Abstract
Healthcare-associated infections (HCAIs) in patients admitted with acute conditions remain a major challenge to healthcare services. Here, we assessed the impact of HCAIs acquired within 7-days of acute stroke on indicators of care-quality outcomes and dependency. Data were prospectively collected (2014–2016) from the Sentinel Stroke National Audit Programme for 3309 patients (mean age = 76.2 yr, SD = 13.5) admitted to four UK hyperacute stroke units (HASU). Associations between variables were assessed by multivariable logistic regression (odds ratios, 95% confidence intervals), adjusted for age, sex, co-morbidities, pre-stroke disability, swallow screening, stroke type and severity. Within 7-days of admission, urinary tract infection (UTI) and pneumonia occurred in 7.6% and 11.3% of patients. Female (UTI only), older age, underlying hypertension, atrial fibrillation, previous stroke, pre-stroke disability, intracranial haemorrhage, severe stroke, and delay in swallow screening (pneumonia only) were independent risk factors of UTI and pneumonia. Compared to patients without UTI or pneumonia, those with either or both of these HCAIs were more likely to have prolonged stay (> 14-days) on HASU: 5.1 (3.8–6.8); high risk of malnutrition: 3.6 (2.9–4.5); palliative care: 4.5 (3.4–6.1); in-hospital mortality: 4.8 (3.8–6.2); disability at discharge: 7.5 (5.9–9.7); activity of daily living support: 1.6 (1.2–2.2); and discharge to care-home: 2.3 (1.6–3.3). In conclusion, HCAIs acquired within 7-days of an acute stroke led to prolonged hospitalisation, adverse health consequences and risk of care-dependency. These findings provide valuable information for timely intervention to reduce HCAIs, and minimising subsequent adverse outcomes.
Similar content being viewed by others
Introduction
Healthcare-associated (nosocomial) infections (HCAIs) are those that are acquired in a healthcare facility while patients are receiving treatment for another condition [1]. HCAIs remain a constant challenge to healthcare services [2] and have further been exacerbated by coronavirus disease (COVID)-19 [3]. Patients admitted with an acute stroke represent one of the highest risk groups for HCAIs because of their older age, multiple underlying health conditions [4], weakness and hyperacute cognitive stroke syndromes with distressing symptoms such as disorientation and delirium [5]. Brain-induced immunosuppression associated with stroke has also been implicated in the development of infections such as pneumonia for such patients [6, 7]. Patients with an acute stroke are highly susceptible to two major HCAIs: i) urinary tract infections (UTIs), associated with lower urinary tract dysfunction and which often require catheterisation [7, 8], and ii) pneumonia due to dysphagia [9]. Despite advances in stroke management, UTIs and pneumonia remain highly prevalent [7, 9].
Although studies have demonstrated that HCAIs are associated with a greater risk of death [10], several key indicators of the burden of disease and dependency, such as disability, malnutrition and care-support, have not been well-documented. Furthermore, the timing of HCAIs onset has often been poorly defined or they develop at the later stages of hospitalisation, thus making it difficult to interpret cause-and-effect relationships between HCAIs and outcome measures. In this study, we examined the impact of HCAIs acquired within 7-days of admission for an acute stroke on indicators of healthcare quality outcome and care-dependency, including: length of stay (LOS) on hyperacute stroke units (HASU); risk of malnutrition; requirement for palliative care; in-patient mortality; disability; as well as care-support on discharge, with a comprehensive adjustment for important confounding factors.
Methods
Study design, participants and setting
This study was part of the Sentinel Stroke National Audit Programme (SSNAP) [11]. We prospectively collected data from 3309 patients with an acute stroke who were consecutively admitted to four HASU in the south of England, from January 2014 to February 2016 [12].
Socio-demographic factors and medical history
Socio-demographic factors were documented in detail by the stroke team, including: age at onset of stroke; sex; and co-morbidities including congestive heart failure; atrial fibrillation; hypertension; diabetes mellitus; and a history of previous stroke [11, 12].
Diagnosis and severity of acute stroke
Diagnosis of stroke was based on clinical presentation and neuroimaging [11, 12] and classified as ischaemic stroke or intracranial haemorrhage. The severity of stroke symptoms at arrival was based on the National Institutes of Health for Stroke Scale (NIHSS), ranging from no symptoms (minimum NIHSS score = 0) to severe stroke symptoms (maximum NIHSS score = 42) [11, 12].
