Abstract
Autophagy is an evolutionarily conserved process that plays a pivotal role in the maintenance of cellular homeostasis and its impairment has been implicated in the pathogenesis of various metabolic diseases including obesity, type 2 diabetes (T2D), and metabolic dysfunction-associated steatotic liver disease (MASLD). This review synthesizes the current evidence from human studies on autophagy alterations under these metabolic conditions. In obesity, most data point to autophagy upregulation during the initiation phase of autophagosome formation, potentially in response to proinflammatory conditions in the adipose tissue. Autophagosome formation appears to be enhanced under hyperglycemic or insulin-resistant conditions in patients with T2D, possibly acting as a compensatory mechanism to eliminate damaged organelles and proteins. Other studies have proposed that prolonged hyperglycemia and disrupted insulin signaling hinder autophagic flux, resulting in the accumulation of dysfunctional cellular components that can contribute to β-cell dysfunction. Evidence from patients with MASLD supports autophagy inhibition in disease progression. Nevertheless, given the available data, it is difficult to ascertain whether autophagy is enhanced or suppressed in these conditions because the levels of autophagy markers depend on the overall metabolism of specific organs, tissues, experimental conditions, or disease duration. Owing to these constraints, determining whether the observed shifts in autophagic activity precede or result from metabolic diseases remains challenging. Additionally, autophagy-modulating strategies are shortly discussed. To conclude, more studies investigating autophagy impairment are required to gain a more comprehensive understanding of its role in the pathogenesis of obesity, T2D, and MASLD and to unveil novel therapeutic strategies for these conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing prevalence of metabolic diseases is a growing global health concern with significant implications for public health [1,2,3]. According to global World Health Organization (WHO) estimates, in 2016, 650 million adults worldwide (18 years old and older) were obese, and 340 million children and adolescents (age: 5–19) were either overweight or obese. The global prevalence of obesity nearly tripled between 1975 and 2016, and if this trend continues, even 1 billion adults may develop obesity by 2025 [4]. In line with these estimates, in May 2022, the WHO released a European Regional Obesity Report, which assessed that up to 60% of adults in the European WHO region are either overweight or obese [5]. The rising prevalence of obesity is often accompanied by other comorbidities, such as type 2 diabetes (T2D). At the end of 2021, the International Diabetes Federation accounted for approximately 500 million diabetes mellitus patients worldwide, of which over 90% were diagnosed with T2D. This number is predicted to continue growing in the following years [6]. Furthermore, even 50–70% of T2D patients, as well as 30–76% of obese and up to 90% of morbidly obese individuals, can also be affected by metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD) [7, 8], which in recent years became the most common chronic liver disease worldwide [1]. Importantly, there is a direct relationship between these three conditions, as the existence of one condition increases the risk of the other. Their coexistence is related to, among others, common risk factors (e.g., insulin resistance and metabolic syndrome), some of which may be modifiable (e.g., physical inactivity or unhealthy diet).
Unsurprisingly, the pathogenesis of obesity, T2D, and MASLD shares common mechanisms such as mitochondrial dysfunction or oxidative and endoplasmic reticulum (ER) stress, chronic inflammation, gut dysbiosis, and altered autophagy [9, 10]. Autophagy is a key evolutionarily conserved cellular process essential for degrading and recycling damaged or malfunctioning cellular components, which can be reused for biosynthetic purposes or energy generation [11]. As such, at basal levels, autophagy contributes to maintaining cellular homeostasis, survival, and various aspects of metabolic health [12]. Autophagy has been intensively studied since its discovery in 1963 [13] and is still a subject of growing interest among researchers and clinicians [14]. Its regulation by an array of signaling pathways depends on nutrient and energy availability, thus it is inherently related to the metabolism of amino acids, glucose, and lipids. Understanding these interrelations is crucial for elucidating the complex mechanisms underlying a variety of diseases, including genetically inherited metabolic disorders, such as lysosomal storage disorders, as well as non-communicable diseases (e.g., obesity, T2D, and MASLD). This review aims to provide an overview of the clinical evidence regarding the role of autophagy impairments in the development of obesity, T2D, and MASLD. Describing detailed molecular mechanisms is outside the scope of this review; however, for more details, the reader can refer to [15,16,17].
Autophagy and its regulation
Autophagy, as a process of self-degradation, plays a significant role in maintaining cellular homeostasis. It is active under basal conditions and can be further stimulated in response to cellular stress such as starvation, enabling the cell to mobilize essential energy sources through the degradation of glycogen (storage of glucose), lipid droplets (storage of triacylglycerols), or other intracellular structures [18]. The physiological regulation of autophagy has been elegantly reviewed in a paper by Rabinowitz and White so the reader can refer to this work for more information [11]. Autophagy also serves as a protective mechanism by eliminating damaged organelles or misfolded proteins that can exert cytotoxic effects [16, 19].
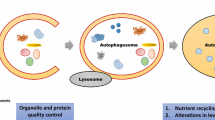
In mammalian cells, three types of autophagy can be identified: microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA) [20]. Despite morphological differences, all these processes culminate in delivering cargo to the lysosome for degradation and recycling. Microautophagy relies on the invagination or protrusions of the lysosomal membrane to capture cargo. CMA utilizes chaperones to identify the proteins targeted for degradation. Meanwhile, macroautophagy, to which in this review, we refer simply as "autophagy", involves isolation of the cargo by forming a double-membraned structure called an autophagosome, which subsequently fuses with a lysosome and its content undergoes degradation [18]. Furthermore, autophagy can be either non-selective or selective [16]. Non-selective macroautophagy plays a crucial role during starvation, involving the random engulfment of cytoplasmic fragments into autophagosomes. Subsequently, lysosome fusion with the autophagosome provides luminal acid hydrolases that degrade captured proteins, lipids, carbohydrates, nucleic acids, and organelles. Such degraded material supplies nutrients that are then secreted back into the cytoplasm by lysosomal permeases to provide the cell with essential energy sources under conditions of cellular stress [21]. Conversely, selective macroautophagy specifically recognizes and degrades a defined cargo, such as protein aggregates (aggrephagy), organelles (e.g., mitophagy, lysophagy, ER-phagy, ribophagy, lipophagy, pexophagy), and pathogens (xenophagy) [16]. Hence, selective autophagy plays a significant role in cellular quality control. Despite the common mechanisms involved in organelle removal, the degradation signals and molecules used in selective autophagy are diverse and specific to each organelle. A comprehensive understanding of these pathways is crucial because defects in the selective autophagy of various organelles have been associated with conditions such as metabolic disorders, neurodegeneration, and aging [16]. Detailed knowledge of how cargos are recognized is necessary for developing specific therapies that precisely target individual stages of autophagy in clinical practice [22].
