Abstract
Hyperkalemia is common in clinical practice and can be caused by medications used to treat cardiovascular diseases, particularly renin–angiotensin–aldosterone system inhibitors (RAASis). This narrative review discusses the epidemiology, etiology, and consequences of hyperkalemia, and recommends strategies for the prevention and management of hyperkalemia, mainly focusing on guideline recommendations, while recognizing the gaps or differences between the guidelines. Available evidence emphasizes the importance of healthcare professionals (HCPs) taking a proactive approach to hyperkalemia management by prioritizing patient identification and acknowledging that hyperkalemia is often a long-term condition requiring ongoing treatment. Given the risk of hyperkalemia during RAASi treatment, it is advisable to monitor serum potassium levels prior to initiating these treatments, and then regularly throughout treatment. If RAASi therapy is indicated in patients with cardiorenal disease, HCPs should first treat chronic hyperkalemia before reducing the dose or discontinuing RAASis, as reduction or interruption of RAASi treatment can increase the risk of adverse cardiovascular and renal outcomes or death. Moreover, management of hyperkalemia should involve the use of newer potassium binders, such as sodium zirconium cyclosilicate or patiromer, as these agents can effectively enable optimal RAASi treatment. Finally, patients should receive education regarding hyperkalemia, the risks of discontinuing their current treatments, and need to avoid excessive dietary potassium intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperkalemia is common in clinical practice, especially in patients with cardiovascular diseases (e.g., hypertension, heart failure [HF] or coronary artery disease), renal impairment, and/or diabetes [1] and can have fatal consequences by causing cardiac arrhythmias [2].
Hyperkalemia can be caused by medications used to treat cardiovascular disease, particularly renin–angiotensin–aldosterone system inhibitors (RAASis) [1]. Since these agents are recommended to reduce target organ damage and improve major clinical outcomes in patients with hypertension, HF, diabetes, and chronic kidney disease (CKD) [3,4,5,6,7,8,9], it is important to determine how to prevent and manage hyperkalemia in a way that does not negatively impact treatment for the patient’s underlying condition.
The aim of the current narrative review is to describe the epidemiology, etiology, and consequences of hyperkalemia and to recommend strategies for the prevention and management of hyperkalemia, with a focus on guideline recommendations while recognizing the gaps or differences between guidelines.
Epidemiology of hyperkalemia
There is no internationally agreed definition of hyperkalemia, and the thresholds for defining the severity of hyperkalemia differ between guidelines (Table 1) [1, 10,11,12,13]. The European Society of Cardiology (ESC) defines hyperkalemia as serum potassium levels > 5.0 mmol/L [1], which is the most common definition; this level, while not in general a risk factor for cardiac arrhythmias, should trigger a more careful and frequent monitoring because it poses, per se, a higher risk of severe hyperkalemia [1, 10,11,12,13]. Depending on the definition, the estimated prevalence of hyperkalemia is 2–3% in the general population and 1–10% in hospitalized patients [14]. Hyperkalemia prevalence increases with age [15, 16], worsening renal function [15,16,17,18], the presence of diabetes or HF [15, 16], a history of myocardial infarction [15], the use of RAASis [15, 17], or resistant hypertension treated with mineralocorticoid receptor antagonists (MRAs) [19,20,21]. Therefore, the prevalence of hyperkalemia can be as high as 40–50% in these high-risk groups [18, 22]. Conversely, use of sodium-glucose cotransporter 2 inhibitors (SGLT2is) decreases the risk of hyperkalemia in RAASi-treated patients with CKD, likely because of the kaliuresis induced by osmotic diuresis, the increased distal sodium delivery and the preservation of renal function [21, 23].
Clinical impact of hyperkalemia
Cohort studies from Denmark and Italy have indicated that patients with HF and CKD tend to have multiple recurrent episodes of hyperkalemia, with the period between hyperkalemia episodes decreasing with each subsequent event [24,25,26]. A database analysis of US patients with CKD who were not on dialysis reported that patients spent between 13.2 and 32.4% of their time in a hyperkalemic state during a median of 2.76 years of follow-up [27]. Indeed, the more severe the renal impairment, the longer patients spent in a hyperkalemic state [27].
