Abstract
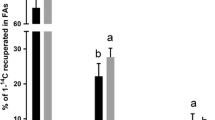
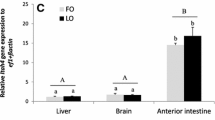
The overall objective is to test the hypothesis that conjugated linoleic acid (CLA) has beneficial effects in Atlantic salmon as a result of affecting lipid and FA metabolism. The specific aims of the present study were to determine the effects of CLA on some key pathways of FA metabolism, including FA oxidation and highly unsaturated FA (HUFA) synthesis. Salmon smolts were fed diets containing two levels of fish oil (low, ∼17%, and high, ∼34%) containing three levels of CLA (a 1∶1 mixture of cis-9,trans-11 and trans-10,cis-12 at 0, 1, and 2% of diet) for 3 mon. The effects of dietary CLA on HUFA synthesis and β-oxidation were measured, and the expression of key genes in the FA oxidation and HUFA synthesis pathways, and the potentially important transcription factors peroxisome proliferators activated receptors (PPAR), were determined in selected tissues. Liver HUFA synthesis and desaturase gene expression was increased by dietary CLA and decreased by high diatary oil content. Carnitine palmitoyltransferase-I (CPT-I) activity and gene expression were generally increased by CLA in muscle tissues although they were relatively unaffected by dietary oil content. In general CPT-1 activity or gene expression was not correlated with β-oxidation. Dietary CLA tended to increase PPARα and β gene expression in both liver and muscle tissues, and PPARγ in liver. In summary, gene expression and activity of the FA pathways were altered in response to dietary CLA and/or oil content, with data suggesting that PPAR are also regulated in response to CLA. Correlations were observed between dietary CLA, liver HUFA synthesis and desaturase gene expression, and liver PPARα expression, and also between dietary CLA, CPT-I expression and activity, and PPARα expression in muscle tissues. In conclusion, this study suggests that dietary CLA has effects on FA metabolism in Atlantic salmon and on PPAR transcription factors. However, further work is required to assess the potential of CLA as a dietary supplement, and the role of PPAR in the regulation of lipid metabolism in fish.
Similar content being viewed by others
Abbreviations
- CPT-1:
-
carnitine palmitoyl transferase-I
- HUFA:
-
highly unsaturated FA
- PPAR:
-
peroxisome proliferator-activated receptor
- rtqPCR:
-
real-time quantitative polymerase chain reaction
- SCD:
-
stearoyl CoA desaturase
- SGR:
-
specific growth rate
- SREBP:
-
steryl regulatory element binding protein
References
Belury, M.A. (2002) Dietary Conjugated Linoleic Acid in Health: Physiological Effects and Mechanisms of Action, Annu. Rev. Nutr. 22, 505–531.
Wang, Y., and Jones, P.J. (2004) Dietary Conjugated Linoleic Acid and Body Composition, Am. J. Clin. Nutr. 79, 1153S-1158S.
Terpstra, A.H. (2004) Effect of Conjugated Linoleic Acid on Body Composition and Plasma Lipids in Humans: An Overview of the Literature, Am. J. Clin. Nutr. 79, 352–361.
Choi, Y., Park, Y., Storkson, J.M., Pariza, M.W., and Ntambi, J.M. (2002) Inhibition of Stearoyl-CoA Desaturase Activity by the cis-9,trans-11 Isomer and the trans-10,cis-12 Isomer of Conjugated Linoleic Acid in MDA-MB-231 and MCF-7 Human Breast Cancer Cells, Biochem. Biophys. Res. Commun. 294, 785–790.
Eder, K., Slomma, N., and Becker, K. (2002) Trans-10,cis-12 Conjugated Linoleic Acid Suppresses the Desaturation of Linoleic and α-Linolenic Acids in HepG2 Cells, J. Nutr. 132, 1115–1121.
Chuang, L.T., Leonard, A.E., Liu, J.W., Mukerji, P., Bray, T.M., and Huang, Y.S. (2001) Inhibitory Effect of Conjugated Linoleic Acid on Linoleic Acid Elongation in Transformed Yeast with Human Elongase, Lipids 36, 1099–1103.