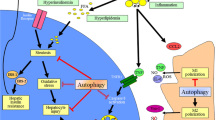
In most cases, autophagy consists of five steps: initiation, nucleation of the autophagosome, expansion, and elongation of the autophagosome membrane, closure and fusion with the lysosome, as well as degradation of intravesicular products [23]. Autophagy is controlled by over 30 autophagy-related proteins encoded by autophagy-related genes (ATGs), originally identified in the yeast Saccharomyces cerevisiae [21]. A crucial regulator of autophagy activation is the mammalian target of rapamycin (mTOR) kinase, which acts as the central component of two functionally and structurally distinct complexes: mTORC1 and mTORC2. mTORC1 activity is inhibited in response to amino acid starvation, growth factor deprivation, decreased ATP or oxygen levels, and enhanced reactive oxygen species (ROS) production. As such, mTORC1 inhibition promotes the initiation of autophagy [24]. The role of mTORC2 in autophagy is not well understood; however, studies suggest that this complex may promote both the activation [25, 26] and inhibition of autophagy [27,28,29]. The process from autophagy initiation to cargo degradation is illustrated in Fig. 1.
The primary mechanism initiating autophagy involves activation of the Unc-51-like kinase (ULK) complex, consisting of ULK1/ATG1, ATG13, FIP200, and ATG101. The key regulators of autophagy initiation are the mTORC1 complex and AMP-activated protein kinase (AMPK), which act in opposition to each other; however, both control autophagy through ULK1 phosphorylation. AMPK, the primary sensor of cellular energy state, is activated when intracellular AMP levels rise (indicating starvation) and then promotes autophagy by directly activating ULK1 through the phosphorylation of Ser317 and Ser777. Under conditions of nutrient sufficiency, mTORC1 prevents ULK1 activation by phosphorylating Ser757 on ULK1 and disrupting the interaction between ULK1 and AMPK [30, 31]. The ULK1 complex further activates the BECN1-VPS34-ATG14L-p150 complex through the phosphorylation of Beclin 1 (BECN1). Activation of the BECN1 complex leads to the generation of phosphatidylinositol-3-phosphate (PI3P), which is crucial for the nucleation of autophagic vesicles by promoting membrane elongation through the recruitment of the ATG2-WIPI (WD‐repeat protein interacting with phosphoinositides) protein complex. The elongation and maturation of autophagosomes involve two conjugation systems similar to the ubiquitination system: the microtubule-associated protein 1 light chain 3 (LC3/ATG8) system and the ATG12 system [32]. LC3 is modified by ATG4, resulting in LC3-I with an exposed glycine residue at the C-terminus. This allows the conjugation of LC3-I with ATG7 (an E1-like enzyme) and then with ATG3 (an E2-like enzyme) [23]. ATG3-LC3 is recognized by the ATG5-ATG12 complex associated with the ATG16L protein (ATG16L complex), which catalyzes the conjugation of LC3 with phosphatidylethanolamine (PE), forming insoluble LC3-II that is stably incorporated into the autophagosomal membrane [33, 34]. Interestingly, cargo selection for the autophagy process can be facilitated by adaptor proteins, such as p62/SQSTM1, which possess a ubiquitin-binding domain and an LC3-II interacting domain. The fusion of autophagosomes and lysosomes is regulated by several molecules including soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and lysosome-associated membrane proteins (LAMPs) [21, 32]. Finally, in the last step of autophagy, the encapsulated cargo is degraded by lysosomal proteases, and the products are released back into the cytosol through lysosomal permeases [32]
Autophagy in metabolic diseases
The pathological mechanisms that drive the development of obesity, T2D, and MASLD include a combination of predisposing genetic background and accompanying environmental and behavioral factors, such as excessive caloric intake and lack of physical activity [35]. Despite their impact on, among others, systemic hormonal and metabolic regulation, these factors are also known to either enhance or suppress autophagy. Autophagy alterations can further affect both adipogenesis and metabolism at the tissue level, potentially manifesting observable effects at the systemic level [36]. Considering the complex and multifactorial pathogenesis of metabolic diseases, in this review, we discuss autophagy alterations in obese patients in the context of T2D and MASLD, as many obese patients commonly suffer from these comorbidities [37].
Obesity
The first observations of changes in autophagy in obese patients were reported in 2010 by Öst et al., who with the use of transmission electron microscopy (TEM) reported an increased number of autophagosomes in adipocytes obtained from obese patients with T2D [38]. This increase occurred together with enhanced levels and turnover of the microtubule-associated protein 1 light chain 3 (LC3) protein, one of the main autophagy markers, as demonstrated in the presence and absence of an autophagy inducer (rapamycin) and inhibitor of lysosomal degradation (chloroquine). The reduced activity of mTORC1 further supported these findings. Furthermore, the observed stimulation of autophagy was associated with the fragmentation of large lipid droplets. In the studied cohort, the average BMI of patients with T2D was approximately 40, pointing to obesity class II/III; however, overweight subjects (BMI ≥ 27) were also included. Patients without T2D included in this study as controls were overweight on average [38]. However, Kovsan et al. showed that autophagophore formation was upregulated in adipose tissue (AT) obtained from obese patients, regardless of their glycemic status. This effect was particularly pronounced in the omental compared to the subcutaneous fat depot, as observed at both mRNA and protein levels (Table 1). This upregulation was positively correlated with the degree of obesity, visceral fat distribution, and adipocyte hypertrophy [39]. In the following years, more studies reported similar findings of upregulation of autophagy markers in AT from patients with obesity and obesity-related T2D, compared to lean controls without T2D [40, 41]. Strong positive correlations between the expression levels of autophagy-related genes and glucometabolic status [42], or higher transcript levels of autophagic genes in the AT of patients with obesity and T2D compared to those with normal glycemia, have also been reported [43]. The increase in autophagosome formation was also shown to be more pronounced in the visceral AT than in the subcutaneous AT [38, 39]. In contrast to patients with obesity and T2D, LC3 was not detected in subcutaneous and visceral AT samples from lean individuals without T2D [41]. Accordingly, as shown in another study, an initially increased number of autophagosomes in the subcutaneous AT of obese patients with or without T2D was undetectable 1 year after they were subjected to bariatric surgery [40]. It has been suggested that stimulated autophagy in AT of individuals with obesity probably plays a role in the modulation of obesity-induced AT inflammation. This assumption is supported by the findings that the inhibition of autophagy significantly enhanced the transcriptional expression of proinflammatory cytokines in obese human and murine AT samples [44].
It is important to emphasize that AT biopsy samples are highly heterogeneous, owing to the combination of numerous cell types. Besides adipocytes, AT also contains the stromal-vascular fraction, which includes obesity-associated inflammatory cells whose role in autophagy is related to immune function. Therefore, it is unclear, which cell type presented an upregulated autophagy profile in some of the previous reports. However, some discrepancies may exist, even between studies performed using the same cell type. For example, in contrast with the results reported by Ost et al. [38], Soussi et al. demonstrated that autophagic flux was greatly diminished in adipocytes freshly isolated from subcutaneous AT of obese patients compared with control adipocytes [45]. Moreover, decreased autophagic flux was inversely correlated with the amount of lipids accumulated in these adipocytes. Accordingly, this outcome was partially reversed after bariatric surgery, proportionally to the reduction of adipocyte size [45]. The mechanism of autophagy attenuation was shown to be dependent on death-associated protein kinase 2 (DAPK2), reported to be one of the most downregulated genes in the AT transcriptome in human morbid obesity [46].
