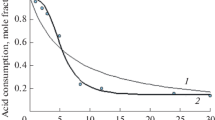
Abstract
The better to characterize enzymes hydrolyzing carboxyl ester bonds (carboxyl ester hydrolases), we have compared the kinetic behavior of various lipases and esterases against solutions and emulsions of vinyl esters and TAG. Shortchain vinyl esters are hydrolyzed at comparable rates by esterases and lipases and have higher limits of solubility in water than corresponding TAG. Therefore, they are suited to study the influence of the physical state of the substrate on carboxyl ester hydrolase activity within a large concentration range. Enzymes used in this study are TAG lipases from microorganisms, lipases from human and guinea pig pancreas, pig liver esterase, and acetylcholinesterase. This study also includes cutinase, a fungal enzyme that displays functional properties between esterases and lipases. Esterases display maximal activity against solutions of short-chain vinyl esters (vinyl acetate, vinyl propionate, and vinyl butyrate) and TAG (triacetin, tripropionin, and tributyrin). Half-maximal activity is reached at ester concentrations far below the solubility limit. The transition from solution to emulsion at substrate concentrations exceeding the solubility limit has no effect on esterase activity. Lipases are active on solutions of short-chain vinyl esters and TAG but, in contrast to esterases, they all display maximal activity against emulsified substrates and half-maximal activity is reached at substrate concentrations near the solubility limit of the esters. The kinetics of hydrolysis of soluble substrates by lipases are either hyperbolic or deviate from the Michaelis-Menten model and show no or weak interfacial activation. The presence of molecular aggregates in solutions of short-chain substrates, as evidenced by a spectral dye method, likely accounts for the activity of lipases against soluble esters. Unlike esterases, lipases hydrolyze emulsions of water-insoluble medium- and long-chain vinyl esters and IAG such as vinyl laurate, trioctanoin, and olive oil. In conclusion, comparisons of the kinetic behavior of carboxyl ester hydrolases against solutions and emulsions of vinyl esters and TAG allows the distinction between lipases and esterases. In this respect, it clearly appears that guinea pig pancreatic lipase and cutinase are unambiguously classified as lipases.
Similar content being viewed by others
References
Tsujita, T., Shirai, K., Saito, Y., and Okuda, H. (1990) Relationship Between Lipase and Esterase, in Isozymes: Structure Function, and Use in Biology and Medicine, pp. 915–933, Wiley-Liss, New York.
Jaeger, K.E., Ransac, S., Dijkstra, B.W., Colson, C., Van Heuvel, M., and Misset, O. (1994) Bacterial Lipases, FEMS Microbiol. Rev. 15, 29–63.
Rosenstein, R., and Götz, F. (2000) Staphylococcal Lipases: Biochemical and Molecular Characterization, Biochimie 82, 1005–1014.
Fojan, P., Jonson, P.H., Petersen, M.T.N., and Petersen, S.B. (2000) What Distinguishes an Esterase from a Lipase: A Novel Structural Approach, Biochimie 82, 1033–1041.
Brady, L., Brzozowski, A.M., Derewenda, Z.S., Dodson, E., Dodson, G.G., Tolley, S., Turkenburg, J.P., Christiansen, L., Huge-Jensen, B., Norskov, L., et al. (1990) A Serine Protease Triad Forms the Catalytic Centre of a Triacylglycerol Lipase, Nature 343, 767–770.
Winkler, F.K., D'Arcy, A., and Hunziker, W. (1990) Structure of Pancreatic Lipase, Nature 343, 771–774.
Derewenda, Z.S., Derewenda, U., and Dodson, G.G. (1992) The Crystal and Molecular Structure of the Rhizomucor miehei Triglyceride Lipase at 1.9 Å Resolution, J. Mol. Biol. 227, 818–839.
Brzozowski, A.M., Derewenda, U., Derewenda, Z.S., Dodson, G.G., Lawson, D.M., Turkenburg, J.P., Bjorkling, F., Huge-Jensen, B., Patkar, S.A., and Thim, L. (1991) A Model for Interfacial Activation in Lipases from the Structure of a Fungal Lipase-Inhibitor Complex, Nature, 351, 761–764.
Schrag, J.D., Yunge, L., Shan, W., and Cygler, M. (1991) Ser-His-Glu Forms the Catalytic Site of a Lipase from Geotrichum candidum, Nature 351, 761–764.
Schrag, J.D., and Cygler, M. (1993) A Refined Structure of the Lipase from Geotrichum candidum, J. Mol. Biol. 230, 575–591.
Van Tilbeurgh, H., Sarda, L., Verger, R., and Cambrillau, C. (1992) Structure of the Lipase-Procolipase Complex, Nature 359, 159–162.
Van Tilbeurgh, H., Egloff, M.P., Martinez, C., Rugani, N., Verger, R., and Cambillau, C. (1993) Interfacial Activation of the Lipase-Procolipase Complex by Mixed Micelles Revealed by X-ray Crystallography, Nature 362, 814–820.
Longhi, S., and Cambillau, C. (1999) Structure-Activity of Cutinase, a Small Lipolytic Enzyme, Biochim. Biophy. Acta 1441, 185–196.
Hjorth, A., Carrière, F., Cudrey, C., Woldike, H., Boel, E., Lawson, D.M., Ferrato, F., Cambillau, C., Dodson, G.G., Thim, L., and Verger, R. (1993) A Structural Domain (the lid) Found in Pancreatic Lipases Is Absent in the Guinea Pig (phospho)Lipase, Biochemistry 32, 4702–4707.