Swallow screening and nutritional status
The target for conducting swallow screening was within 4-h of stroke diagnosis [11]. Oral fluid, food or medications were allowed if the patient had no risk of aspiration. Those with high risk of malnutrition were diagnosed according to the Malnutrition Universal Screening Test protocol [13].
Healthcare-associated infections
Both UTI and pneumonia were diagnosed in the first 7-days following the initial admission for stroke. UTI was defined as patients who had a positive urine culture or were clinically treated, and newly acquired pneumonia was diagnosed on the basis of clinical examination and chest X-ray and treated with antibiotics [11, 12].
Disability, mortality, palliative care, and care-support at discharge
Disability before the occurrence of stroke as well as at discharge was evaluated by the modified Rankin Scale (mRS). The mRS scores range from 0–6, with a higher score indicating a greater severity (mRS score = 6 indicates death) [14]. Mortality and palliative care at discharge were documented to reflect poor outcomes [11]. The level of care-support was planned for patients on discharge included help for activity of daily living (ADL), and discharge to a care-home [11, 12].
Categorisation of variables
Moderately-severe to severe disability was defined as an mRS score ≥ 4. Severity of stroke was classified as: “no stroke symptoms” (NIHSS score = 0), “minor stroke” (NIHSS score = 1–4), “moderate stroke” (NIHSS score = 5–15), “moderate to severe stroke” (NIHSS score = 16–20), and “severe stroke” (NIHSS score = 21–42). Prolonged LOS was defined as those who spent longer than 14 days on HASU. Swallow screening status was categorised into groups: screening performed within 4 h, 4–72 h, and > 72 h of stroke diagnosis [11, 12]. Age stratification was based on three groups: < 70, 70–79, and ≥ 80 years.
Statistical analysis
Kruskal–Wallis tests were conducted to test HCAIs in non-parametric data (LOS on HASU). Multivariable logistic regression was conducted to examine the association between HCAIs (patients without HCAIs as reference) and healthcare outcomes and dependency (LOS in hospital > 14 days; palliative care decision by discharge; in-patient mortality; mRS ≥ 4 at discharge; risk of malnutrition; ADL support and discharge to care-home), with adjustment for risk factors (age; sex; co-morbidities; mRS scores; type of stroke; NIHSS scores; and time taken for swallow screening). The results were expressed as odds ratios (OR) and 95% confidence intervals (CI). The goodness-of-fit for logistic regression was assessed by Hosmer–Lemeshow tests. Analyses were performed using SPSS Statistics for Windows, v.28.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 3309 patients (1656 men and 1653 women) were studied, with men younger (mean ± SD) at of onset of stroke (73.1 ± 13.2 years) than women (79.3 ± 13.0 years). Hypertension represented the highest proportion of patients with underlying risk factors for stroke (52.3%), followed by history of previous stroke (23.1%), atrial fibrillation (20.1%), diabetes (16.0%), and congestive heart failure 5.9%). There were 5.5% of patients with pre-stroke disabilities (mRS score ≥ 4). Most patients were diagnosed with ischaemic stroke (83.3%, with the remainder mostly as intracranial haemorrhage (15.7%), and 7.7% had moderate-to-severe stroke (NIHSS score = 16–20) and 6.9% had severe stroke (NIHSS score > 20) on arrival. Within 7-days of admission, UTI and pneumonia occurred in 7.6% and 11.3% of patients, respectively. There were 33.9% of patients staying on HASUs > 14 days, 25.8% at risk of malnutrition, 14.5% in-patient deaths and 7.6% with a decision made for palliative care on discharge. At discharge, 29.9% of patients had disabilities (mRS score ≥ 4), 20.4% required ADL support and 5.3% required new care-home discharge (Table 1).
Table 2 shows the main features of patients with different HCAI status. Compared to those without either UTI or pneumonia, proportionally there were: more women; older patients (≥ 80 years); greater prestroke disabilities and comorbidities; more severe stroke, and poorer outcomes. These proportions were generally higher amongst those with pneumonia only than those with UTI only, and further increased (except for congestive heart failure, risk of malnutrition and new discharge to care homes) in those with both HCAIs.