To summarize, it could be expected that the impairment of systemic metabolism due to excessive food intake together with insufficient energy expenditure, which are inherently associated with obesity, lead to autophagy inhibition due to enhanced mTORC1 signaling. However, the evidence provided so far points to the fact that the impact of overnutrition on autophagy is much more complex. Autophagy can be either enhanced or inhibited, depending on changes in the global metabolism of a specific type of investigated organ, or tissue, as well as the experimental conditions or duration of the disease [36]. Most reports have provided evidence that autophagy in obesity is upregulated at the initiation stage of autophagosome biogenesis, which might serve as an autophagic response to proinflammatory conditions in AT [44]. Of note, the majority of these studies observed autophagy alterations based on the changes in the mRNA levels of only several genes (mostly ATG5, ATG7, and LC3B) [47]. Only a few of these alterations were confirmed also at the protein level. The exception was LC3B, whose protein levels were evaluated by Western blotting in almost all the studies.
Type 2 diabetes
Two pathological conditions underlying T2D development, which are also associated with impaired autophagy, are insulin resistance and β-cell failure [48, 49]. Elevated glucose levels have been shown to disturb autophagy in vitro and in vivo, resulting in the aggravation of diabetes-related metabolic derangements in insulin-target tissues, such as skeletal muscle, which contribute to the worsening of insulin resistance [50, 51]. Accordingly, Møller et al. reported that the mRNA and protein levels of autophagy-related molecules were downregulated in the skeletal muscle biopsies of patients with T2D and severe insulin resistance [52]. Given that the studied patients were infused with insulin and glucose during biopsy sampling, it is difficult to conclude whether the observed effects reflect the disease state, treatment, or disease complications [52]. This limitation was overcome by Kruse et al., who showed that neither obesity nor T2D affected the expression of numerous autophagy-related genes at both mRNA and protein levels in muscle and AT biopsies, despite hyperglycemia (Table 2) [53]. Skeletal muscle biopsies were obtained under basal and insulin-stimulated states during euglycemia and hyperglycemia, and these experiments showed that physiological insulin concentrations reduced the levels of markers of autophagosome formation in an mTOR-independent manner. The impact of insulin in patients with T2D was no longer detectable under euglycemia but was restored under a hyperglycemic state. These results implied that the autophagic process may be adapted to hyperglycemic conditions in patients with T2D [53].
In 2009, Liang et al. showed that β-cells obtained from patients with T2D exhibited a major accumulation of autophagic phagosomes. The expression of genes involved in the initial phases of autophagy was not affected in diabetic islets, however, a lower expression of lysosomal genes suggested the presence of alterations at later stages, which could disrupt autophagosome removal capacity. These findings were associated with β-cell dysfunction and failure [54]. These results were in line with the observations of Mizukami et al., who reported that p62/SQSTM1 protein levels were increased in pancreases from patients with T2D (obtained post-mortem), suggesting defective autolysosomal degradation, but only if severe β-cell loss was observed [55]. Further studies have shown that the structure of pancreatic islets is maintained by the autophagy process, which contributes to the survival of pancreatic β-cells, exerting protective effects on them and insulin-target tissues [56, 57]. For example, autophagy has been shown to prevent β-cell death during hypoxia caused by a rapid metabolic rate to supply insulin production. Immunofluorescence staining of pancreatic tissue from patients with T2D revealed decreased protein expression of LC3B and p62/SQSTM1, two key autophagic markers, which may be related to chronic hypoxic conditions in diabetic islets [58]. To further support the connection between autophagy impairment and the glycemic state of patients, the levels of autophagic markers and the extent of β-cell loss were reported to be negatively correlated with the levels of HbA1c [55, 58]. Decreased autophagic flux in patients with T2D may also be related to enhanced mTOR signaling and inflammation, as reported by Alizadeh, who evaluated the association between T2D and inflammation in peripheral blood mononuclear cells (PBMCs) [59]. In contrast, elevated levels of Beclin 1 (BECN1) and LC3B, suggesting enhanced autophagosome biogenesis, were positively correlated with the extent of oxidative and ER stress markers in leukocytes obtained from T2D patients. These findings point to a potential cell-dependent divergence that can occur when studying autophagic flux among patients with T2D [60].
Diabetic complications
To date, it has been ascertained that aberrant regulation of autophagy, which occurs in patients with T2D, may be responsible for the occurrence and progression of diabetic complications (Table 2). Persistent hyperglycemia and insulin resistance trigger disruption of cellular metabolism, resulting in a decline in tissue and organ function. This further contributes to the development of diverse diabetic complications that affect the cardiovascular system, kidneys, nerves, and eyes, such as diabetic heart disease, diabetic kidney disease, diabetic nephropathy, diabetic peripheral neuropathy, and diabetic retinopathy (DR) [61, 62]. For example, patients with T2D have a higher risk of cardiovascular complications than healthy individuals. High blood glucose levels induce oxidative stress and chronic inflammation in endothelial cells, favoring endothelial injury with an augmented risk of atherosclerosis, myocardial infarction, stroke, and limb amputation [63]. Alterations in cardiac metabolism result in the inhibition of lysosomal degradation, leading to the accumulation of defective organelles in diabetic cardiomyocytes with consequent cardiac damage [64, 65]. The defective terminal phase of autophagy in endothelial cells isolated from patients with T2D was demonstrated by increased levels of p62/SQSTM1 and LAMP2A proteins. In parallel, an increased level of ATG7 protein suggested enhanced autophagosome formation; however, the level of BECN1, which is involved in the initiation of autophagosome biogenesis, was not significantly affected [66]. This report is in contrast to the findings of Munasinghe et al., who reported an increased number of autophagosomes and significantly elevated levels of LC3B-II and BECN1 proteins in diabetic cardiomyocytes. At the same time, the level of p62/SQSTM1 was reduced, implying stimulation rather than inhibition of the autophagic process [67].
Another chronic complication associated with diabetes is diabetic nephropathy, a pathological condition, in which the renal tissue is highly susceptible to irreversible damage due to prolonged high blood glucose and lipid levels, which favor microvascular injury and lead to oxidative stress, resulting in podocyte toxicity [68, 69]. Persistent hyperglycemia contributes to mTORC1 activation, which disrupts autophagosome formation in podocytes of mice and patients with T2D, resulting in exacerbated proteinuria [70]. Tagawa et al. showed that the level of p62/SQSTM1 protein was elevated in kidney biopsies from patients with diabetic nephropathy and massive proteinuria, suggesting defective lysosomal degradation [71]. The level of BECN1 in the serum of patients with diabetic kidney disease was reported to be decreased, which was inversely correlated with the severity of albuminuria, stage of nephropathy, and duration of diabetes [72]. Accordingly, lowered autophagosome biogenesis, reflected by decreased levels of ATG5 and LC3B proteins in patients with T2D, including those with nephropathy or retinopathy, has also been reported [73].
Altogether, some studies indicate that autophagosome formation is enhanced in response to metabolic stressors, such as insulin resistance and hyperglycemia, potentially serving as a compensatory mechanism to remove damaged organelles and proteins. Conversely, other studies suggest that chronic hyperglycemia and dysregulated insulin signaling impair autophagic flux, leading to the accumulation of dysfunctional cellular components and contributing to the pathogenesis of insulin resistance and β-cell dysfunction. These equivocal outcomes underscore the complex role of autophagy in metabolic dysregulation implicated in the pathogenesis of T2D.