Verger, R. (1997) Interfacial Activation of Lipases: Facts and Artifacts, TIBTECH. 15, 32–38.
Chahinian, H., Nini, L., Boitard, E., Dubès, J.P., Sarda, L., and Comeau, L. (2000) Kinetic Properties of Penicillium cyclopium Lipase Studied with Vinyl Esters, Lipids 35, 919–926.
Nini, L., Sarda, L., Comeau, L.C., Boitard, E., Dubès, J.P., and Chahinian, H. (2001) Lipase-Catalysed Hydrolysis of Short-Chain Substrates in Solution and in Emulsion: A Kinetic Study, Biochim. Biophys. Acta, 1534, 34–44.
Brockerhoff, H. (1968) Substrate Specificity of Pancreatic Lipase, Biochim. Biophys. Acta 159, 296–303.
Brockerhoff, H. (1970) Substrate Specificity of Pancreatic Lipase. Influence of the Structure of the Fatty Acids on the Reactivity of Esters, Biochim. Biophys. Acta 212, 92–101.
Hiol, A., Jonzo, M.D., Rugani, N., Druet, D., Sarda, L., and Comeau, L.C. (2000) Purification and Characterization of an Extracellular Lipase from a Thermophilic Rhizopus oryzae Strain Isolated from Palm Fruit, Enzyme Microb. Technol. 26, 421–430.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem. 193, 265–275.
Ferrato, F., Carrière, F., Sarda, L., and Verger, R. (1997) A Critical Reevaluation of the Phenomenon of Interfacial Activation, Methods Enzymol. 286, 327–347.
Berg, O.G., Cajal, Y., Butterfoss, G.L., Grey, R.L., Alsina, M.A., Yu, B.Z., and Jain, M.K. (1998) Interfacial Activation of Triglyceride Lipase from Thermomyces (Humicola) lanuginosa: Kinetic Parameters and a Basis for the Control of the Lid, Biochemistry 37, 6615–6627.
Canioni, P., Julien, R., Rathelot, J., and Sarda, L. (1977) Pancreatic and Microbial Lipases: A Comparison of the Interaction of Pancreatic Colipase with Lipases of Various Origins, Lipids 12, 393–397.
Entressangles, B., and Desnuelle, P. (1968) Action of Pancreatic Lipase on Aggregated Glyceride Molecules in an Isotropic System, Biochim. Biophys. Acta 159, 285–295.
Sarda, L., and Desnuelle, P. (1958) Action de la lipase pancréatique sur les esters en émulsion, Biochim. Biophys. Acta 30, 513–521.
Desnuelle, P., Ailhaud, G., and Sarda, L. (1960) Inhibition de la lipase pancréatique par le diéthyl-p-nitrophénylphosphate en émulsion, Biochim. Biophys. Acta 37, 570–571.
Deever, A.M. (1992) Mechanism of Activation of Lipolytic Enzymes. Ph.D. Thesis, University of Utrecht, The Netherlands, 192 pp.
Noble, M.E.M., Cleasby, A., Johnson, L.N., Egmond, M.R., and Frenken, L.G. (1993) The Crystal Structure of Triacylglycerol Lipase from Pseudomonas glumae Reveals a Partially Redundant Catalytic Aspartate, FEBS Lett. 331, 123–128.
Jaeger, K.E., Ransac, S., Koch, H.B., Ferrato, F., and Dijkstra, B.W. (1993) Topological Characterization and Modeling of the 3D Structure of Lipase from Pseudomonas aeruginosa, FEBS Lett. 332, 143–149.
Jaeger, K.E., Ransac, S., Dijkstra, B.W., Colson, C., Vanheuvel, M., and Misset, O. (1994) Bacterial Lipases, FEMS Microbiol. Rev. 15, 29–63.
Martinelle, M., Holmquist, M., and Hult, K. (1995) On the Interfacial Activation of Candida antarctica Lipase A and B as Compared with Thermomyces lanuginosa Lipase, Biochim. Biophys. Acta 1258, 272–276.
Thirstrup, K., Verger, R., and Carrière, F. (1994) Evidence for a Pancreatic Lipase Subfamily with New Kinetic Properties, Biochemistry 33, 2748–2756.
Van Oort, M.G., Deever, A.M., Dijkman, R., Tjeenk, M.L., Vereij, H.M., De Haas, G.H., Wenzig, E., and Götz, F. (1989) Purification and Substrate Specificity of Staphylococcus hyicus Lipase, Biochemistry 28, 9278–9285.
Arpigny, J.L., and Jaeger, K.E. (1999) Bacterial Lipolytic Enzymes: Classification and Properties, Biochem. J. 343, 177–183.
Prompers, J.J., Groenewegen, A., Hilbers, C.W., and Pepermans, H.A.M. (1999) Backbone Dynamics of Fusarium solani pisi Cutinase Probed by NMR. The Lack of Interfacial Activation Revisited, Biochemistry 38, 5315–5327.
Egmond, M.R., and de Vlieg, J. (2000) Fusarium solani pisi Cutinase, Biochimie 82, 1015–1021.
Author information
Authors and Affiliations
About this article
Cite this article
Chahinian, H., Nini, L., Boitard, E. et al. Distinction between esterases and lipases: A kinetic study with vinyl esters and TAG. Lipids 37, 653–662 (2002). https://doi.org/10.1007/s11745-002-0946-7
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-002-0946-7