Abstract
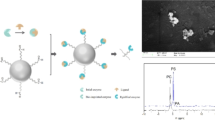
The purpose of the study was to assess a fluorimetric assay for the determination of total phospholipase A2 (PLA2) activity in biological samples introducing the innovation of immobilized substrates on crosslinked polymeric membranes. The immobilized C12-NBD-PtdCho, a fluorescent analogue of phosphatidylcholine, exhibited excellent stability for 3 months at 4 °C and was not desorbed in the aqueous reaction mixture during analysis. The limit of detection was 0.5 pmol FA (0.2 pg) and the linear part of the response curve extended from 1 up to 190 nmol FA/h/mL sample. The intra- and inter-day relative standard deviations (%RSD), were ≤6 and ≤9 %, respectively. Statistical comparison with other fluorescent methods showed excellent correlation and agreement. Semiempirical calculations showed a fair amount of electrostatic interaction between the NBD-labeled substrate and the crosslinked polyvinyl alcohol with the styryl pyridinium residues (PVA-SbQ) material, from the plane of which, the sn-2 acyl chain of the phospholipid stands out and is accessible by PLA2. Atomic Force Microscopy revealed morphological alterations of the immobilized substrate after the reaction with PLA2. Mass spectrometry showed that only C12-NBD-FA, the PLA2 hydrolysis product, was detected in the reaction mixture, indicating that PLA2 recognizes PVA-SbQ/C12-NBD-PtdCho as a surface to perform catalysis.








Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- ARDS:
-
Acute respiratory distress syndrome
- BAL fluid:
-
Bronchoalveolar lavage fluid
- BSA:
-
Bovine serum albumin
- C12-NBD-FA:
-
12-[(7-Nitro-2-1,3-benzoxadiazol-4-yl)amino}dodecanoic acid
- C12-NBD-PtdCho:
-
1-Palmitoyl-2-{12-[(7-nitro-2-1,3benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3-phosphocholine
- C6-NBD-PtdCho:
-
1-Palmitoyl-2-{6-[7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine
- EDTA:
-
Ethylenediaminetetraacetic acid
- FA:
-
Fatty acid
- HPLC:
-
High performance liquid chromatography
- MS:
-
Mass spectrometry
- PAF:
-
Platelet activating factor
- PLA2 :
-
Phospholipase A2
- PVA-SbQ:
-
Photocrosslinkable polyvinyl alcohol with steryl pyridinium residues
- RSD:
-
Relative standard deviation
- XRD:
-
X-ray diffraction analysis
References
Gijon MA, Leslie CC (1999) Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol 65:330–336
Balsinde J, Winstead MV, Dennis EA (2002) Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett 531:2–6
Six DA, Dennis EA (2000) The expanding superfamily of phospholipase A2: classification and characterization. Biochim Biophys 1488:1–19
Burke JE, Dennis EA (2009) Phospholipase A2 structure/function, mechanism and signaling. J Lipid Res 50:237–242
Nakos G, Malamou-Mitsi VD, Lachana A, Karassavoglou A, Kitsiouli E, Agnandi N, Lekka ME (2002) Immunoparalysis in patients with severe trauma and the effect of inhaled interferon-gamma. Crit Care Med 30:1488–1494
Kostopanagiotou G, Routsi C, Smyrniotis V, Lekka ME, Kitsiouli E, Arkadopoulos N, Nakos G (2003) Alterations in bronchoalveolar lavage fluid during ischemia-induced acute hepatic failure in the pig. Hepatology 37:1130–1138
Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME (2005) Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med 33:772–779
Nevalainen TJ (2003) Serum phospholipases A2 in inflammatory diseases. Clin Chem 39:2453–2459
Nakos G, Pneumatikos J, Tsangaris H, Tellis C, Lekka ME (1997) Protein and phospholipids in BAL from patients with hydrostatic pulmonary edema. Am J Respir Crit Care Med 155:945–951
Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB (1990) Interfacial catalysis: the mechanism of phospholipase A2. Science 250:1541–1546
Berg OG, Yu BJ, Rogers J, Jain MK (1991) Interfacial catalysis by phospholipase A2: determination of the interfacial kinetic rate constants. Biochemistry 30:7283–7297
Kitsiouli EI, Nakos G, Lekka ME (1999) Differential determination of phospholipase A(2) and PAF-acetylhydrolase in biological fluids using fluorescent substrates. J Lipid Res 40:2346–2356
Thuren T, Vertanen JA, Lalla M, Kinnunen PKJ (1985) Fluorimetric assay for phospholipase A2 in serum. Clin Chem 31:714–717
Karkabounas A, Kitsiouli EI, Nakos G, Lekka ME (2011) HPLC-fluorimetric assay of phospholipase A2. Application to biological samples with high protein content and various reaction conditions. J Chromatogr B 879:1557–1564
Tsiafoulis CG, Florou AB, Trikalitis PN, Bakas T, Prodromidis MI (2005) Electrochemical study of ferrocene intercalated vanadium pentoxide xerogel/polyvinyl alcohol composite films: application in the development of amperometric biosensors. Electrochem Commun 7:781–788
King CHR, Margolin AL (1993) Immobilization of substrates in enzyme–catalyzed hydrolysis. Tetrahedron Asymmetry 4:943–946
Ong S, Cai SJ, Bernal C, Rhee D, Qiu X, Pidgeon C (1994) Phospholipid immobilization on solid surfaces. Anal Chem 66:782–792