Metabolic dysfunction-associated steatotic liver disease
The prevalence of MASLD is highly associated with the presence of both obesity and T2D. In contrast to obesity (or adipocytes, to be more specific), where the current evidence implies enhanced autophagy, data gathered from MASLD patients suggest that autophagy in the liver is mostly inhibited [36]. In 2014, Fukuo et al. demonstrated by TEM that the number of autophagic vesicles in liver samples obtained from MASLD patients was three times higher than that observed in histopathologically normal livers [74]. On the other hand, TEM analysis of liver sinusoidal endothelial cells (LSECs) from liver biopsies carried out by Hammoutene et al. revealed that autophagic vacuoles are notably smaller and less numerous in patients with metabolic dysfunction-associated steatohepatitis (MASH) compared to those with simple steatosis or no liver abnormalities, implying defects in autophagic processes (Table 3). Furthermore, in vitro mechanistic experiments performed on immortalized liver endothelial cells suggest that defective autophagy may promote the generation of proinflammatory factors, such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), leading to endothelial inflammation and cell death [75]. Additional analysis based on immunohistochemical staining performed by Fukuo et al. revealed that in 15 out of 22 MASLD patients, p62/SQSTM1 levels were significantly elevated compared to the complete absence of this protein in the liver samples of the control group [74]. Significantly decreased expression of lysosomal enzymes (cathepsin B, D, and L) in the livers of MASLD patients supports the hypothesis of impaired autophagy, as an accumulation of p62/SQSTM1 indicates dysfunctional lysosomal degradation of autophagic cargo. Moreover, p62/SQSTM1 protein levels were significantly correlated with alanine aminotransferase levels, lobular inflammation, and NAS scores (especially scores higher than 5, indicating the presence of MASH) [74]. Other authors have also observed a significant accumulation of p62/SQSTM1 protein in the livers of patients with MASLD compared to individuals with histologically normal livers, with a more pronounced increase in p62/SQSTM1 protein aggregation observed in MASH compared to simple steatosis [76, 77]. Accordingly, Wang et al. reported increased p62/SQSTM1 levels in serum and liver tissue obtained from MASH patients, as well as in liver tissue samples from patients with simple steatosis [78]. The blockade of the autophagic flux was also supported by an increased LC3-II/LC3-I ratio, where LC3-II serves as a recognition site for p62/SQSTM1 [76]. An increased LC3-II/LC3-I ratio was also observed in liver samples from MASLD patients with prediabetes, nonetheless, the level of p62/SQSTM1 protein was not affected in the studied cohort, which complicates the interpretation of these results [79]. No changes in p62/SQSTM1 levels along with an increased LC3A/B-II ratio were also reported by Lee et al., however, the evidence can be considered weak due to the extremely low sample size [80].
BECN1 is implicated in the initiation process of the formation of autophagic vesicles. According to González-Rodríguez et al., a decrease in hepatic mRNA levels of BECN1 was observed in patients with simple steatosis compared to patients with histologically normal liver or patients with MASH [76]. In line with these findings, decreased levels of BECN1 as well as other proteins, i.e., the Unc-51-like autophagy-activating kinase 1 (ULK1), p-ULKs555, ATG5, p62/SQSTM1, and BCL2 Interacting Protein 3 (BNIP3), were reported in liver samples from MASLD subjects compared to controls with healthy liver, which implies the impairment of initiation of autophagy and autophagosome formation [81]. On the other hand, González-Rodríguez et al. found no significant changes in BECN1 protein expression in liver tissue of MASLD patients, despite the aforementioned decrease in its mRNA levels [76]. Interestingly, Ezquerro et al. did not observe any changes in BECN1 transcript levels in the liver tissue of MASLD patients, regardless of their glycemic status (distinction of BECN1 levels between simple steatosis and MASH within the MASLD group was not performed) [79].
ATG5, ATG7, and LC3 proteins are involved in the maturation of the autophagosome. It has been reported that ATG5 transcript levels were not altered in patients with morbid obesity and MASLD, who exhibited different levels of insulin resistance [79]. In line with these results, both transcript and protein levels of ATG7, as well as protein levels of LC3-II and the LC3-II/I ratio, did not differ between controls and MASLD patients [81]. On the other hand, higher hepatic mRNA and protein expression of ATG7 was reported in the MASH group, especially when mild lobular inflammation was present, within the cohort of morbidly obese women. However, the differences were revealed only when comparing MASH to non-MASH groups, but not MASLD vs. non-MASLD [82]. This finding requires further investigation and confirmation since recently it has been shown that loss-of-function mutations in the ATG7 gene increased the risk of developing severe liver diseases among MASLD patients [83]. On the other hand, the gene set enrichment analysis performed by Lake et al. showed that the set of genes associated with autophagy was enriched among upregulated genes in liver biopsy samples obtained from patients with simple steatosis and MASH [84]. The authors suggest that the upregulation of the autophagy gene set may reflect an adaptation to hepatic lipotoxicity or ER-stress response [84], as also shown by González-Rodríguez et al. [76].
Most of the studies reported autophagy alterations at the protein level through the evaluation of LC3B, p62/SQSTM1, or both. Only a few studies investigated the mRNA levels of genes involved in the initiation of autophagosome formation, such as BECN1, ATG5, or ATG7. To conclude, the available evidence from patients supports the role of inhibition of the autophagic process in the development and progression of MASLD, however, more evidence regarding the stage of autophagy impairment is needed to better understand the pathophysiology of this disease.
Autophagy modulators
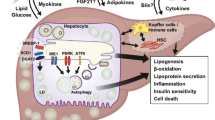
Autophagy-modulating strategies (Fig. 2) have gained significant attention as potential candidate therapies for numerous diseases including metabolic conditions such as obesity, MASLD, and T2D [14, 85, 86]. These strategies aim to restore the proper function of the autophagy process, which is critical for the maintenance of cellular homeostasis by degrading and recycling damaged organelles and proteins. Lifestyle interventions such as caloric restriction, intermittent fasting, and exercise have been shown to stimulate autophagy and improve metabolic health [87, 88]. Weight loss and exercise are known to be effective in the management of obesity, MASLD, and T2D [36, 89]. Even though controlled human studies assessing the impact of lifestyle interventions on autophagy markers among patients with obesity, MASLD or T2D are limited [87], Nunez et al. have shown that the elevated number of autophagosomes in the subcutaneous AT of patients with obesity became undetectable one year after bariatric surgery [40].
Besides lifestyle-based interventions, autophagy impairments in metabolic diseases could also be targeted with the use of pharmacological agents, for example, caloric restriction mimetics, which imitate caloric restriction-like benefits without following a dietary restriction [90, 91], AMPK activators, mTOR inhibitors or compounds restoring proper lysosomal function. Among drugs commonly used in clinical practice, metformin is a known autophagy modulator that regulates the AMPK pathway [92, 93]. According to the study by Abad-Jimenez et al., obese patients with T2D treated with metformin exhibited an improved inflammatory and redox status accompanied by the attenuation of inflammasome complex and autophagy in the visceral AT compared to metabolically healthy obese subjects. Markers of autophagy inhibition included decreased protein levels of ATG5, BECN1, and elevated levels of p62/SQSTM1 [94].
