Abstract
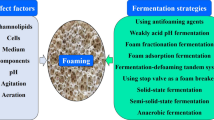
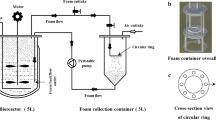
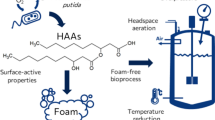
Although the biosurfactant rhamnolipid has been previously characterized as having low foam ability, its fermentation is largely impeded by severe foaming. Hence, the investigation of this paradox is critically important for improving the mass production of rhamnolipid. Unexpectedly, the hydrophobic cell, instead of rhamnolipid, has been claimed to explain such severe foaming in rhamnolipid fermentation. This study tried to systematically investigate the severe foaming in fermentation, aiming to propose an effective strategy for foam control. The overflowing foam sustained a super high stability in terms of half-time for over 30 min. The major product of rhamnolipid largely contributed to the severe foaming in the fermentation process whereas other products like cells elicited much more limited effects. Furthermore, the foam stability of the fermentation broth increased with rhamnolipid concentration and noticeably increased with agitation speed. In the classic Bikerman foam test system without stirring, rhamnolipid showed foam stability as low as Tween 20 which is well known for its poor foam stability. However, in a stirring Bikerman system, rhamnolipid exhibited a foam stability almost as high as sodium dodecyl sulfate (SDS) at 10 g/L and even surpassed SDS at a higher concentration of 20 g/L. Hence, the extraordinarily increased foam stability of rhamnolipid with both agitation and concentration could explain the severe foaming at its late-stage fermentation when rhamnolipid-rich solution is mechanically agitated.






Similar content being viewed by others
References
Jadhav M, Kalme S, Tamboli D, Govindwar S (2011) Rhamnolipid from Pseudomonas desmolyticum NCIM-2112 and its role in the degradation of Brown 3REL. J Basic Microbiol 51:385–396
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87:167–174
Reis RS, Pereira AG, Neves BC, Freire DM (2011) Gene regulation of rhamnolipid production in Pseudomonas aeruginosa—a review. Bioresour Technol 102:6377–6384
Sha R, Jiang L, Meng Q, Zhang G, Song Z (2012) Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J Basic Microbiol 52:458–466
Long X, Zhang G, Han L, Meng Q (2013) Dewatering of floated oily sludge by treatment with rhamnolipid. Water Res 47:4303–4311
Long X, Zhang G, Shen C, Sun G, Wang R, Yin L, Meng Q (2013) Application of rhamnolipid as a novel biodemulsifier for destabilizing waste crude oil. Bioresour Technol 131:1–5
Lang S, Wullbrandt D (1999) Rhamnose lipids–biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Mukherjee S, Das P, Sen R (2006) Towards commercial production of microbial surfactants. Trends Biotechnol 24:509–515
Chen S-Y, Wei YH, Chang JS (2007) Repeated pH-stat fed-batch fermentation for rhamnolipid production with indigenous Pseudomonas aeruginosa S2. Appl Microbiol Biotechnol 76:67–74
Timmis KN, McGenity T, Van Der Meer J, De Lorenzo V (2010) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin Heidelberg
Pansiripat S, Pornsunthorntawee O, Rujiravanit R, Kitiyanan B, Somboonthanate P, Chavadej S (2010) Biosurfactant production by Pseudomonas aeruginosa SP4 using sequencing batch reactors: effect of oil-to-glucose ratio. Biochem Eng J 49:185–191
Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol 99:1157–1164
Zhao F, Shi R, Zhao J, Li G, Bai X, Han S, Zhang Y (2015) Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J Appl Microbiol 118:379–389
Neto DC, Meira JA, de Araújo JM, Mitchell DA, Krieger N (2008) Optimization of the production of rhamnolipids by Pseudomonas aeruginosa UFPEDA 614 in solid-state culture. Appl Microbiol Biotechnol 81:441–448
Camilios-Neto D, Bugay C, de Santana-Filho AP, Joslin T, de Souza LM, Sassaki GL, Mitchell DA, Krieger N (2011) Production of rhamnolipids in solid-state cultivation using a mixture of sugarcane bagasse and corn bran supplemented with glycerol and soybean oil. Appl Microbiol Biotechnol 89:1395–1403
