Abstract
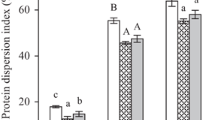
The objective of this work was to evaluate the kinetics and thermodynamics parameters and the effects of anionic, cationic and nonionic surfactants and polyethylene glycol on the activity and stability of a crude esterase extracted from soybeans (Glycine max L.). The activation energy for thermal inactivation was calculated from the Arrhenius plot was found to be 59.4 kJ mol−1 and the ΔH* 56.82 kJ mol−1 at 40 °C, which was the optimum temperature for enzyme activity. The ΔS* and ΔG* of the enzyme were found to be 61.67 kJ mol−1 and 15.50 J mol−1 K−1, respectively, at the optimum temperature. The activity was only enhanced by the cationic surfactants cetyltrimethylammonium bromide and tetradecylmethylammonium bromide at a concentration of 3.0 mM. The anionic surfactant showed a positive effect on enzyme activity at the concentrations of 1.5 and 3.0 mM. Aqueous PEG (polyethylene glycols) solutions activated the esterase, and maximum activation (170 %) occurred with the addition of 6 kDa PEG. PEG with molecular weights of 0.4 and 10 kDa enhanced enzyme stability at 40 °C.








Similar content being viewed by others
References
Polizelli PP, Tiera MJ, Bonilla-Rodriguez GO (2008) Effect of surfactants and polyethylene glycol on the activity and stability of a lipase from oilseeds of Pachira aquatica. J Am Oil Chem Soc 85:749–753
Paques FW, Macedo GA (2006) Lipases de Látex vegetais: propriedades e Aplicações industriais: a review. Quim Nova 29:93–102
Barros M, Fleuri LF, Macedo GA (2010) Seed lipases: sources, applications and properties—a review. Braz J Chem Eng 27:15–29
Barros M, Macedo GA (2011) Biochemical characterization and biocatalytic potential of esterase from Brazilian Glycine max L. J Food Sci Biotechnol 20:1195–1201
Chahiniana H, Ninib L, Boitardc E, Dubèsc JP, Comeaub LC, Sardad L (2002) Distinction between esterases and lipases: a kinetic study with vinyl esters and TAG. Lipids 37:653–662
Mendes AA, Oliveira PC, de Castro HF (2012) Properties and biotechnological applications pancreatic lipase. J Mol Catal B-Enzym 78:119–134
Houde A, Kademi A, Leblanc D (2004) Lipases and their industrial applications: an overview. Appl Biochem Biotechnol 118:155–170
Davranov K (1994) Microbial lipases in biotechnology. Appl Biochem Micro 30:427–432
Stamatis H, Xenakis A, Kolisis FN (1999) Bioorganic reactions in microemulsions the case of lipase. Biotechnol Adv 17:293–318
Holmberg K (2003) Organic reactions in microemulsions. Curr Opin Colloid Interface Sci 8:187–196
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye biding. Anal Biochem 39:239–247
Aizono Y, Funatsu M, Sugano M, Hayashi K, Fujiki Y (1973) Enzymatic properties of rice bran lipase. Agr Biol Chem Tokyo 37:2031–2036
Pio TF, Macedo GA (2007) Optimizing the production of cutinase by Fusarium oxysporum using response surface methodology. Enzyme Microb Tech 41:613–619
Marangoni AG (2003) Enzyme kinetics: a modern approach. Hoboken, New Jersey
Ludikhuyze L, Ooms V, Weemaes C, Hendrickx M (1999) Kinetic of the irreversible and inactivations of myrosinase form broccoli (Brassica oleracea L. Cv. Italica). J Agric Food Chem 47:1794–1800
Longo MA, Combes D (1999) Thermostability of modified enzymes: a detailed study. J Chem Technol Biotechnol 74:25–32
Bhatti HN, Amin F (2013) Kinetic and hydrolytic characterization of newly isolated alkaline lipase form Ganoderma lucidum using canola oil cake as substrate. J Chem Soc Pak 35(3):33–38
Staubmam R, Ncube I, Gübitz GM, Steiner W, Read JS (1999) Esterase and lipase activity in Jatropha curcas L. seeds. J Biotechnol 75:117–126
Rhee JK, Dg Ahn, Kim YG, Oh JW (2005) New thermophilic and thermostable esterase with sequence similarity to the hormone-sensitive lipase family, cloned from a metagenomic library. Appl Environ Microbiol 71(2):817–825
Souza CFV, Faccin DJL, Mertins O, Heck JX, Silveira NP, Secchi AR, Ayub MAS (2009) Kinetics of thermal inactivation of transglutaminase from a newly isolated Bacillus circulansBL32. J Chem Technol Biotechnol 84:1567–1575
Cobos A, Estrada P (2003) Effect of polyhydroxylic solvents on the thermostability and activity of xylanase from Trichoderma reesei QM 9414. Enzyme Microb Technol 33:810–818
Ghori MI, Iqbal MJ, Hameed A (2011) Characterization of a novel lipase from Bacillus sp. isolated from tannery wastes. Braz J Microbiol 42:22–29
Ortega N, Diego S, Rodriguez-Nogalez JM, Perez-Mateos M, Busto MD (2004) Kinetics behavior and thermal inactivation of pectin lyase used in food processing. Int J Food Sci Technol 39:631–639
Delorme V, Dhouib R, Canaan S, Fotiadu F, Carrière F, Cavalier JF (2011) Effects of surfactants on lipase structure, activity, and inhibition. Pharmaceut Res 28:1831–1842
Pancera SM, da Silva LHW, Loh W, Itri R Jr, Pessoa A, Petri FS (2002) The effect of poly(ethylene glycol) on the activity and structure of glucose-6-phosphate dehydrogenase in solution. Colloid Surface B 26:291–300
Reis P, Holmberg K, Watzke H, Leser ME, Miller R (2009) Lipases at interfaces: a review. Adv Colloid Interface 147:237–250
Mendes AA, Barbosa BC, Soares CMF, da Silvaand MLC, de Castro HF (2006) Atividade e estabilidade operacional de lipase imobilizada em fosfato de zircônio na ausência e presença de polietilenoglicol. Acta Sci Technol 28:133–140
Fahmy AS, Abo-Zeid AZ, Mohamed TM, Ghanem HM, Borai HI, Mohamed SA (2008) Characterization of esterases from Cucurbita pepocv Eskandrani. Bioresour Technol 99:437–443
Diaz JC, Cordova J, Baratti J, Carriere F, Abousalham A (2007) Effect of nonionic surfactants on Rhizopus homothallicus lipase activity. Mol Biotechnol 35:205–214
Verger R, Haas GH (1976) Interfacial enzyme kinetics of lipolysis. Annu Biophys Bioeng 5:77–1176
Aguiar J, Carpena P, Molina-Bolívar JA, Ruiz CC (2003) On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J Colloid Interface Sci 258:116–122
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
de Barros, M., Celligoi, M.A.P.C. & Macedo, G.A. Kinetics of Denaturation and Effects of Surfactants and Polyethylene Glycol on Soybean Esterase (Glycine max L) Stability. J Am Oil Chem Soc 93, 37–44 (2016). https://doi.org/10.1007/s11746-015-2755-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-015-2755-8