviewed and edited manually. We first noted any completely sporadic rate count that continued for more than 30 s, and we employed detailed records for diagnosis of AF. AF was diagnosed according to the advice of a cardiologist.
Secondary assessments to evaluate predictive factors for POAF in patients undergoing lung resection included those described in Table 1 as well as plasma concentration of interleukin-6 (IL-6), serum magnesium (Mg), serum calcium (Ca), C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT Pro-BNP) by blood sampling, and three fractions of catecholamines (adrenaline, noradrenaline, and dopamine) by sampling from stored urine for 24 h at selected time points throughout this study. After centrifuging the blood samples, we separated the plasma and stored it at −80 °C until it was analyzed.
IL-6 was measured by a commercially available assay [Quantikine human IL-6 (high sensitivity), R&D Systems, Minneapolis, Minnesota]. The minimum detectable dose (MDD) of IL-6 ranged from 0.016 to 0.110 pg/mL. The mean MDD was 0.039 pg/mL.
Sample size
The sample size for this study was based on the previous manuscript data, showing that perioperative landiolol infusion could decrease the incidence of POAF among the patients undergoing thoracic surgery [15]. At 80 % power, using a two-sided significance level of 5 %, log-rank test for Kaplan–Meier method showed that 99 patients were required, and randomized in a 1:1 ratio in each treatment arm. We, therefore, needed 100 participants at least. As a preliminary study, we decided to enforce key open when half of the needed population (50 patients) had been enrolled and completed the study course. The analysis was by intention-to-treat and involved all patients who were randomly assigned.
Data analysis and statistical methods
Continuous data are expressed as the mean ± standard deviation (SD) and categorical data as counts or percentages. We analyzed continuous variables using the Student t test or the Mann–Whitney non-parametric test when appropriate, and we evaluated categorical variables using the Chi-square test. Atrial fibrillation-free survival curves were generated by means of Kaplan–Meier estimates, and differences in survival were compared with the use of the log-rank test. Independent predictors of POAF were examined by the multivariate logistic regression analysis using the perioperative parameters detected with the univariate analysis.
We considered P values of less than 0.05 to be significant. Statistical analyses were performed using JMP® version 10.0.2. (SAS Institute, Inc., Cary, NC).
Results
Patient recruitment commenced in September 2009. To investigate the futility of this study, we enforced key open as a preliminary study when half of the needed population (50 cases) had been enrolled and completed the study course, because the prior study was examined in the population undergoing CABG surgery. That was concluded in November 2010. The 50 patients, all ASA physical status I or II, were randomly assigned to the two infusion groups. No refusals to participate were received after randomization, and all patients completed the study requirements. We recorded no event of AF during general anesthesia.
We completed Holter electrocardiographic monitoring of all 50 eligible patients. The cumulative total duration of Holter monitoring for all patients was 345 days, 0 h, 56 min, and 47 s.
Patient background and findings of preoperative 12-lead electrocardiogram, transthoracic echocardiogram, and spirogram were similar between the groups (Table 1). Holter records revealed comparable average, and maximum and minimum heart rate values between the groups on postoperative days 0 through 6.
The events of AF were recorded in 5 (20 %) of 25 patients in the landiolol group and 4 (16 %) of 25 patients in the control group. Cumulative total durations of AF during the 7 postoperative days were 3382 min in the control group and 7713 min in the landiolol group.
Kaplan–Meier analysis showed that patients who were continuously infused with low-dose landiolol during general anesthesia could not avoid the incidence of POAF in comparison with the placebo saline group (Fig. 1).
Assuming that the incidence rate of POAF in the control group was as much as investigated in these 50 series and that no more AF would occur in the landiolol group in another attempt, we expected no significant difference in all 100 cases that we intended to examine with the Chi-square test (P = 0.372). Hence, we abandoned further investigation, because of the statistical futility.