Inhibitors of sodium-glucose cotransporter 2 (SGLT2) constitute another class of drugs used to treat T2D, which apart from reducing blood glucose levels and body mass, enhance autophagy and lysosomal degradation [95]. To investigate the impact of dapagliflozin, one of the SGLT2 inhibitors, on the liver, Furuya et al. performed in vivo experiments followed by a small clinical study among hospitalized patients with T2D (n = 12) [96]. In an animal model of obesity and T2D (KK-Ay mouse strain), dapagliflozin increased the LC3-II/LC3-I ratio and the pool of several amino acids (valine, leucine, tryptophan, and tyrosine) in the liver suggesting the occurrence of enhanced proteolysis. Accordingly, plasma valine and leucine levels were significantly elevated among T2D patients, who received 5 mg of dapagliflozin per day together with other antidiabetic drugs compared to those who received antidiabetic drugs excluding dapagliflozin [96]. Also, empagliflozin, another SGLT-2 inhibitor, has demonstrated beneficial effects against hepatic steatosis under both in vitro and in vivo conditions [97]. In addition to its glucose-lowering effect, this drug alleviated hepatic steatosis through activation of the AMPK-TET2 pathway in hepatocytes, while in liver macrophages it potentiated autophagy in an AMPK/mTOR-dependent manner to attenuate the inflammatory response [97].
GLP-1 receptor agonists (GLP-1 RAs), primarily known for their efficacy in the treatment of T2D and obesity, have recently gained attention for their potential effects on autophagy [98]. One of the GLP-1 RAs, liraglutide, was found to reduce lipid accumulation in the liver by restoring autophagic flux via the AMPK/mTORC1 and transcription factor EB (TFEB)-mediated lysosomal autophagic pathways in both in vitro and in vivo experiments [99,100,101]. TFEB is recognized as a central transcriptional regulator of autophagy, a master regulator of lysosomal biogenesis, and a key regulator of lipid metabolism [102], which makes it a promising target for therapeutic intervention in metabolic diseases. Exenatide is another GLP-1 RA that has been studied for its autophagy-modulating effects. Shao et al. reported that exenatide targets the NLRP3 inflammasome via the autophagy/mitophagy pathway, delaying the progression of MASLD in mice [103]. Nevertheless, these findings are limited solely to in vitro and in vivo animal experiments.
Additionally, natural compounds like resveratrol and spermidine have demonstrated their potential in vivo in modulating autophagy and improving metabolic outcomes [104,105,106]. However, while resveratrol has shown potential metabolic benefits in in vitro and animal studies, evidence from human clinical trials remains limited and inconclusive. Most human studies have demonstrated only modest metabolic improvements, so many questions about its bioavailability, optimal dosing, and overall efficacy remain unanswered [107, 108]. Concerning evidence of autophagy modulation in humans, a 30-day supplementation with resveratrol (150 mg per day) was reported to induce TFEB expression in the subcutaneous AT of healthy obese men [109]. Another autophagy inducer, spermidine, has shown promising effects on metabolic health, including reducing weight gain, obesity-related complications, and improving insulin resistance in both humans and mice [110]. In particular, studies have demonstrated that spermidine intake negatively correlates with obesity-associated parameters and enhances gut barrier function, indicating its potential as a therapy for obesity and its complications [111]. So far, clinical evidence that spermidine supplementation may have beneficial effects through the regulation of autophagy in patients with obesity, T2D, and MASLD is lacking [112].
Therapeutic approaches targeting autophagy may hold promise for mitigating the progression of metabolic diseases and improving overall health. Nevertheless, further research is needed to fully understand and validate their potential for enhancing metabolic health in clinical settings through autophagy modulation.
Conclusions
Autophagy is a fundamental cellular process that plays a pivotal role in the maintenance of metabolic homeostasis. In metabolic diseases such as obesity, T2D, and MASLD, dysregulation of autophagy has emerged as a key contributing factor. Autophagy regulates lipid metabolism, insulin sensitivity, and glucose homeostasis, affecting the development and progression of these metabolic disorders. In this review, we gathered evidence of autophagy impairments from human studies, which clearly shows that autophagy alterations may differ depending on the type of tissue or cells. Due to methodological limitations, it is difficult to conclude whether the observed changes in autophagic flux are a cause or a consequence of the metabolic diseases we have discussed. Understanding the intricate interplay between autophagy and different metabolic pathways is crucial for developing effective therapies for metabolic diseases, nevertheless, further research is needed to identify optimal targets for potential interventions.
Data availability
Not applicable.
References
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA (2022) The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 7:851–861
Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PALu, Ma Y, Mainoo J, Mensah NK, Merriman GA, Mokdad TR, Moschandreas AH, Naghavi J, Naheed M, Nand A, Narayan D, Nelson KM, Neuhouser EL, Nisar ML, Ohkubo MI, Oti T, Pedroza SO et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781
Collaborators GBDD (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402:203–234
Boutari C, Mantzoros CS (2022) A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 133:155217
Organization, W. H. (2022) WHO European Regional Obesity Report 2022
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, Rhee EJ (2019) Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J 43:31–45
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64:73–84
Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184:2537–2564
Burgos-Moron E, Abad-Jimenez Z, Maranon AM, Iannantuoni F, Escribano-Lopez I, Lopez-Domenech S, Salom C, Jover A, Mora V, Roldan I, Sola E, Rocha M, Victor VM (2019) Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med 8:1385
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348
Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cadwell K, Cecconi F, Choi AMK, Choi ME, Chu CT, Codogno P, Colombo MI, Cuervo AM, Deretic V, Dikic I, Elazar Z, Eskelinen EL, Fimia GM, Gewirtz DA, Green DR, Hansen M, Jaattela M, Johansen T, Juhasz G, Karantza V, Kraft C, Kroemer G, Ktistakis NT, Kumar S, Lopez-Otin C, Macleod KF, Madeo F, Martinez J, Melendez A, Mizushima N, Munz C, Penninger JM, Perera RM, Piacentini M, Reggiori F, Rubinsztein DC, Ryan KM, Sadoshima J, Santambrogio L, Scorrano L, Simon HU, Simon AK, Simonsen A, Stolz A, Tavernarakis N, Tooze SA, Yoshimori T, Yuan J, Yue Z, Zhong Q, Galluzzi L, Pietrocola F (2021) Autophagy in major human diseases. EMBO J 40:e108863