Esker AR, Brode PF III, Rubingh DN, Rauch DS, Yu H, Gast AP, Robertson CR, Trigiante G (2000) Protease activity on an immobilized substrate modified by polymers: subtilisin BPN. Langmuir 16:2198–2206
Halling PJ, Ulijn RV, Flitsch SL (2005) Understanding enzyme action on immobilized substrates. Curr Opin Biotechnol 16:385–392
Pantazi D, Drougas E, Loppinet B, Tellis C, Kosmas AM, Lekka ME (2006) Hydrolysis of phospholipase D of phospholipids in solution state or adsorbed on a silica matrix. Chem Phys Lip 139:20–31
Rouillon R, Euzet P, Carpentier R (2004) Stabilization of photosynthetic materials. Method Mol Biol 274:261–269
Barthelmebs L, Carpentier R, Rouillon R (2011) Physical and chemical immobilization methods of photosynthetic materials. Method Mol Biol 684:247–256
Laberge D, Rouillon R, Carpentier R (2000) Comparative study of thylakoid membranes sensitivity for herbicide detection after physical or chemical immobilization. Enzyme Microb Technol 26:332–336
Tsiafoulis CG, Prodromidis MI, Karayannis MI (2004) Development of an amperometric biosensing method for the determination of L-fucose in pretreated urine. Biosens Bioelectron 20:620–627
Setwart JJP (1989) Optimazation of parameters for semiempirical methods. I Methods. J Comput Chem 10:209–220
Ichimura K, Iwata S, Mochizuki S, Ohmi M, Adachi D (2012) Revisit to the photocrosslinking behavior of PVA-SbQ as a water-soluble photopolymer with anomalously low contents of quaterized stilbazol side chains. J Polym Sci A Polym Chem 50:4094–4102
Mazères S, Schram V, Tocanne JF, Lopez A (1996) 7-nitrobenz-2-oxa-1,3-diazole-4-yl-labeled phospholipids in lipid membranes: differences in fluorescence behavior. Biophys J 71:327–335
Mukherjee S, Raghuraman H, Dasgupta S, Chattopadhyay A (2004) Organization and dynamics of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: a fluorescence approach. Chem Phys Lipid 127:91–101
Filipe HA, Moreno MJ, Loura LM (2011) Interaction of 7-nitrobenz-2-oxa-1,3-diazol-4-yl-labeled fatty amines with 1-palmitoyl, 2-oleoyl-sn-glycero-3-phosphocholine bilayers: a molecular dynamics study. J Phys Chem B 115:10109–10119
Loura LM, Fernandes F, Fernandes AC, Ramalho JP (2008) Effects of fluorescent probe NBD-PC on the structure, dynamics and phase transition of DPPC. A molecular dynamics and differential scanning calorimetry study. Biochim Biophys Acta 1778:491–501
Buitrago-Rey R, Olarte J, Gomez-Marin JE (2002) Evaluation of two inhibitors of invasion: lY311727 [3-(3-acetamide-1-benzyl-2-ethyl-indolyl-5-oxy)propane phosphonic acid] and AEBSF [4-(2-aminoethyl)-benzenesulphonyl fluoride] in acute murine toxoplasmosis. J Antimicrob Chemother 49:871–874
Martin OC, Pagano RE (1986) Normal and reverse-phase HPLC separations of fluorescent (NBD) lipids. Anal Biochem 159:101–108
Wu H, Yu L, Tong Y, Ge A, Yau S, Osawa M, Ye S (2013) Enzyme-catalyzed hydrolysis of the supported phospholipid bilayers studied by atomic force microscopy. Biochim Biophys Acta 1828:642–651
Tatulian SA (2001) Toward understanding interfacial activation of secretory phospholipase A2 (PLA2): membrane surface properties and membrane-induced structural changes in the enzyme contribute synergistically to PLA2 activation. Biophys J 50:789–800
Petrovic N, Grove C, Langton PE, Misso NL, Thompson PJ (2001) A simple assay for a human serum phospholipase A2 that is associated with high-density lipoproteins. J Lipid Res 42:1706–1713
Sapirstein A, Spech RA, Witzgall R, Bonventre JV (1996) Cytosolic phospholipase A2 (PLA2), but not secretory PLA2, potentiates hydrogen peroxide cytotoxicity in kidney epithelial cells. J Biol Chem 271:21505–21513
Kitsiouli E, Nakos G, Lekka ME (2009) Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta 1792:941–953
Taverniers I, Loose MD, Bockstaele EV (2004) Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends. Anal Chem 23:535
Lasch J, Willhardt I, Kinder D, Sauer H, Smesny S (2003) Fluorometric assays of phospholipase A2 with three different substrates in biological samples of patients with schizophrenia. Clin Chem Lab Med 41:908–914
Lekka ME, Karkabounas A, University of Ioannina research committee (2011) Immobilization of lipid substrates in order to develop and evaluate a fluorimetric assay for the determination of phospholipase A2 activity in biological samples. Greek Patent Code Nr 1007801
Acknowledgments
The authors would like to thank the Unit of Environmental, Organic and Biochemical high resolution analysis-ORBITRAP-LC–MS of the University of Ioannina for providing access to the facilities. The authors would like to thank the Confocal Laser Scanning Microscopy Unit of the University of Ioannina for providing access to the facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Karkabounas, A., Georgiadou, D.G., Argitis, P. et al. Immobilization of Lipid Substrates: Application on Phospholipase A2 Determination. Lipids 50, 1259–1271 (2015). https://doi.org/10.1007/s11745-015-4076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4076-y