Regarding the secondary endpoint, the plasma concentration of IL-6 sampled at the end of surgery was only significantly lower in the landiolol group, not the control group (Fig. 2). However, the plasma concentration of IL-6 at other points, serum Mg, Ca, CRP, NT Pro-BNP, and fractions of adrenaline, noradrenaline, and dopamine in stored urine for 24 h at all points were similar between the groups.
Plasma concentration of interleukin-6 at before operation (baseline), just after resection (resected), end of operation (end of op), 6 h after end of operation (6 h post-op), and postoperative day 1 and 4 in control normal saline group and landiolol group, respectively. An asterisk indicates statistical significance (P < 0.05). NS normal saline, L landiolol, POD postoperative day
We entered 53 covariates into a univariate logistic regression analysis to identify independent predictors of POAF. The analysis identified five statistically significant covariates. Next, we performed the multivariate logistic regression analysis for prevalence of POAF with these five predictors. Finally, we determined that only one predictor (IL-6 sampled at 6 h after end of surgery) was statistically significant (Table 2).
Throughout this series, we experienced no major adverse event or mortality.
Discussion
The result of this double-blind randomized controlled preliminary study fails to demonstrate that the incidence of POAF after lung resection can be prevented by continuous administration of low-dose landiolol infusion during general anesthesia. The previous studies revealed that prophylactic landiolol administration significantly lowered the incidence of AF after CABG, which tended to occur in the same period as after pulmonary resection but to have a higher incidence rate (25.0–37.8 % in the control group) [15–21] compared with that in this trial. In addition, the population who undergo CABG would more frequently need many more inotropic agents during and/or after surgery than those who undergo lung resection. Certainly, postoperative administration of inotropes and vasopressors would raise the incidence of AF after cardiac surgery [25]. Takahashi et al. [26] demonstrated that landiolol has an antiarrhythmic effect on epinephrine-induced ventricular arrhythmias in halothane-anesthetized dogs. Consequently, among cardiac surgical patients, landiolol minimizes the unpleasant effect of inotropes in the environment with exposure to some exogenous or endogenous catecholamines, and can act effectively against AF. Therefore, it could significantly suppress the AF rate after cardiac surgery.
On reconsidering the matter in the population undergoing lung resection, Okita et al. [27] reported that a 5-μg/kg/min infusion of landiolol during surgery could decrease the incidence rate of AF after lung resection. However, their retrospective trial was not blinded and each electrocardiographic monitor was turned off on the first postoperative day. Afterward, the detection of dysrhythmia (including AF) exclusively depended on patient complaint of symptoms, such as palpitation or chest discomfort, at which time an electrocardiogram was recorded to determine what happened. In our investigation, AF sustained for only 30 s was picked up by close 24-h electrocardiographic monitoring. Such short-term AF might not be detected by their method.
In regard to the dose of landiolol infusion, it is comparable to or higher than one of the previous trials aimed at CABG patients [15–21]. Therefore, the dose (5 μg/kg/min) could bear comparison with other prior investigations as a preventive administration.
AF could be caused by multiple factors (e.g., hyper-activity of the autonomic nerve system, myocardial ischemia, inflammatory reaction, atrial fibrosis, and abnormal electrolyte level). It is very interesting that only the IL-6 sampled just after the operation in this study was significantly lower in the landiolol group than in the control group, although we examined to find out how the landiolol would act.
Landiolol has higher selectivity to beta-1 receptors than esmolol and takes effect very quickly, with a 4-min half-life in the blood. The significant decrease of IL-6 just after the surgical procedure in our results could be accounted for by this very short-acting characteristic of landiolol. Furthermore, continuous administration of landiolol during general anesthesia could not significantly affect average, minimum, or maximum heart rates measured by Holter monitor on each postoperative day for 1 week after surgery. This could also account for the very short duration of action of landiolol.