Serum potassium levels have a U-shaped relationship with all-cause mortality in both healthy and high-risk individuals [27,28,29,30,31,32], with a significant increase in mortality for patients with serum potassium levels < 4.0 mmol/L and ≥ 5.0 mmol/L [28]. While diabetes, HF, and CKD increase the risk of mortality associated with hyperkalemia, the risk is highest when all three are present [28].
Hyperkalemia also increases the risk of hospitalization among patients with HF or CKD [24, 25], as well as major adverse cardiovascular events (MACE) in patients with CKD [30]. These events contribute to significant economic costs and poor clinical outcomes. In Italy, it has been estimated that maintaining normal serum potassium levels (i.e., normokalemia) in a patient with CKD would save more than €16,000 over the patient’s lifetime, mainly due to a delayed need for dialysis (by an average of 2.29 years) and a longer life expectancy (by an average of 1.79 years) [33]. Indeed, by maintaining normokalemia, clinicians do not need to down-titrate or discontinue RAASis, which translates into a delayed need for dialysis and longer life expectancy.
Among patients undergoing dialysis, the presence of hyperkalemia significantly increases the risk of hospitalization [34, 35], emergency department visits [34], cardiovascular mortality [35, 36], all-cause mortality [34,35,36], and sudden death [37, 38]. The risk of sudden death is also 2.7-fold higher in patients with a predialytic serum potassium ≥ 6.0 mmol/L than in those with serum potassium < 6.0 mmol/L [38]. The risk of sudden death or hospitalization associated with hyperkalemia is increased at the end of a long interdialytic interval (i.e., 60–72 h, such as over weekends), consistent with the adverse outcomes that result from serum potassium accumulation [34, 37].
Clinical impact of renin–angiotensin–aldosterone system inhibitor withdrawal
For patients receiving RAASis, the risk posed by hyperkalemia must be balanced against the risk of avoiding or withdrawing these agents, which have a proven benefit in terms of cardiovascular outcomes. Despite guideline recommendations, a high proportion of patients receiving RAASis in the clinical practice setting receive suboptimal doses [39,40,41,42].
Data from the US CHAMP-HF registry show that a minority of patients with HF receive stable target doses of RAASis over 12 months of follow-up; in fact, 73.0% of patients receiving angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) had stable sub-target doses over this time, and 11.5% had a dose reduction or discontinued the ACEi/ARB [40]. For patients on MRAs, the corresponding percentages were 67.3% and 4.4% [40]. Medical reasons were the most common cause of dose decrease or discontinuation [40].
Hyperkalemia is a common reason for reducing the dose or discontinuing RAASis [39, 43]. An analysis of data from a large US database of electronic medical records found that, in patients who developed hyperkalemia during RAASi treatment, the RAASi dose was titrated downwards in 16–21% of events, and RAASi was discontinued in 22–27% of hyperkalemic events [39]. However, both RAASi discontinuation and submaximal doses of RAASis were associated with an increased risk of adverse cardiovascular outcomes (composite) or death over a median of 3.4 years of follow-up [39]. BLITZ-HF, a cross-sectional study in patients with acute or chronic HF, found hyperkalemia to be one of the main factors underlying a lack of RAASi implementation in this population [44]. In a Canadian population-based cohort study, discontinuation of RAASis in patients with hyperkalemia and CKD was associated with an increased risk of dialysis initiation and mortality [45].
In Europe, the ESC-HFA-EORP Heart Failure Long-term Registry showed that hyperkalemia was associated with an increased risk of mortality, as well as an increased risk of discontinuing RAASi treatment [16]. However, hyperkalemia was no longer associated with an increased risk of death after adjustment for RAASi discontinuation, suggesting that treatment withdrawal was the true cause of the increased mortality risk, and hyperkalemia was a marker for treatment discontinuation [16].