Moya-Camarena, S.Y., Van den Heuvel, J.P., Blanchard, S.G., Leesnitzer, L.A., and Belury, M.A. (1999) Conjugated Linoleic Acid Is a Potent naturally Occurring Ligand and Activator of PPARα, J. Lipid Res. 40, 1426–1433.
Belury, M.A., Moya-Camarena, S.Y., Lu, M., Shi, L., Leesnitzer, L.M., and Blanchard, S.G. (2002) Conjugated Linoleic Acid Is an Activator and Ligand for Peroxisome Proliferator-Activated Receptor-Gamma (PPARγ), Nutr. Res. 22, 817–824.
Smith, S.A. (2002) Peroxisome Proliferator-Activated Receptors and the Regulation of Mammalian Lipid Metabolism, Biochem. Soc. Trans. 30, 1086–1090.
Roche, H.M., Noone, E., Sewter, C., McBennett, S., Savage, D., Gibney, M.J., O'Rahilly, S., and Vidal-Puig, A.J. (2002) Isomer-Dependent Metabolic Effects of Conjugated Linoleic Acid: Insights from Molecular Markers Sterol Regulatory Element Binding Protein-1 c and LXRα, Diabetes 51, 2037–2044
Matsuzaka, T., Shimano, H., Yahagi, N., Amemiya-Kudo, M., Yoshikawa, T., Hasty, A.H., Tamura, Y., Osuga, J., Okazaki, H., Iizuka, Y., Takahashi, A., Sone, H., Gotoda, T.; Ishibashi, S., and Yamada, N. (2002) Dual Regulation of Mouse Δ5- and Δ6-Desaturase Gene Expression by SREBP-1 and PPARα, J. Lipid Res. 43, 107–114.
Moon, Y., Shah, N.A., Mohapatra, S., Warrington, J.A., and Horton, J.D. (2001) Identification of a Mammalian Long Chain Fatty Acyl Elongase Regulated by Sterol Regulatory Element-Binding Proteins, J. Biol. Chem. 276, 45358–45366.
U.S. National Research Council. (1993) Nutrient Requirements of Fish, Washington, DC: National Academy.
Whelan, J.A., Russell, N.B., and Whelan, M.A. (2003) A Method for Absolute Quantification of cDNA Using Real-Time PCR, J. Immun. Method. 278:261–269.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem. 193, 265–275.
Kennedy, S.R., Campbell, P.J., Porter, A., and Tocher, D.R. (2005) Influence of Dietary Conjugated Linoleic Acid (CLA) on Lipid and Fatty Acid Composition in Liver and Flesh of Atlantic Salmon (Salmo salar), Comp. Biochem. Physiol. 141B, 168–178.
Stubhaug, I., Tocher, D.R., Bell, J.G., Dick, J.R., and Torstensen, B.E. (2005) Fatty Acid Metabolism in Atlantic Salmon (Salmo salar L.) Hepatocytes, and Influence of Dietary Vegetable Oil, Biochim. Biophys. Acta 1734, 277–288.
Saggerson, E.D., and Carpenter, C.A. (1986) Carnitine Palmitoyltransferase in Liver and Five Extrahepatic Tissues in the Rat. Inhibition by DL-2-Bromopalmitoyl-CoA and Effect of Hypothyroidism, Biochem. J. 236, 137–141.
Torstensen, B.E., Li, Ø, and Frøyland, L. (2000) Lipid Metabolism and Tissue Composition in Atlantic Salmon (Salmo salar L.): Effects of Capelin-, Palm- and Oleic Acid Enriched Sunflower Oil as Dietary Lipid Sources, Lipids 35, 653–664.
Zar, J.H. (1994) Biostatistical Analysis, 2nd edn, Englewood Cliffs, NJ: Prentice Hall.
Oku, H., Wongtangtintharn, S., Iwasaki, H., and Toda, T. (2003) Conjugated Linoleic Acid (CLA) Inhibits Fatty Acid Synthetase Activity in vitro, Biosci. Biotechnol. Biochem. 67, 1584–1586.