Outcome measures
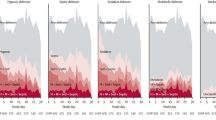
For any given severity of stroke, the LOS on HASU was longer (Fig. 1A), whilst there were increasingly higher proportions of patients spending > 14 days on HASU and a high risk of malnutrition (Fig. 1B, C); in-patient mortality; palliative care; ADL support; and new discharge to a care-home (except for the group with most severe strokes) (Fig. 2A–C).
Event rates, unadjusted and adjusted OR and 95%CI are shown in Table 3. After adjusting for potential confounding factors (age; sex; co-morbidities; pre-stroke disabilities; swallow screening; stroke type and severity) and compared to patients without UTI, patients with UTI were more likely to have (OR, 95%CI) prolonged stay (> 14-days) on HASU: 3.3 (2.4–4.8); high risk of malnutrition: 1.5 (1.1–2.0); palliative care decision: 2.5 (1.7–3.7); in-hospital mortality: 3.0 (2.2–4.1); disability at discharge: 3.9 (2.8–5.4); ADL support: 1.8 (1.2–2.6); and discharge to care-home: 1.9 (1.2–3.0). Compared to patients without pneumonia, those with pneumonia were also more likely to have prolonged stay on HASU: 7.4 (4.9–11.4); high risk of malnutrition: 4.6 (3.6–5.9); palliative care decision by discharge: 7.7 (5.6–10.6); in-hospital mortality: 8.2 (6.2–10.8); disability at discharge: 12.5 (9.0–17.2); ADL support: 1.6 (1.1–2.4); and discharge to care-home: 1.9 (1.3–2.8).
Further analysis was conducted comparing those without UTI or pneumonia. Patients with UTI and/or pneumonia had greater adjusted risk for having prolonged length of stay on HASU: 5.1 (3.8–6.8); high risk of malnutrition: 3.6 (2.9–4.5); palliative care decision: 4.5 (3.4–6.1); in-hospital mortality: 4.8 (3.8–6.2); disability at discharge: 7.5 (5.9–9.7); ADL support: 1.6 (1.2–2.2); and discharge to care-home: 2.3 (1.6–3.3) (Fig. 3).
Discussion
In summary, for acute stroke patients hospital-acquired UTI increased the risk of prolonged stay on HASUs, death, palliative care, or disability by 2.5–4 times, and a greater risk of malnutrition, ADL support or discharge to care-homes by 1.5–2 times. Pneumonia increased the risk of prolonged stay on HASUs, death, palliation, or disability by 7.4–12.5 times, and the risk of malnutrition, ADL support or discharge to care-homes by 1.6–4.6 times. Furthermore, these HCAIs exerted their effects on outcomes at every level of stroke severity.
The influence of these HCAIs on an increased LOS is consistent with previous studies of stroke [15,16,17] and other medical conditions [18]. A prolonged hospital stay induces loss of bodily functions [19] and predisposes patients to infections, risk of malnutrition and death [20]. This vicious cycle also increases healthcare costs. A study in Scotland estimated that 58,010 hospital bed-days were lost to HCAIs, at an annual cost of £46.4 million, which extrapolated to £774 million overall in the UK [21]. With another study in England, 52,085 UTI and 7,529 bloodstream infections were associated with bladder catheterisation, of which 38,084 and 2,524 respectively were HCAIs. Catheter-associated UTI (CAUTI) incurred 45,717 excess bed-days, 1,467 deaths and 10,471 lost quality-adjusted life-years (QALYs). Estimated total direct hospital costs amounted to £54.4 million, with an additional £209.4 million in economic value of lost QALYs [22].
With acute stroke patients, associations of HCAIs with increased risk of death [8, 16, 17, 23] and disability [15, 17, 23, 24] are well documented and consistent with this study, but little information is available for palliative care. Overall they indicate the seriousness of HCAIs and the need for early identification of those at greatest risk as they continue to have an impact on disability [17] and mortality [25] for many years.
The burden of stroke is enormous globally [26] and we found acute stroke patients with HCAIs were more likely to require ADL support and care-homes. This area is poorly studied, but such patients impose high burdens of disease to caregivers and healthcare services [27]. Moreover, the total health and social care cost for acute stroke patients in the UK (except Scotland) is about £3.60 billion a year in the first 5-years after admission, with an average cost of £46,039 per patient [28]. However, there is a lack of information on the impact of HCAIs on the overall cost of post-stroke care.