Prior manuscripts documented that various predictors were associated with POAF after thoracic surgery. In our randomized controlled trial, we addressed some perioperative factors and performed the logistic regression analysis with these factors. At last, our logistic regression method revealed that only IL-6 sampled 6 h after surgery was associated significantly with the incidence of POAF. In light of the significant difference mentioned earlier of IL-6 just after surgery between the landiolol group and the control group, prolongation of the dosing duration should be considered, since landiolol infusion over additional hours might reduce significantly the IL-6 sampled 6 h after surgery.
Between our trial and the other studies that demonstrated the efficacy of landiolol as the prophylaxis for POAF, one of the definite major disparities is the surgical procedure used on the subjects; another possible disparity is the duration of landiolol administration. Sezai et al. [17] started the administration of landiolol at the time of central anastomosis during CABG and discontinued after 48 h. In another investigation [19], they started landiolol infusion intravenously from the completion of central anastomosis of CABG and continued for 3 days, together with oral or gavage administration of bisoprolol, from the day after surgery. In these studies, they showed that beta-antagonists, including landiolol infusion, were useful to prevent POAF. We demonstrated that a 5-μg/kg/min infusion of landiolol just during general anesthesia could not prevent AF after pulmonary resection. From this point of view, it is debatable whether intraoperative landiolol infusion is really indispensable. Otherwise, it might be suggested that the administration of beta blocker just after surgery can inhibit POAF.
This study had several limitations. First, the study population was small. More subjects are needed to document that intraoperative small-dose landiolol infusion is useless as prevention for POAF. However, we had no choice, but to terminate the trial, because of statistical futility.
Second, we did not arrange the study group of postoperatively long-term infusion of landiolol. The administration of landiolol was restricted only when vigilant monitoring was available. This means that, ideally, the patient under the administration of landiolol should have occupied an intensive-care bed or high-care bed for several days. Researchers should also investigate a dosing duration long enough to prevent POAF by comparing three groups of different dosing durations of landiolol infusion. We were forced to end our study to make effective use of medical resources.
We must avoid useless administration in consideration of effective economical availability. Then, it is worthwhile to conduct trials, regarding the prevention of POAF by low-dose landiolol infusion administered after surgery or by only perioral dosing of beta-antagonists after lung surgery. At any rate, the subject requires further study.
Conclusions
We failed to demonstrate that low-dose infusion of landiolol during general anesthesia could prevent the incidence of AF after lung resection. Only IL-6 sampled at 6 h after surgery significantly predicted POAF among pulmonary surgical patients.
References
Roselli EE, Murthy SC, Rice TW, Houghtaling PL, Pierce CD, Karchmer DP, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg. 2005;130:438–44.
Asamura H, Naruke T, Tsuchiya R, Goya T, Kondo H, Suemasu K. What are the risk factors for arrhythmias after thoracic operations? A retrospective multivariate analysis of 267 consecutive thoracic operations. J Thorac Cardiovasc Surg. 1993;106:1104–10.
Wahi R, McMurtrey MJ, DeCaro LF, Mountain CF, Ali MK, Smith TL, et al. Determinants of perioperative morbidity and mortality after pneumonectomy. Ann Thorac Surg. 1989;48:33–7.
Cardinale D, Martinoni A, Cipolla CM, Civelli M, Lamantia G, Fiorentini C, et al. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg. 1999;68:1827–31.
Barbetakis N, Vassiliadis M. Is amiodarone a safe antiarrhythmic to use in supraventricular tachyarrhythmias after lung cancer surgery? BMC surg. 2004;4:7.
Krowka MJ, Pairolero PC, Trastek VF, Payne WS, Bernatz PE. Cardiac dysrhythmia following pneumonectomy. Clinical correlates and prognostic significance. Chest. 1987;91:490–5.
Amar D, Roistacher N, Burt M, Reinsel RA, Ginsberg RJ, Wilson RS. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995;108:349–54.
Polanczyk CA, Goldman L, Marcantonio ER, Orav EJ, Lee TH. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann Intern Med. 1998;129:279–85.