Similar findings were reported in a cohort study among outpatients with HF in Italy [46]. In this analysis, hyperkalemia was a common cause of MRA dose reduction or withdrawal in patients with HF. While hyperkalemia per se was not associated with an increased risk of mortality, MRA withdrawal secondary to hyperkalemia was associated with a fivefold increased risk of mortality after adjustment for baseline risk factors [46].
The Randomized Aldactone Evaluation Study (RALES) found that, in patients with HF, the use of spironolactone 25 mg/day was associated with lower mortality rates even for those with moderate hyperkalemia (up to 6.0 mmol/L); at higher potassium levels, the dependent negative effects, including MRA withdrawal, outweighed the cardioprotective efficacy of this drug [47]. These data argue against automatic discontinuation of MRAs when potassium concentrations rise to > 5.0 mmol/L. Nevertheless, patients must be carefully followed for hyperkalemia when treated with MRAs; this concept is supported by a real-world analysis examining trends in the rate of spironolactone prescriptions and the rate of hospitalization for hyperkalemia in ambulatory patients before and after the publication of RALES [48]. The analysis found that following the increase in the prescription rate of spironolactone in HF after publication of RALES, hospitalizations and mortality due to hyperkalemia did actually increase by 4 and 7 times, respectively.
Guideline recommendations for hyperkalemia
A number of guidelines on the management of cardiorenal diseases contain recommendations on hyperkalemia, including the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for diabetes management in CKD [9], the KDIGO guidelines for blood pressure management in CKD [8], the ESC HF guidelines [5], and the American College of Cardiology (ACC) HF guidelines [49].
In addition, there are several sets of guidelines specifically on hyperkalemia management, including international consensus recommendations [50], guidelines from the UK Renal Association [10], and the Italian Society of Nephrology (ISN) position paper [14].
Monitoring serum potassium during renin–angiotensin–aldosterone system inhibitor treatment
The KDIGO guidelines recommend that ACEis or ARBs should be used in patients with advanced CKD, but note that close monitoring of serum potassium is required in patients with CKD to aid early identification of even moderate hyperkalemia, in order to prevent more severe degrees of this complication [8, 9]. The ESC HF guidelines also recommend close monitoring of serum potassium, with optimization of RAASi treatment when potassium levels are 4.5–5.0 mmol/L [5]. Once an elevated serum potassium level has been recorded, it is advisable to repeat the test for confirmation. For example, the UK guidelines recommend repeating the test within 3 days if the initial level was 5.5–5.9 mmol/L and within 1 day if the level was 6.0–6.4 mmol/L [10]. Patients with serum potassium ≥ 6.5 mmol/L should be considered as having acute hyperkalemia and be admitted for immediate assessment and treatment [10].
Diet
The KDIGO guidelines, the ISN position paper on hyperkalemia, and the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines on nutrition in patients with CKD all recommend limiting foods rich in potassium among patients with hyperkalemia, but also note the importance of considering the patient’s need for adequate fiber intake [8, 9, 14, 51]. The KDOQI guidelines suggest identifying the most important sources of potassium in the patient’s diet and reducing them, ideally with the assistance of a renal dietician [51]. However, there is generally a lack of high-quality evidence demonstrating the effectiveness of dietary potassium restriction as a management strategy for hyperkalemia [2, 51]. In fact, a low-potassium diet may deprive patients of important nutrients and could conflict with a heart-healthy diet, such as the Dietary Approaches to Stopping Hypertension (DASH) diet [52, 53]. For example, patients on hemodialysis generally have a low intake of fruits and vegetables, but increasing their consumption reduced both the risk of all-cause mortality (by 5.0%) and the risk of death due to non-cardiovascular causes (by 3.0%) [54]. A study by the Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team showed that increased consumption of fruits and vegetables led to a slower rate of decline of kidney function among CKD and non-CKD individuals [55]. Patients who are trying to limit their dietary potassium intake may also face many practical barriers and psychosocial issues [56], which means that other potassium-lowering strategies are often needed for effective hyperkalemia management.