Direct causal links between HCAIs and subsequent outcomes cannot be established by this study. However, unique features include: (i) a sequential timeline of variables of interest: starting from underlying risk factors occurring prior to development of HCAIs, followed by HCAIs acquired during the early phase of admission, and subsequent outcome measures that developed after the occurrence of HCAIs; (ii) risk factors were independently associated with HCAIs, and (iii) these risk (confounding) factors, which could influence the association between HCAIs and outcomes, were accounted for. Poor outcomes were further accentuated by the presence of HCAIs, at any stroke severity. To recognise HCAIs at a specific time of hospitalisation, especially during the initial phase of acute stroke is therefore crucial, with respect to timely treatment and evaluation of the possible causal links between HCAIs and outcomes.
Although HCAIs are particularly detrimental to patients admitted with an acute stroke, they are widespread across medical and surgical patients, imposing a huge burden of disease on healthcare services. Amongst 13.8 million adult patients admitted to National Health Service (NHS) hospitals in England in 2016–2017, 653,000 (4.73%) developed HCAIs, of whom 22,800 (3.49%) died as a result [29]. For patients admitted with acute general medical conditions, bloodstream, respiratory and urinary tract infections are amongst the most common HCAIs across high-income countries [30, 31], with older age and underlying health conditions being the major risk factors [32]. HCAIs are associated with increased mortality and morbidity [33], LOS in hospital [18] and burden of disease [34].
Indwelling urinary catheters are used frequently in older adults admitted to hospital, but introduce CAUTI. In a 2019 study of 5,203,496 patients admitted for acute medical conditions, 19.2% were catheterised, of whom 3.8% developed CAUTI in hospital [22]. Stroke patients are at a 3.5-fold greater risk of UTI than acute medical patients [35], possibly exacerbated by catheterisation [7]. The rates of CAUTI in this study are not known as the SSNAP protocol does not record information on the use of indwelling urinary catheters. However, Stott et al.reported that amongst stroke patients, catheters were used in 18% of those without and 63.1% with UTI [8], whilst another study found the risk of UTI amongst stroke patients was increased by 2.7-fold for those with a catheter placement compared to those without [36]. However, catheterisation rates in acute stroke patients were higher than those with acute medical conditions, but have been somewhat declining over time. In an earlier study of 3,756 stroke patients admitted to London hospitals in 1995–2011, 31.2% were catheterised [37], whilst a 2004–2005 study of 404 acute stroke patients in Scotland showed 25.7% had catheter insertion [8]. A 2006–2008 study of 2,893 Taiwanese stroke patients showed 25% received catheterisation [38] and a smaller 2013 study of 212 French stroke patients, reported 21.2% had urinary catheters inserted [39].
However, despite their widespread use, routine documentation of indwelling urinary catheters in stroke patients is lacking at a national level, including SSNAP [40, 41]. Thus, up-to-date progress on reducing CAUTI in stroke patients is unclear. Given the impact of UTI on poor outcomes, the use of indwelling urinary catheters should be documented routinely to monitor and minimise avoidable catheterisation, or reduce the duration of their use, and consequently improve the quality of patient care [42].
Early nutritional support is also crucial in stroke survival. Early identification of dysphagia allows timely delivery of parenteral or enteral nutritional support through a central venous line or a nasogastric tube respectively. Although these are routinely used in a clinical setting, the SSNAP protocol does not record the rates of nasogastric tube (NGT) insertion; therefore its risks and benefits could not be assessed. However, a systemic review of eleven articles (60–1088 patients) showed no clear evidence for an association between NGT placement and stroke-associated pneumonia [9]. Further prospective studies are therefore warranted to assess the risk of stroke-associated pneumonia from a NGT compared to other routes of nutritional support.
Summaries of UK stroke data between 2013 and 2023 [40, 41] showed that age, sex distribution and co-morbidities of acute stroke remain generally unchanged. However, certain aspects of stroke management have improved over time. National SSNAP data through 2013/14 to 2022/23 revealed that brain imaging within 1-h of arrival had increased from 42 to 57%, and access to specialist care for intracranial haemorrhage increased from 66 to 76%. This was linked to lower in-hospital mortality from 33 to 29%, whilst discharge to an Early Supported Discharge or Community Rehabilitation Team increased from 41 to 61%. However, an initial rise of patients receiving 45 min of occupational therapy, physiotherapy, speech and language therapy and psychology five days a week declined during the Coronavirus-19 pandemic [40, 41]. These changes over time may have some bearing on the data analyses from this study which collected data from 2014–2016. However our study examined the risk of HCAIs on health outcomes, as opposed to measuring prevalence or incidence. Therefore results were less temporally-dependent, especially when the data were fully adjusted for time-dependent confounding factors. Thus, in this study the relative risk of an outcome due to HCAIs would be expected to be similar to that at the present time.