Ohsumi Y (2014) Historical landmarks of autophagy research. Cell Res 24:9–23
Kitada M, Koya D (2021) Autophagy in metabolic disease and ageing. Nat Rev Endocrinol 17:647–661
Yorimitsu T, Klionsky DJ (2005) Autophagy: molecular machinery for self-eating. Cell Death Differ 12(Suppl 2):1542–1552
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12
Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: cellular and molecular mechanisms. Autophagy 14:207–215
Parzych KR, Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20:460–473
Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: mechanism, regulation and research progress. Bull Cancer 108:304–322
Tabibzadeh S (2023) Role of autophagy in aging: the good, the bad, and the ugly. Aging Cell 22:e13753
Moulis M, Vindis C (2018) Autophagy in metabolic age-related human diseases. Cells 7:149
Anding AL, Baehrecke EH (2017) Cleaning house: selective autophagy of organelles. Dev Cell 41:10–22
Mulcahy Levy JM, Thorburn A (2020) Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ 27:843–857
Zhang J (2015) Teaching the basics of autophagy and mitophagy to redox biologists–mechanisms and experimental approaches. Redox Biol 4:242–259
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11:859–871
Cybulski N, Hall MN (2009) TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34:620–627
Sun Y, Wang H, Qu T, Luo J, An P, Ren F, Luo Y, Li Y (2023) mTORC2: a multifaceted regulator of autophagy. Cell Commun Signal 21:4
Zhou B, Kreuzer J, Kumsta C, Wu L, Kamer KJ, Cedillo L, Zhang Y, Li S, Kacergis MC, Webster CM, Fejes-Toth G, Naray-Fejes-Toth A, Das S, Hansen M, Haas W, Soukas AA (2019) Mitochondrial permeability uncouples elevated autophagy and lifespan extension. Cell 177(299–314):e16
Aspernig H, Heimbucher T, Qi W, Gangurde D, Curic S, Yan Y, Donner von Gromoff E, Baumeister R, Thien A (2019) Mitochondrial perturbations couple mTORC2 to autophagy in C. elegans. Cell Rep 29:1399-1409.e5
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Holczer M, Hajdu B, Lorincz T, Szarka A, Banhegyi G, Kapuy O (2020) Fine-tuning of AMPK-ULK1-mTORC1 regulatory triangle is crucial for autophagy oscillation. Sci Rep 10:17803
Sun K, Deng W, Zhang S, Cai N, Jiao S, Song J, Wei L (2013) Paradoxical roles of autophagy in different stages of tumorigenesis: protector for normal or cancer cells. Cell Biosci 3:35
Noda T, Fujita N, Yoshimori T (2009) The late stages of autophagy: how does the end begin? Cell Death Differ 16:984–990
Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell 55:238–252
Rojano A, Sena E, Manzano-Nunez R, Pericas JM, Ciudin A (2023) NAFLD as the metabolic hallmark of obesity. Intern Emerg Med 18:31–41
Zhang Y, Sowers JR, Ren J (2018) Targeting autophagy in obesity: from pathophysiology to management. Nat Rev Endocrinol 14:356–376
Powell EE, Wong VW, Rinella M (2021) Non-alcoholic fatty liver disease. Lancet 397:2212–2224
Ost A, Svensson K, Ruishalme I, Brannmark C, Franck N, Krook H, Sandstrom P, Kjolhede P, Stralfors P (2010) Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med 16:235–246
Kovsan J, Bluher M, Tarnovscki T, Kloting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schon MR, Greenberg AS, Elazar Z, Bashan N, Rudich A (2011) Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab 96:E268–E277
Nunez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, Chaim EA, Pareja JC, Geloneze B, Velloso LA, Araujo EP (2013) Defective regulation of adipose tissue autophagy in obesity. Int J Obes 37:1473–1480
Kosacka J, Kern M, Kloting N, Paeschke S, Rudich A, Haim Y, Gericke M, Serke H, Stumvoll M, Bechmann I, Nowicki M, Bluher M (2015) Autophagy in adipose tissue of patients with obesity and type 2 diabetes. Mol Cell Endocrinol 409:21–32
Xu Q, Mariman ECM, Roumans NJT, Vink RG, Goossens GH, Blaak EE, Jocken JWE (2018) Adipose tissue autophagy related gene expression is associated with glucometabolic status in human obesity. Adipocyte 7:12–19
Rodriguez A, Gomez-Ambrosi J, Catalan V, Rotellar F, Valenti V, Silva C, Mugueta C, Pulido MR, Vazquez R, Salvador J, Malagon MM, Colina I, Fruhbeck G (2012) The ghrelin O-acyltransferase-ghrelin system reduces TNF-alpha-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 55:3038–3050
Jansen HJ, van Essen P, Koenen T, Joosten LA, Netea MG, Tack CJ, Stienstra R (2012) Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology 153:5866–5874
Soussi H, Reggio S, Alili R, Prado C, Mutel S, Pini M, Rouault C, Clement K, Dugail I (2015) DAPK2 downregulation associates with attenuated adipocyte autophagic clearance in human obesity. Diabetes 64:3452–3463
Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, Poitou C, Basdevant A, Stich V, Viguerie N, Langin D, Bedossa P, Zucker JD, Clement K (2008) Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9:R14
Haim Y, Bluher M, Slutsky N, Goldstein N, Kloting N, Harman-Boehm I, Kirshtein B, Ginsberg D, Gericke M, Guiu Jurado E, Kovsan J, Tarnovscki T, Kachko L, Bashan N, Gepner Y, Shai I, Rudich A (2015) Elevated autophagy gene expression in adipose tissue of obese humans: a potential non-cell-cycle-dependent function of E2F1. Autophagy 11:2074–2088
Cheng AYY, Gomes MB, Kalra S, Kengne AP, Mathieu C, Shaw JE (2023) Applying the WHO global targets for diabetes mellitus. Nat Rev Endocrinol 19:194–200
Doria A, Gatto M, Punzi L (2013) Autophagy in human health and disease. N Engl J Med 368:1845
Barutta F, Bellini S, Kimura S, Hase K, Corbetta B, Corbelli A, Fiordaliso F, Bruno S, Biancone L, Barreca A, Papotti MG, Hirsh E, Martini M, Gambino R, Durazzo M, Ohno H, Gruden G (2023) Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy. Autophagy 19:505–524
Barlow AD, Thomas DC (2015) Autophagy in diabetes: beta-cell dysfunction, insulin resistance, and complications. DNA Cell Biol 34:252–260
Moller AB, Kampmann U, Hedegaard J, Thorsen K, Nordentoft I, Vendelbo MH, Moller N, Jessen N (2017) Altered gene expression and repressed markers of autophagy in skeletal muscle of insulin resistant patients with type 2 diabetes. Sci Rep 7:43775
Kruse R, Vind BF, Petersson SJ, Kristensen JM, Hojlund K (2015) Markers of autophagy are adapted to hyperglycaemia in skeletal muscle in type 2 diabetes. Diabetologia 58:2087–2095
Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, Marselli L, Masiello P, Marchetti P (2009) Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia 52:1083–1086