Pharmacological treatment
The ESC HF guidelines recommend initiating pharmacological potassium-lowering treatment with potassium binders as soon as serum potassium exceeds 5.0 mmol/L [5]. RAASi treatment should only be reduced or discontinued in patients whose serum potassium exceeds 6.5 mmol/L. On the other hand, the ACC HF guidelines advocate a higher threshold for identifying hyperkalemia (serum potassium ≥ 5.5 mmol/L) [49]. These guidelines note that potassium binders facilitate the continuation of RAASi treatment, but that the effect of this treatment continuation on cardiovascular outcomes is uncertain [49].
The KDIGO guidelines and international consensus recommendations for hyperkalemia recommend that RAASi treatment should not be de-escalated or discontinued unless alternative measures for hyperkalemia management have been optimized or these measures have failed to normalize serum potassium [8, 9, 50]. Oral supplementation with sodium bicarbonate can be used in patients with metabolic acidosis, and potassium-wasting diuretics may be an option only in patients with extracellular volume expansion [10, 14]. However, prolonged supplementation with sodium bicarbonate may increase the sodium load, which may worsen fluid overload in patients with CKD and HF [10]. Similarly, the use of diuretics requires careful monitoring and management to prevent worsening hypovolemia or renal function with subsequent increase in serum potassium [14].
Most guidelines (i.e., those of the ESC, ACC, international consensus recommendations, and the UK Renal Association) advocate the use of newer potassium binders (i.e., patiromer sorbitex calcium [patiromer] and sodium zirconium cyclosilicate [SZC]) to manage hyperkalemia while maintaining RAASi treatment [5, 10, 49, 50].
Pharmacological and clinical profile of potassium binders
The earlier generation of potassium binders was potassium exchange resins based on polystyrene, either sodium polystyrene sulfonate (SPS) or calcium polystyrene sulfonate (CPS), which act in the gastrointestinal tract to swap potassium for sodium or calcium, respectively, thereby increasing fecal potassium excretion [57]. While both are effective in reducing serum potassium [58], SPS is more widely used [57]. Both CPS and SPS are associated with gastrointestinal adverse events, particularly constipation, nausea, and vomiting, but also potentially serious adverse events, including ulcers, perforation, and ischemia/thrombosis [58,59,60,61,62]. For example, in a Canadian cohort study among 27,704 elderly patients initiating SPS treatment, serious gastrointestinal adverse events requiring emergency department presentation or hospitalization developed in the first 30 days at a rate of ~ 23 per 1000 patient-years; the risk was highest for gastrointestinal ischemia or thrombosis (fourfold increase in the risk compared with non-use of SPS) [62]. The risk of intestinal necrosis is increased when these agents are used with sorbitol, a common laxative for constipation [2, 57].
As with the older potassium binders, patiromer and SZC are not systemically absorbed but act in the gastrointestinal tract to trap potassium ions for subsequent fecal elimination; however, the newer potassium binding agents show minimal water absorption or swelling in the gastrointestinal tract, resulting in fewer adverse effects [57, 63, 64].
Patiromer consists of a non-absorbed cation exchange polymer with a calcium-sorbitol counter-ion complex that increases stability [63]. While SPS exchanges potassium for sodium ions, patiromer exchanges potassium for calcium ions; therefore, it may be safer than SPS in patients who should avoid even small increases in sodium loads, such as those with severe hypertension, CKD, or HF [63]. Patiromer is designed to be maximally ionized (i.e., have the greatest binding capacity) at the physiological pH of the gastrointestinal tract, where potassium concentrations are the highest [63].
SZC is a microporous zirconium silicate that mimics the action of physiological potassium ion channels [64]. It is highly selective for potassium, with minimal effect on the absorption of other cations such as calcium or magnesium [64]. SZC has a rapid onset of effect (within 1 h) [65], and, therefore, is the preferred option in instances where a relatively rapid reduction in serum potassium levels is needed [66]. By comparison, the onset of action for patiromer is 4–7 h and is not suited to emergency treatment [67].