Vaporciyan AA, Correa AM, Rice DC, Roth JA, Smythe WR, Swisher SG, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127:779–86.
Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. 2012;7:4.
Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109:219–25.
El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–6.
Mariscalco G, Engström KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg. 2009;88:1871–6.
Bradley D, Creswell LL, Hogue CW, Epstein AE, Prystowsky EN, Daoud EG. Pharmacologic prophylaxis american college of chest physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:39S–47S.
Fujiwara H, Sakurai M, Namai A, Kawamura T. Effect of low-dose landiolol, an ultrashort-acting β-blocker, on postoperative atrial fibrillation after CABG surgery. Gen Thorac Cardiovasc Surg. 2009;57:132–7.
Wakamatsu H, Takase S, Sato Y, Seto Y, Kurosawa H, Yokoyama H. Effect of intra-operative low-dose infusion of landiolol hydrochloride on post-operative atrial fibrillation after off-pump coronary artery bypass grafting. Kyobu geka. 2010;63:764–8.
Sezai A, Minami K, Nakai T, Hata M, Yoshitake I, Wakui S, et al. Landiolol hydrochloride for prevention of atrial fibrillation after coronary artery bypass grafting: new evidence from the PASCAL trial. J Thorac Cardiovasc Surg. 2011;141:1478–87.
Fujii M, Bessho R, Ochi M, Shimizu K, Terajima K, Takeda S. Effect of postoperative landiolol administration for atrial fibrillation after off pump coronary artery bypass surgery. J Cardiovasc Surg. (Torino). 2012;53:369–74.
Sezai A, Nakai T, Hata M, Yoshitake I, Shiono M, Kunimoto S, et al. Feasibility of landiolol and bisoprolol for prevention of atrial fibrillation after coronary artery bypass grafting: a pilot study. J Thorac Cardiovasc Surg. 2012;144:1241–8.
Nakanishi K, Takeda S, Kim C, Kohda S, Sakamoto A. Postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting or cardiac valve surgery: intraoperative use of landiolol. J Cardiothorac Surg. 2013;8:19.
Ogawa S, Okawa Y, Goto Y, Aoki M, Baba H. Perioperative use of a beta blocker in coronary artery bypass grafting. Asian Cardiovasc Thorac. 2013;21:265–9.
Nagaoka E, Arai H, Tamura K, Makita S, Miyagi N. Prevention of atrial fibrillation with ultra-low dose landiolol after off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2014;20:129–34.
Nakashima M, Kanamaru M. Phase I study of ONO-1101, a new ultra short acting β 1-blocking agent in healthy volunteers. Rinsho Iyaku. 2000;16:1531–56.
Iguchi S, Iwamura H, Nishizaki M, Hayashi A, Senokuchi K, Kobayashi K, et al. Development of a highly cardioselective ultra short-acting beta-blocker, ONO-1101. Chem Pharm Bull. (Tokyo). 1992;40:1462–9.
Salaria V, Mehta NJ, Abdul-Aziz S, Mohiuddin SM, Khan IA. Role of postoperative use of adrenergic drugs in occurrence of atrial fibrillation after cardiac surgery. Clin Cardiol. 2005;28:131–5.
Takahashi S, Fujii Y, Inomata S, Miyabe M, Toyooka H. Landiolol decreases a dysrhythmogenic dose of epinephrine in dogs during halothane anesthesia. Can J Anesth. 1999;46:599–604.
Okita T, Uji M, Shinjo T, Morioka M, Kumano H, Ishimura N, et al. Use of landiolol hydrochloride for the prevention of atrial fibrillation after lung resection. Masui. 2008;57:953.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Aoyama, H., Otsuka, Y. & Aoyama, Y. Landiolol infusion during general anesthesia does not prevent postoperative atrial fibrillation in patients undergoing lung resection. Gen Thorac Cardiovasc Surg 64, 735–741 (2016). https://doi.org/10.1007/s11748-016-0707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-016-0707-3