Mizukami H, Takahashi K, Inaba W, Tsuboi K, Osonoi S, Yoshida T, Yagihashi S (2014) Involvement of oxidative stress-induced DNA damage, endoplasmic reticulum stress, and autophagy deficits in the decline of beta-cell mass in Japanese type 2 diabetic patients. Diabetes Care 37:1966–1974
Riahi Y, Wikstrom JD, Bachar-Wikstrom E, Polin N, Zucker H, Lee MS, Quan W, Haataja L, Liu M, Arvan P, Cerasi E, Leibowitz G (2016) Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia 59:1480–1491
Lee MS (2014) Role of islet beta cell autophagy in the pathogenesis of diabetes. Trends Endocrinol Metab 25:620–627
Liang R, Liu N, Cao J, Liu T, Sun P, Cai X, Zhang L, Liu Y, Zou J, Wang L, Ding X, Zhang B, Shen Z, Yoshida S, Dou J, Wang S (2022) HIF-1alpha/FOXO1 axis regulated autophagy is protective for beta cell survival under hypoxia in human islets. Biochim Biophys Acta Mol Basis Dis 1868:166356
Alizadeh S, Mazloom H, Sadeghi A, Emamgholipour S, Golestani A, Noorbakhsh F, Khoshniatnikoo M, Meshkani R (2018) Evidence for the link between defective autophagy and inflammation in peripheral blood mononuclear cells of type 2 diabetic patients. J Physiol Biochem 74:369–379
Rovira-Llopis S, Diaz-Morales N, Banuls C, Blas-Garcia A, Polo M, Lopez-Domenech S, Jover A, Rocha M, Hernandez-Mijares A, Victor VM (2015) Is autophagy altered in the leukocytes of type 2 diabetic patients? Antioxid Redox Signal 23:1050–1056
Cole JB, Florez JC (2020) Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol 16:377–390
Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ (2022) Type 2 diabetes. Lancet 400:1803–1820
Muriach M, Flores-Bellver M, Romero FJ, Barcia JM (2014) Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev 2014:102158
Kanamori H, Naruse G, Yoshida A, Minatoguchi S, Watanabe T, Kawaguchi T, Tanaka T, Yamada Y, Takasugi H, Mikami A, Minatoguchi S, Miyazaki T, Okura H (2021) Morphological characteristics in diabetic cardiomyopathy associated with autophagy. J Cardiol 77:30–40
Jia G, Hill MA, Sowers JR (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122:624–638
Fetterman JL, Holbrook M, Flint N, Feng B, Breton-Romero R, Linder EA, Berk BD, Duess MA, Farb MG, Gokce N, Shirihai OS, Hamburg NM, Vita JA (2016) Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 247:207–217
Munasinghe PE, Riu F, Dixit P, Edamatsu M, Saxena P, Hamer NS, Galvin IF, Bunton RW, Lequeux S, Jones G, Lamberts RR, Emanueli C, Madeddu P, Katare R (2016) Type-2 diabetes increases autophagy in the human heart through promotion of Beclin-1 mediated pathway. Int J Cardiol 202:13–20
Atkins RC, Zimmet PZ, International Society of, N., International Federation of Kidney Foundations World Kidney Day Steering, C., International Diabetes, F. (2010) Diabetic kidney disease: act now or pay later. Med J Aust 192:272–274
Rico-Fontalvo J, Aroca G, Cabrales J, Daza-Arnedo R, Yanez-Rodriguez T, Martinez-Avila MC, Uparella-Gulfo I, Raad-Sarabia M (2022) Molecular mechanisms of diabetic kidney disease. Int J Mol Sci. 23
Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mor A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstadt H, Kerjaschki D, Cohen CD, Hall MN, Ruegg MA, Inoki K, Walz G, Huber TB (2011) Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Investig 121:2197–2209
Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, Kim EH, Haneda M, Kajiwara N, Hayashi K, Ohashi H, Ugi S, Maegawa H, Uzu T (2016) Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes 65:755–767
Naguib M, Rashed LA (2018) Serum level of the autophagy biomarker Beclin-1 in patients with diabetic kidney disease. Diabetes Res Clin Pract 143:56–61
Yassin R, Tadmor H, Farber E, Igbariye A, Armaly-Nakhoul A, Dahan I, Nakhoul F, Nakhoul N (2021) Alteration of autophagy-related protein 5 (ATG5) levels and Atg5 gene expression in diabetes mellitus with and without complications. Diab Vasc Dis Res 18:14791641211062050
Fukuo Y, Yamashina S, Sonoue H, Arakawa A, Nakadera E, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S (2014) Abnormality of autophagic function and cathepsin expression in the liver from patients with non-alcoholic fatty liver disease. Hepatol Res 44:1026–1036
Hammoutene A, Biquard L, Lasselin J, Kheloufi M, Tanguy M, Vion AC, Merian J, Colnot N, Loyer X, Tedgui A, Codogno P, Lotersztajn S, Paradis V, Boulanger CM, Rautou PE (2020) A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J Hepatol 72:528–538
Gonzalez-Rodriguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillon J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntane J, Bosca L, Garcia-Monzon C, Martin-Sanz P, Valverde AM (2014) Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD. Cell Death Dis 5:e1179
Fukushima H, Yamashina S, Arakawa A, Taniguchi G, Aoyama T, Uchiyama A, Kon K, Ikejima K, Watanabe S (2018) Formation of p62-positive inclusion body is associated with macrophage polarization in non-alcoholic fatty liver disease. Hepatol Res 48:757–767
Wang X, Zhang X, Chu ESH, Chen X, Kang W, Wu F, To KF, Wong VWS, Chan HLY, Chan MTV, Sung JJY, Wu WKK, Yu J (2018) Defective lysosomal clearance of autophagosomes and its clinical implications in nonalcoholic steatohepatitis. FASEB J 32:37–51
Ezquerro S, Mocha F, Fruhbeck G, Guzman-Ruiz R, Valenti V, Mugueta C, Becerril S, Catalan V, Gomez-Ambrosi J, Silva C, Salvador J, Colina I, Malagon MM, Rodriguez A (2019) Ghrelin reduces TNF-alpha-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab 104:21–37
Lee S, Kim S, Hwang S, Cherrington NJ, Ryu DY (2017) Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget 8:63370–63381
Moore MP, Cunningham RP, Meers GM, Johnson SA, Wheeler AA, Ganga RR, Spencer NM, Pitt JB, Diaz-Arias A, Swi AIA, Hammoud GM, Ibdah JA, Parks EJ, Rector RS (2022) Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology 76:1452–1465
Barrientos-Riosalido A, Real M, Bertran L, Aguilar C, Martinez S, Parada D, Vives M, Sabench F, Riesco D, Castillo DD, Richart C, Auguet T (2023) Increased hepatic ATG7 mRNA and ATG7 protein expression in nonalcoholic steatohepatitis associated with obesity. Int J Mol Sci 24:1324
Baselli GA, Jamialahmadi O, Pelusi S, Ciociola E, Malvestiti F, Saracino M, Santoro L, Cherubini A, Dongiovanni P, Maggioni M, Bianco C, Tavaglione F, Cespiati A, Mancina RM, D’Ambrosio R, Vaira V, Petta S, Miele L, Vespasiani-Gentilucci U, Federico A, Pihlajamaki J, Bugianesi E, Fracanzani AL, Reeves HL, Soardo G, Prati D, Romeo S, Valenti LV, Investigators ES (2022) Rare ATG7 genetic variants predispose patients to severe fatty liver disease. J Hepatol 77:596–606
Lake AD, Novak P, Hardwick RN, Flores-Keown B, Zhao F, Klimecki WT, Cherrington NJ (2014) The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicol Sci 137:26–35
Pietrocola F, Bravo-San Pedro JM (2021) Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants 10:102