Both patiromer and SZC have demonstrated their efficacy in short-term correction of serum potassium, as well as maintaining levels within the normokalemic range over the long term, in patients with hyperkalemia (Table 2), including those with CKD, HF, hypertension, or type 2 diabetes [68,69,70,71,72,73,74,75,76,77,78,79,80]. However, as noted in the ACC guidelines [49], no data are yet available regarding the impact of patiromer or SZC on cardiovascular outcomes.
Importantly, the newer potassium binding agents allow for the continued use of RAASis at stable or increased doses in most patients [68, 69, 76, 77, 80]. SZC treatment is associated with increases in serum bicarbonate, which can reduce the risk of metabolic acidosis and the need for alkali supplementation (e.g., with sodium bicarbonate) [81]. Patiromer and SZC are both generally well tolerated; common adverse events during patiromer treatment are hypomagnesemia, constipation, diarrhea, abdominal pain, and flatulence [67], while the most common adverse events during SZC treatment are constipation and edema-related events [65]. The latter effect, resulting from the presence of ~ 400 mg of sodium for each 5 g dose of SZC, must be especially considered in patients with non-dialysis CKD and HF [82].
Recommendations for hyperkalemia management
Clear differences exist between sets of guidelines on hyperkalemia management in patients with cardiorenal disease, and it may be helpful to have a comprehensive and unified guideline that considers the potential for multiple comorbidities in these patients. Based on the available evidence, we advocate that physicians take a proactive approach to hyperkalemia management in clinical practice that focuses on patient identification and recognizes that hyperkalemia is often a long-term condition that needs ongoing treatment.
Patient identification
Hyperkalemia is simple to diagnose but may go undetected in an outpatient setting because it is usually asymptomatic. Therefore, given the risk of hyperkalemia during RAASi treatment, it is advisable to check serum potassium prior to initiating these treatments, and then regularly throughout treatment [10, 14]. This is particularly important in older patients and those who have a history of hyperkalemia, who are at increased risk of subsequent hyperkalemic events [15, 24,25,26, 47]. Currently, there are no internationally agreed criteria for the magnitude, duration, and frequency of elevated serum potassium that define chronic hyperkalemia [52].
ESC HF guidelines and the position paper of the Italian ISN recommend starting potassium binders when serum potassium is ≥ 5.0 mmol/L [5, 14], while in the UK, the threshold of serum potassium for the use of potassium binders is higher (> 6.0 mmol/L) [10]. Based on the most recent KDIGO guidelines in diabetic and nondiabetic CKD [9] (personal communication, M Madero), we recommend that potassium binder treatment is initiated with a serum potassium level of at least 5.5 mmol/L confirmed in two tests and after excluding pseudohyperkalemia. Indeed, this is the threshold for initiating potassium binders according to the Italian Drug Agency [83].
Treatment
All healthcare professionals (HCPs) involved in the management of patients with cardiovascular or renal disease should recognize that hyperkalemia is a predictable and manageable adverse effect of RAASi-containing treatment regimens. Therefore, HCPs need to be familiar with the current guideline recommendations for the management of hyperkalemia, as well as of the patient’s cardiorenal disease.
Based on the known benefits of RAASis in patients with cardiorenal disease, clinical practice guidelines such as those from the ESC consistently recommend treating chronic hyperkalemia first before reducing the dose or discontinuing RAASi treatment if serum potassium levels exceed 6.5 mmol/L [5, 10, 14, 50]. The only exception may be a temporary interruption of RAASis during an acute intercurrent illness, such as sepsis, hypovolemia, or acute kidney injury [10]. Alternatively, the ACC guidelines recommend discontinuing MRAs if serum potassium levels cannot be maintained < 5.5 mmol/L [49]. Nevertheless, a recent randomized open-label crossover trial in patients with CKD suggested that adding an SGLT2i to MRA therapy provides increased kidney and cardiovascular protection and reduces the risk of MRA-related hyperkalemia [84].