Zhu L, Yang X, Xue C, Chen Y, Qu L, Lu W (2012) Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Bioresour Technol 117:208–213
Sodagari M, Ju LK (2014) Cells were a more important foaming factor than free rhamnolipids in fermentation of Pseudomonas aeruginosa E03-40 for high rhamnolipid production. J Surfactants Deterg 17:573–582
Salwa M, Asshifa MN, Amirul A, Yahya AR (2009) Different feeding strategy for the production of biosurfactant from Pseudomonas aeruginosa USM AR2 in modified bioreactor. Biotechnol Bioprocess Eng 14:763–768
Zhang Q, Ju LK (2011) Rhamnolipids as affinity foaming agent for selective collection of β-glucosidase from cellulase enzyme mixture. Enzyme Microb Technol 48:175–180
Hori K, Marsudi S, Unno H (2002) Simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Biotechnol Bioeng 78:699–707
Chayabutra C, Wu J, Ju LK (2001) Rhamnolipid production by Pseudomonas aeruginosa under denitrification: effects of limiting nutrients and carbon substrates. Biotechnol Bioeng 72:25–33
Wu J, Ju LK (1998) Extracellular particles of polymeric material formed in n-hexadecane fermentation by Pseudomonas aeruginosa. J Biotechnol 59:193–202
Urum K, Pekdemir T, Ross D, Grigson S (2005) Crude oil contaminated soil washing in air sparging assisted stirred tank reactor using biosurfactants. Chemosphere 60:334–343
Urum K, Pekdemir T (2004) Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere 57:1139–1150
Tang X, Zhu Y, Meng Q (2007) Enhanced crude oil biodegradability of Pseudomonas aeruginosa ZJU after preservation in crude oil-containing medium. World J Microbiol Biotechnol 23:7–14
Long X, Meng Q, Sha R, Huang Q, Zhang G (2012) Two-step ultrafiltration of rhamnolipids using PSU-g-PEG membrane. J Membr Sci 409:105–112
Sha R, Meng Q, Jiang L (2012) The addition of ethanol as defoamer in fermentation of rhamnolipids. J Chem Technol Biotechnol 87:368–373
Zhang JC, Zhang L, Wang XC, Zhang L, Zhao S, Yu JY (2011) A surface rheological study of polyoxyethylene alkyl ether carboxylic salts and the stability of corresponding foam. J Dispers Sci Technol 32:372–379
Gong Z, Peng Y, Wang Q (2015) Rhamnolipid production, characterization and fermentation scale-up by Pseudomonas aeruginosa with plant oils. Biotechnol Lett 37:1–6
Lee BS, Kim EK (2004) Lipopeptide production from Bacillus sp. GB16 using a novel oxygenation method. Enzyme Microb Technol 35:639–647
Bikerman JJ (1965) Foams and emulsion—formation, properties, and breakdown. Ind Eng Chem 57:56–62
Murray BS, Ettelaie R (2004) Foam stability: proteins and nanoparticles. Curr Opin Colloid Interface Sci 9:314–320
Mulligan CN, Wang S (2006) Remediation of a heavy metal-contaminated soil by a rhamnolipid foam. Eng Geol 85:75–81
Gutwald S, Mersmann A (1997) Mechanical foam breaking – a physical model for impact effects with high speed rotors. Chem Eng Technol 20:76–84
Pelton R (2002) A review of antifoam mechanisms in fermentation. J Ind Microbiol Biotechnol 29:149–154
Sarachat T, Pornsunthorntawee O, Chavadej S, Rujiravanit R (2010) Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour Technol 101:324–330
Helvacı Ş, Peker S, Özdemir G (2004) Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloid Surface B 35:225–233
Cohen R, Exerowa D (2007) Surface forces and properties of foam films from rhamnolipid biosurfactants. Adv Colloid Interface Sci 134:24–34
Chen CY, Baker SC, Darton RC (2006) Continuous production of biosurfactant with foam fractionation. J Chem Technol Biotechnol 81:1915–1922
Acknowledgments
We gratefully acknowledge the financial support of this study by the National High-Technology R&D Program (863 program) of China (No. SS2014AA022104) and National Natural Science Foundation of China (No. 21406195).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Long, X., Sha, R., Meng, Q. et al. Mechanism Study on the Severe Foaming of Rhamnolipid in Fermentation. J Surfact Deterg 19, 833–840 (2016). https://doi.org/10.1007/s11743-016-1829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1829-4