Evans, M., Lin, X., Odle, J., and McIntosh, M. (2002) Trans-10,cis-12 Conjugated Linoleic Acid Increases Fatty Acid Oxidation in 3T3-L1 Preadipocytes, J. Nutr. 132 450–455.
Berge, G.M., Ruyter, B., and Asgard, T. (2004) Conjugated Linoleic Acid in Diets for Juvenile Atlantic Salmon (Salmo salar): Effects on Fish Performance, Proximate Composition, Fatty Acid and Mineral Content, Aquaculture 237, 365–380.
Takahashi, Y., Kushiro, M., Shinohara, K., and Ide, T. (2003) Activity and mRNA Levels of Enzymes Involved in Hepatic Fatty Acid Synthesis and Oxidation in Mice Fed Conjugated Linoleic Acid, Biochim. Biophys. Acta. 1631, 265–273.
Twibell, R.G., Watkins, B.A., Rogers, L., and Brown, P.B. (2000) Effects of Dietary Conjugated Linoleic Acids on Hepatic and Muscle Lipids in Hybrid Striped Bass, Lipids 35, 155–161.
Nakamura, M.T., and Nara, T.Y. (2004) Structure, Function, and Dietary Regulation of Δ6, Δ5 and Δ9 Desaturases, Annu. Rev. Nutr. 24, 345–376.
Torstensen, B.E., Bell, J.G., Rosenlund, G., Henderson, R.J., Graff, I.E., Tocher, D.R., Lie, Ø., and Sargent, J.R. (2005) Tailoring of Atlantic Salmon (Salmo salar L.) Flesh Lipid Composition and Sensory Quality by Replacing Fish Oil with a Vegetable Oil Blend, J. Agric. Food Chem. 53, 10166–10178.
Tocher, D.R. (2003) Metabolism and Functions of Lipids and Fatty Acids in Teleost Fish, Rev. Fish. Sci. 11, 107–184.
Nanton, D.A., Lall, S.P., Ross, N.W., and McNiven, M.A. (2003) Effect of Dietary Lipid Level on Fatty Acid β-Oxidation and Lipid Composition in Various Tissues of Haddock, Melanogrammus aeglefinus L., Comp. Biochem. Physiol. 135B, 95–108.
Rahman, S.M., Wang, Y.M., Yotsumoto, H., Cha, Y., Han, S.Y., Inoue, S., and Yanagita, T. (2001) Effects of Conjugated Linoleic Acid on Serum Leptin Concentration, Body-Fat Accumulation, and β-Oxidation of Fatty Acid in OLETF Rats, Nutrition 17, 385–390.
Degrace, P., Demizieux, L., Gresti, J., Chardigny, J.M., Sebedio, J.L., and Clouet, P. 2004. Hepatic Steatosis Is Not Due to Impaired Fatty Acid Oxidation Capacities in C57BL/6J Mice Fed the Conjugated trans-10,cis-12-Isomer of Linoleic Acid, J. Nutr. 134, 861–867.
Frøyland, L., Lie, Ø., and Berge, R.K. (2000) Mitochondrial and Peroxisomal β-Oxidation Capacities in Various Tissues from Atlantic Salmon Salmo salar, Aquaculture Nutr. 6, 85–89.
Bouthegourd, J.C., Even, P.C., Gripois, D., Toffon, B., Blouquit, M.F., Roseau, S., Lutton, C., Tome, D., and Martin, J.C. (2002) A CLA Mixture Prevents Body Triglyceride Accumulation Without Affecting Energy Expenditure in Syrian Hamsters, J. Nutr. 132, 2682–2689.
Frøyland, L., Madsen, L., Eckhoff, K.M., Lie, Ø., and Berge, R.K. (1998) Carnitine Palmitoyltransferase I, Carnitine Palmitoyltransferase II, and Acyl-CoA Oxidase Activities in Atlantic Salmon (Salmo salar), Lipids 33, 923–930.
Rasmussen, B.B., and Wolfe, R.R. (1999) Regulation of Fatty Acid Oxidation in Skeletal Muscle, Annu. Rev. Nutr. 19, 463–484.