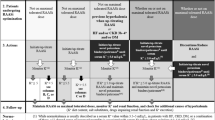
Treatment should involve the use of newer potassium binders (i.e., SZC or patiromer), based on evidence that these agents can allow patients to effectively achieve normokalemia while optimizing RAASi treatment [68, 69, 76, 77]. An optimal serum potassium level of 4.0–4.5 mmol/L has been suggested by the ISN position paper because these levels are associated with the lowest mortality rate [14]; however, we suggest a target serum potassium of 4.0–4.9 mmol/L, which may be more feasible in clinical practice. There is no consensus (considering clinical trial evidence) on the specific treatment of hyperkalemia tailored to a given potassium level; therefore, in Fig. 1, we propose a pragmatic step-by-step approach to hyperkalemia in non-dialysis CKD patients that summarizes indications by current guidelines and position papers [2, 8, 10, 14, 49, 52] (personal communication, M Madero), where each additional step contains the interventions indicated in the previous steps. This approach can be implemented in all outpatients with CKD and chronic hyperkalemia receiving RAASi therapy, regardless of the main comorbidities (hypertension, diabetes, HF). In-hospital treatment of acute severe hyperkalemia, i.e., in the setting of emergency units, cardiac arrest, and resuscitation, goes beyond the scope of this review; this topic has been extensively addressed elsewhere [10]. It is worth noting that SZC and patiromer might also allow the effective treatment of hyperkalemia in some patients who are not deemed to be candidates for dialysis treatment [85].
Pragmatic step-by-step intervention for the treatment of hyperkalemia in patients with hyperkalemia. ECG electrocardiogram, IV intravenous, MRA mineralocorticoid receptor antagonist, NSAID non-steroidal anti-inflammatory drug, RAASi renin–angiotensin–aldosterone system inhibitor, sK serum potassium, SGLT2i sodium-glucose cotransporter 2 inhibitor, SZC sodium zirconium cyclosilicate
Patients should receive education on hyperkalemia, as well as the risks of discontinuing their current RAASi treatments, and the need to avoid excessive dietary potassium intake. In patients with CKD who experience hyperkalemia frequently, the recommended potassium intake is < 3 g/day—corresponding to an approximate urinary potassium excretion of < 78 mmol/24 h [86]—while maintaining a healthy diet rich in fruits and vegetables [86, 87]. If patients are suitable for potassium binder treatment, they must understand how important it is to follow this therapy, as a successful combination of optimal RAASi plus potassium binder can avoid/delay the need for dialysis and other serious clinical outcomes. Patients and clinicians need to understand that liberalization/normalization of their diet is possible, but that potassium excess should be still avoided.
A plea for stakeholder engagement and research
Institutions and payers need to be aware of the risk of hyperkalemia and RAASi down-titration, and they should promote, through all available channels, the effective management of hyperkalemia without RAASi dose adjustment wherever possible. HCPs need to encourage all colleagues to learn the guideline recommendations and apply them in clinical practice. Closer cross-specialist collaboration will help to optimize outcomes for people with cardiorenal disease. Patient groups and associations need to educate patients of all risks associated with hyperkalemia and RAASi down-titration, and should encourage patient and caregiver education. Scientific societies need to be a reliable partner of the above-mentioned stakeholders and encourage a patient-centric approach to hyperkalemia management. Finally, further research is needed, including head-to-head comparisons of individual potassium binders and real-world clinical studies of patients who are treated according to the evidence-based recommendations, such as the TRACK study (ClinicalTrials.gov identifier: NCT05408039), which aims to increase understanding of hyperkalemia management, treatment models and decision making for the management of hyperkalemia.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, Ceconi C, Coats AJS, Drexel H, Filippatos G, Kaski JC, Lund L, Niessner A, Ponikowski P, Savarese G, Schmidt TA, Seferovic P, Wassmann S, Walther T, Lewis BS (2018) Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother 4:180–188. https://doi.org/10.1093/ehjcvp/pvy015