Limitations to this study include the limited number of principal HCAIs (UTI and pneumonia) collected by the SSNAP protocol, and it would be important in future studies to document other HCAIs such as bloodstream infections and gastrointestinal complications. Although the study recruited patients locally, the data were from a relatively large cohort of patients admitted consecutively from one of the largest NHS regions in the UK, and data collection followed national SSNAP guidance, using standardised methods [11].
In conclusion, our study provided further insights into the impact of HCAIs on healthcare outcomes in acute stroke patients. Timely intervention is therefore necessary to reduce/prevent HCAIs.
Data sharing statement
No additional data are available.
Data availability
Not applicable.
Abbreviations
- ADL:
-
Activity of daily living
- CAUTI:
-
Catheter-associated urinary tract infection
- CI:
-
Confidence intervals
- COVID:
-
Coronavirus disease
- HASU:
-
Hyperacute stroke units
- HCAIs:
-
Healthcare-associated infections
- LOS:
-
Length of stay
- mRS:
-
Modified Rankin Scale
- NGT:
-
Nasogastric tube
- NHS:
-
National Health Service
- NIHSS:
-
National Institutes of Health for Stroke Scale
- OR:
-
Odds ratios
- QALYs:
-
Quality-adjusted life-years
- SSNAP:
-
Sentinel Stroke National Audit Programme
- UTI:
-
Urinary tract infection
References
Haque M, Sartelli M, McKimm J, Bakar MA (2018) Health care-associated infections–an overview. Infect Drug Resist 15(11):2321–2333. https://doi.org/10.2147/IDR.S177247
NHS England. Healthcare associated infections https://www.england.nhs.uk/patient-safety/healthcare-associated-infections/ (Accessed 20 September 2023)
Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, Bellani G, Biagioni E, Bonfanti P, Bottino N, Coloretti I (2021) Hospital-acquired infections in critically ill patients with COVID-19. Chest 160(2):454–465. https://doi.org/10.1016/j.chest.2021.04.002
Fluck D, Fry CH, Rankin S, Gulli G, Affley B, Robin J, Kakar P, Sharma P, Han TS (2022) Comparison of characteristics, management and outcomes in hospital-onset and community-onset stroke: a multi-centre registry-based cohort study of acute stroke. Neurol Sci 43(8):4853–4862. https://doi.org/10.1007/s10072-022-06015-w.(a)
Ferro JM (2001) Hyperacute cognitive stroke syndromes. J Neurol 248(10):841–849. https://doi.org/10.1007/s004150170067
Faura J, Bustamante A, Miró-Mur F, Montaner J (2021) Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation 18(1):127. https://doi.org/10.1186/s12974-021-02177-0
Poisson SN, Johnston SC, Josephson SA (2010) Urinary tract infections complicating stroke: mechanisms, consequences, and possible solutions. Stroke 41(4):e180–e184
Stott DJ, Falconer A, Miller H, Tilston JC, Langhorne P (2009) Urinary tract infection after stroke. QJM 102(4):243–249. https://doi.org/10.1093/qjmed/hcp012
Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Smith CJ, Pownall S (2020) Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: a systematic review. Dysphagia 35(5):735–744. https://doi.org/10.1007/s00455-019-10061-6
Elkind MS, Boehme AK, Smith CJ, Meisel A, Buckwalter MS (2020) Infection as a stroke risk factor and determinant of outcome after stroke. Stroke 51(10):3156–3168. https://doi.org/10.1161/STROKEAHA.120.030429
Royal College of Physicians. Clinical effectiveness and evaluation unit on behalf of the intercollegiate stroke working party. SSNAP April–July 2017. Public Report. https://www.strokeaudit.org/Documents/National/Clinical/AprJul2017/AprJul2017-PublicReport.aspx (Accessed 20 September 2023)
Han TS, Gulli G, Affley B, Fluck D, Fry CH, Barrett C, Kakar P, Sharma S, Sharma P (2019) New evidence-based A1, A2, A3 alarm time zones for transferring thrombolysed patients to hyper-acute stroke units: faster is better. Neurol Sci 40(8):1659–1665. https://doi.org/10.1007/s10072-019-03901-8
Kondrup JE, Allison SP, Elia M, Vellas B, Plauth M (2003) ESPEN guidelines for nutrition screening 2002. Clin Nutr 22(4):415–421. https://doi.org/10.1016/S0261-5614(03)00098-0
Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5):604–607. https://doi.org/10.1161/01.str.19.5.604
Ovbiagele B, Hills NK, Saver JL, Johnston SC, California Acute Stroke Prototype Registry Investigators (2006) Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J Stroke Cerebrovasc Dis 15(5):209–13. https://doi.org/10.1016/j.jstrokecerebrovasdis.2006.05.004
Masrur S, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Zhao X, Olson D, Pan W, Hernandez AF, Fonarow GC, Schwamm LH (2013) Dysphagia screening and hospital-acquired pneumonia in patients with acute ischemic stroke: findings from Get with the Guidelines-Stroke. J Stroke Cerebrovasc Dis 22(8):e301–e309. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.11.013
Vermeij FH, Scholte op Reimer WJ, De Man P, Van Oostenbrugge RJ, Franke CL, De Jong G, De Kort PL, Dippel DW (2009) Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: data from the Netherlands Stroke Survey. Cerebrovasc Dis. 27(5):465–71. https://doi.org/10.1159/000210093.
Stewart S, Robertson C, Pan J, Kennedy S, Haahr L, Manoukian S, Mason H, Kavanagh K, Graves N, Dancer SJ, Cook B (2021) Impact of healthcare-associated infection on length of stay. J Hosp Infect 114:23–31. https://doi.org/10.1016/j.jhin.2021.02.026
National Audit Office (2016) Discharging older patients from hospital. Report by the Comptroller and Auditor General. NAO, London. http://www.nao.org.uk/wp-content/uploads/2015/12/Discharging-older-patients-from-hospital-Summary.pdf. (Accessed 20 September 2023)
Fluck D, Fry CH, Gulli G, Affley B, Robin J, Kakar P, Sharma P, Han TS (2022) Association of risk of malnutrition with adverse outcomes and early support on discharge in acute stroke patients without prestroke disability: a multicenter, registry-based cohort study. Nutr Clin Pract 37(5):1233–1241. https://doi.org/10.1002/ncp.10790.(b)
Manoukian S, Stewart S, Graves N, Mason H, Robertson C, Kennedy S, Pan J, Kavanagh K, Haahr L, Adil M, Dancer SJ (2021) Bed-days and costs associated with the inpatient burden of healthcare-associated infection in the UK. J Hosp Infect 114:43–50. https://doi.org/10.1016/j.jhin.2020.12.027
Smith DR, Pouwels KB, Hopkins S, Naylor NR, Smieszek T, Robotham JV (2019) Epidemiology and health-economic burden of urinary-catheter-associated infection in English NHS hospitals: a probabilistic modelling study. J Hosp Infect 103(1):44–54. https://doi.org/10.1016/j.jhin.2019.04.010
Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G (2011) Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 77(14):1338–1345. https://doi.org/10.1212/WNL.0b013e31823152b1
Sellars C, Bowie L, Bagg J, Sweeney MP, Miller H, Tilston J, Langhorne P, Stott DJ (2007) Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke 38(8):2284–2291. https://doi.org/10.1161/STROKEAHA.106.478156
Yu YJ, Weng WC, Su FC, Peng TI, Chien YY, Wu CL, Lee KY, Wei YC, Lin SW, Zhu JX, Huang WY (2016) Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci 33:124–128. https://doi.org/10.1016/j.jocn.2016.02.039
Norrving B, Kissela B (2013) The global burden of stroke and need for a continuum of care. Neurology 80(3 Suppl 2):S5-12. https://doi.org/10.1212/WNL.0b013e3182762397
Rigby H, Gubitz G, Phillips S (2009) A systematic review of caregiver burden following stroke. Int J Stroke 4(4):285–292
Xu XM, Vestesson E, Paley L, Desikan A, Wonderling D, Hoffman A, Wolfe CD, Rudd AG, Bray BD (2018) The economic burden of stroke care in England, Wales and Northern Ireland: using a national stroke register to estimate and report patient-level health economic outcomes in stroke. Eur Stroke J 3(1):82–91. https://doi.org/10.1177/2396987317746516
Guest JF, Keating T, Gould D, Wigglesworth N (2020) Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJ Open 10(1):e033367. https://doi.org/10.1136/bmjopen-2019-033367
Zarb P, Coignard B, Griskeviciene J, Muller A, Vankerckhoven V, Weist K, Goossens MM, Vaerenberg S, Hopkins S, Catry B, Monnet DL (2012) The European centre for disease prevention and control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill 17(46):20316. https://doi.org/10.2807/ese.17.46.20316-en
Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM (2018) Changes in prevalence of health care–associated infections in US hospitals. N Engl J Med 379(18):1732–1744. https://doi.org/10.1056/NEJMoa1801550
Letica-Kriegel AS, Salmasian H, Vawdrey DK, Youngerman BE, Green RA, Furuya EY, Calfee DP, Perotte R (2019) Identifying the risk factors for catheter-associated urinary tract infections: a large cross-sectional study of six hospitals. BMJ Open 9(2):e022137. https://doi.org/10.1136/bmjopen-2018-022137
Barrasa-Villar JI, Aibar-Remón C, Prieto-Andrés P, Mareca-Doñate R, Moliner-Lahoz J (2017) Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 65(4):644–652. https://doi.org/10.1093/cid/cix411
Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, Haller S, Harder T, Klingeberg A, Sixtensson M, Velasco E (2016) Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med 13(10):e1002150. https://doi.org/10.1371/journal.pmed.1002150
Retelski J, Richardson T, Mahabaleshwarkar R, Gohs FX, Spencer MD (2017) Retrospective analysis of catheter-acquired urinary tract infection: relationship to stroke diagnosis. Clin Nurse Spec. 31(4):E11–E16. https://doi.org/10.1097/NUR.0000000000000307. (PMID: 28594676)
Bogason E, Morrison K, Zalatimo O, Ermak DM, Lehman E, Markley E, Cockroft K (2017) Urinary tract infections in hospitalized ischemic stroke patients: source and impact on outcome. Cureus 9(2):e1014. https://doi.org/10.7759/cureus.1014
John G, Primmaz S, Crichton S, Wolfe C (2018) Urinary incontinence and indwelling urinary catheters as predictors of death after new-onset stroke: a report of the south london stroke register. J Stroke Cerebrovasc Dis 27(1):118–124. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.08.018
Wu CH, Tseng MC, Chen YW, Sung SF, Yeh PS, Lin HJ (2013) Indwelling urinary catheterization after acute stroke. Neurourol Urodyn 32(5):480–485. https://doi.org/10.1002/nau.22317
Net P, Karnycheff F, Vasse M, Bourdain F, Bonan B, Lapergue B (2018) Urinary tract infection after acute stroke: impact of indwelling urinary catheterization and assessment of catheter-use practices in French stroke centers. Rev Neurol 174(3):145–149. https://doi.org/10.1016/j.neurol.2017.06.029
Sentinel Stroke National Audit Programme (SSNAP) Clinical audit April 2013 – March 2018 Annual Public Report. https://www.strokeaudit.org/Documents/National/Clinical/Apr2017Mar2018/Apr2017Mar2018-AnnualReport.aspx (Accessed 12 December 2023)
Sentinel Stroke National Audit Programme (SSNAP) Annual Report 2023. https://www.strokeaudit.org/Documents/National/Clinical/Apr2022Mar2023/Apr2022Mar2023-AnnualReport.aspx (Accessed 12 December 2023)
Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee (2010) Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 31(4):319–26. https://doi.org/10.1086/651091
Acknowledgements
The authors wish to thank patients and all those who were involved in the surveys.
Funding
None.
Author information
Authors and Affiliations
Contributions
TSH and DF reviewed the topic related literature and TSH performed the study concept and analysis design. BA, JR and PK performed the study coordination and data collection. TSH wrote the first draft, analysed, interpreted the data and revised the manuscript. CHF and PS edited the manuscript. DF, BA, JR, PK and PS checked, interpreted results and commented on the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this paper.
Ethical approval
This study does not require NHS Research Ethics Committee approval. This study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fluck, D., Fry, C.H., Robin, J. et al. Impact of healthcare-associated infections within 7-days of acute stroke on health outcomes and risk of care-dependency: a multi-centre registry-based cohort study. Intern Emerg Med (2024). https://doi.org/10.1007/s11739-024-03543-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11739-024-03543-5