Eaton, S., Bartlett, K., and Pourfarzam, M. (1996) Mammalian Mitochondrial β-Oxidation, Biochem. J. 320, 345–357.
Gulick, T., Cresci, S., Caira, T., Moore, D.D., and Kelly, D.P. (1994) The Peroxisome Proliferators-Activated Receptor Regulates Mitochondrial Fatty Acid Oxidative Enzyme Gene Expression. Proc. Natl. Acad. Sci. USA 91, 11012–11016.
Gregoire, F.M., Smas, C.M., and Sul, H.S. (1998) Understanding Adipocyte Differentiation, Physiol. Rev. 78, 783–809.
Boukouvala, E., Antonopoulou, E., Favre-Krey, L., Diez, A., Bautista, J.M., Leaver, M.J., Tocher, D.R., and Krey, G. (2004) Molecular Characterization of Three Peroxisome Proliferator-Activated Receptors from the Sea Bass (Dicentrarchus labrax), Lipids 39, 1085–1092.
Leaver, M.J., Boukouvala, E., Antonopoulou, E., Diez, A., Favre-Krey, L., Ezaz, M.T., Bautista, J.M., Tocher, D.R., and Krey, G. (2005) Three Peroxisomal Proliferator-Activated Receptor (PPAR) Isotypes from Each of Two Species of Marine Fish, Endocrinology 146, 3150–3162.
Brown, J.M., Boysen, M.S., Jensen, S.S., Morrison, R.F., Storkson, J., Lea-Currie, R., Pariza, M., Mandrup, S., and McIntosh, M.K. (2003) Isomer-Specific Regulation of Metabolism and PPARγ Signaling by CLA in Human Preadipocytes, J. Lipid Res. 44, 1287–1300.
Granlund, L., Juvet, L.K., Pedersen, J.I., and Nebb, H.I. (2003) Trans10, cis12-Conjugated Linoleic Acid Prevents Triacylglycerol Accumulation in Adipocytes by Acting as a PPARγ Modulator, J. Lipid Res. 44, 1441–1452.
Clement, L., Poirier, H., Niot, I., Bocher, V., Guerre-Millo, M., Krief, S., Staels, B., and Besnard, P. (2002) Dietary trans-10, cis-12 Conjugated Linoleic Acid Induces Hyperinsulinemia and Fatty Liver in the Mouse, J. Lipid Res. 43, 1400–1409.
Zhou, X., Sun, C., Jiang, L., and Wang, H. (2004) Effect of Conjugated Linoleic Acid on PPAR Gamma Gene Expression and Serum Leptin in Obese Rat, Wei Sheng Yan Jiu 33, 307–309.
Warren, J.M., Simon, V.A., Bartolini, G., Erickson, K.L., Mackey, B.E., and Kelley, D.S. (2003) Trans-10,cis-12 CLA Increases Liver and Decreases Adipose Tissue Lipids in Mice: Possible Roles of Specific Lipid Metabolism Genes, Lipids 38, 497–504.
Macarulla, M.T., Fernandez-Quintela, A., Zabala, A., Navarro, V., Echevarria, E., Churruca, I., Rodriguez, V.M., and Portillo, M.P. (2005) Effects of Conjugated Linoleic Acid on Liver Composition and Fatty Acid Oxidation Are Isomer-Dependent in Hamster, Nutrition 21, 512–519.
Peters, J.M., Park, Y., Gonzalez, F.J., and Pariza, M.W. (2001) Influence of Conjugated Linoleic Acid on Body Composition and Target Gene Expression in Peroxisome-Proliferator-Activated Receptor α-Null Mice, Biochim. Biophys. Acta 1533, 233–241.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kennedy, S.R., Leaver, M.J., Campbell, P.J. et al. Influence of dietary oil content and conjugated linoleic acid (CLA) on lipid metabolism enzyme activities and gene expression in tissues of atlantic salmon (Salmo salar L.). Lipids 41, 423–436 (2006). https://doi.org/10.1007/s11745-006-5116-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-006-5116-4