Zeng J, Acin-Perez R, Assali EA, Martin A, Brownstein AJ, Petcherski A, Fernandez-Del-Rio L, Xiao R, Lo CH, Shum M, Liesa M, Han X, Shirihai OS, Grinstaff MW (2023) Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat Commun 14:2573
Bagherniya M, Butler AE, Barreto GE, Sahebkar A (2018) The effect of fasting or calorie restriction on autophagy induction: a review of the literature. Ageing Res Rev 47:183–197
Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H (2018) Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol Rep 6:e13651
Ezpeleta M, Gabel K, Cienfuegos S, Kalam F, Lin S, Pavlou V, Song Z, Haus JM, Koppe S, Alexandria SJ, Tussing-Humphreys L, Varady KA (2023) Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metab 35(56–70):e3
Marino G, Pietrocola F, Madeo F, Kroemer G (2014) Caloric restriction mimetics: natural/physiological pharmacological autophagy inducers. Autophagy 10:1879–1882
Kepp O, Chen G, Carmona-Gutierrez D, Madeo F, Kroemer G (2020) A discovery platform for the identification of caloric restriction mimetics with broad health-improving effects. Autophagy 16:188–189
Xiang M, Yuan X, Zhang N, Zhang L, Liu Y, Liu J, Gao Y, Xu Y, Sun W, Tang Q, Zhang Y, Lu J (2024) Effects of exercise, metformin, and combination treatments on type 2 diabetic mellitus-induced muscle atrophy in db/db mice: crosstalk between autophagy and the proteasome. J Physiol Biochem 80:235–247
Park JM, Lee DH, Kim DH (2023) Redefining the role of AMPK in autophagy and the energy stress response. Nat Commun 14:2994
Abad-Jimenez Z, Lopez-Domenech S, Diaz-Rua R, Iannantuoni F, Gomez-Abril SA, Perianez-Gomez D, Morillas C, Victor VM, Rocha M (2020) Systemic oxidative stress and visceral adipose tissue mediators of NLRP3 inflammasome and autophagy are reduced in obese type 2 diabetic patients treated with metformin. Antioxidants 9:892
Lopaschuk GD, Verma S (2020) Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 5:632–644
Furuya F, Fujita Y, Matsuo N, Minamino H, Oguri Y, Isomura N, Ikeda K, Takesue K, Li Y, Kondo A, Mano F, Inagaki N (2022) Liver autophagy-induced valine and leucine in plasma reflect the metabolic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin. EBioMedicine 86:104342
Li T, Fang T, Xu L, Liu X, Li X, Xue M, Yu X, Sun B, Chen L (2020) Empagliflozin alleviates hepatic steatosis by activating the AMPK-TET2-autophagy pathway in vivo and in vitro. Front Pharmacol 11:622153
Yaribeygi H, Maleki M, Santos RD, Jamialahmadi T, Sahebkar A (2024) Glp-1 mimetics and autophagy in diabetic milieu: state-of-the-art. Curr Diabetes Rev 20:93–101
He Q, Sha S, Sun L, Zhang J, Dong M (2016) GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun 476:196–203
He Y, Ao N, Yang J, Wang X, Jin S, Du J (2020) The preventive effect of liraglutide on the lipotoxic liver injury via increasing autophagy. Ann Hepatol 19:44–52
Fang Y, Ji L, Zhu C, Xiao Y, Zhang J, Lu J, Yin J, Wei L (2020) Liraglutide alleviates hepatic steatosis by activating the TFEB-regulated autophagy-lysosomal pathway. Front Cell Dev Biol 8:602574
Yu S, Wang Z, Ding L, Yang L (2020) The regulation of TFEB in lipid homeostasis of non-alcoholic fatty liver disease: molecular mechanism and promising therapeutic targets. Life Sci 246:117418
Shao N, Yu XY, Ma XF, Lin WJ, Hao M, Kuang HY (2018) Exenatide delays the progression of nonalcoholic fatty liver disease in C57BL/6 Mice, which may involve inhibition of the NLRP3 inflammasome through the mitophagy pathway. Gastroenterol Res Pract 2018:1864307
Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X (2017) Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 12:e0183541
Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, Weng X (2014) Resveratrol modulates autophagy and NF-kappaB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol 63:166–173
Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018) Spermidine in health and disease. Science 359:eaan2788
Barber TM, Kabisch S, Randeva HS, Pfeiffer AFH, Weickert MO (2022) Implications of resveratrol in obesity and insulin resistance: a state-of-the-art review. Nutrients 14:2870
Gu W, Geng J, Zhao H, Li X, Song G (2022) Effects of resveratrol on metabolic indicators in patients with type 2 diabetes: a systematic review and meta-analysis. Int J Clin Pract 2022:9734738
Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Muller M, Schrauwen P, Mariman EC, Blaak EE (2014) The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes 38:470–473
Zou D, Zhao Z, Li L, Min Y, Zhang D, Ji A, Jiang C, Wei X, Wu X (2022) A comprehensive review of spermidine: Safety, health effects, absorption and metabolism, food materials evaluation, physical and chemical processing, and bioprocessing. Compr Rev Food Sci Food Saf 21:2820–2842
Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F, Zheng A, Hu L, Zhao Y, Zheng L, Fu Z (2020) Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12:1–19
Choksomngam Y, Pattanakuhar S, Chattipakorn N, Chattipakorn SC (2021) The metabolic role of spermidine in obesity: evidence from cells to community. Obes Res Clin Pract 15:315–326
Acknowledgements
Graphic elements in Fig. 2 were sourced from Canva under the Free Content License.
Funding
This work was funded by the National Science Centre, Poland (grant UMO-2021/43/I/NZ3/00510) for B.P. P.J. and M.R.W. This work was also supported by the Czech Science Foundation (22-04100L) for M.R. Additional funding for this work was provided by Unione europea–NextGenerationEU-Italian Ministry of Education, University, and Research, PRIN 2017XA5J5N; PRINP202242AFC, and PRIN2022ALJN73, and from local funds from the University of Ferrara, FIRD-2023 for A. R; the Unione europea–NextGenerationEU-Italian Ministry of Education, University, and Research, PRIN2017E5L5P3, and PRIN2020RRJP5L_003, Italian Association for Cancer Research (AIRC, IG-23670) for P.P. We also acknowledge the financial support from the European Union's Horizon Europe Research and Innovation Programme for the project “PAS GRAS: De-risking metabolic, environmental and behavioral determinants of obesity in children, adolescents and young adults” under grant agreement No. 101080329.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Research involving human participants and/or animals
This is a review article and any studies with human participants or animals were performed by any of the authors.
Informed consent
Taking into account that the authors performed any studies with human participants, the informed consent is not applicable for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jakubek, P., Pakula, B., Rossmeisl, M. et al. Autophagy alterations in obesity, type 2 diabetes, and metabolic dysfunction-associated steatotic liver disease: the evidence from human studies. Intern Emerg Med 19, 1473–1491 (2024). https://doi.org/10.1007/s11739-024-03700-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-024-03700-w