Palmer BF, Carrero JJ, Clegg DJ, Colbert GB, Emmett M, Fishbane S, Hain DJ, Lerma E, Onuigbo M, Rastogi A, Roger SD, Spinowitz BS, Weir MR (2021) Clinical management of hyperkalemia. Mayo Clin Proc 96:744–762. https://doi.org/10.1016/j.mayocp.2020.06.014
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group (2020) 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 41:255–323. https://doi.org/10.1093/eurheartj/ehz486
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, ESC Scientific Document Group (2020) 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 41:407–477. https://doi.org/10.1093/eurheartj/ehz425
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726. https://doi.org/10.1093/eurheartj/ehab368
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B, ESC National Cardiac Societies, ESC Scientific Document Group (2021) 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 42:3227–3337. https://doi.org/10.1093/eurheartj/ehab484
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39:3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group (2021) KDIGO 2021 Clinical Practice Guideline for the management of blood pressure in chronic kidney disease. Kidney Int 99:S1–S87. https://doi.org/10.1016/j.kint.2020.11.003
Kidney Disease: Improving Global Outcomes Diabetes Work Group (2022) KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int 102:S1–S127. https://doi.org/10.1016/j.kint.2022.06.008
Alfonzo A, Harrison A, Baines R, Chu A, Mann S, MacRury M. UK Kidney Association (2020) Renal association clinical practice guidelines: treatment of acute hyperkalaemia in adults. https://ukkidney.org/health-professionals/guidelines/treatment-acute-hyperkalaemia-adults. Accessed 13 Oct 2022
American Heart Association (2005) Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10.1: life-threatening electrolyte abnormalities. Circulation 112:IV121–IV125. https://doi.org/10.1161/CIRCULATIONAHA.105.166563
Howlett JG, Chan M, Ezekowitz JA, Harkness K, Heckman GA, Kouz S, Leblanc MH, Moe GW, O’Meara E, Abrams H, Ducharme A, Grzeslo A, Hamilton PG, Koshman SL, Lepage S, McDonald M, McKelvie R, Rajda M, Swiggum E, Virani S, Zieroth S, Canadian Cardiovascular Society Heart Failure Guidelines Panels (2016) The Canadian Cardiovascular Society heart failure companion: bridging guidelines to your practice. Can J Cardiol 32:296–310. https://doi.org/10.1016/j.cjca.2015.06.019
National Kidney Foundation (2014) Clinical update on hyperkalemia. https://www.kidney.org/sites/default/files/02-10-6785_HBE_Hyperkalemia_Bulletin.pdf. Accessed 13 Oct 2022
Bianchi S, Aucella F, De Nicola L, Genovesi S, Paoletti E, Regolisti G (2019) Management of hyperkalemia in patients with kidney disease: a position paper endorsed by the Italian Society of Nephrology. J Nephrol 32:499–516. https://doi.org/10.1007/s40620-019-00617-y
Nilsson E, Gasparini A, Ärnlöv J, Xu H, Henriksson KM, Coresh J, Grams ME, Carrero JJ (2017) Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 245:277–284. https://doi.org/10.1016/j.ijcard.2017.07.035
Rossignol P, Lainscak M, Crespo-Leiro MG, Laroche C, Piepoli MF, Filippatos G, Rosano GMC, Savarese G, Anker SD, Seferovic PM, Ruschitzka F, Coats AJS, Mebazaa A, McDonagh T, Sahuquillo A, Penco M, Maggioni AP, Lund LH, Heart Failure Long-Term Registry Investigators Group (2020) Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur J Heart Fail 22:1378–1389. https://doi.org/10.1002/ejhf.1793
Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC (2009) The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 169:1156–1162. https://doi.org/10.1001/archinternmed.2009.132
Sarafidis PA, Blacklock R, Wood E, Rumjon A, Simmonds S, Fletcher-Rogers J, Ariyanayagam R, Al-Yassin A, Sharpe C, Vinen K (2012) Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol 7:1234–1241. https://doi.org/10.2215/CJN.01150112
Chomicki J, Klem P, Marrs J (2014) Evaluation of the incidence of hyperkalemia in patients prescribed spironolactone for the treatment of resistant hypertension. J Am Assoc Hypertens 8:e30–e31. https://doi.org/10.1016/j.jash.2014.03.056
Khosla N, Kalaitzidis R, Bakris GL (2009) Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am J Nephrol 30:418–424. https://doi.org/10.1159/000237742
Luo X, Xu J, Zhou S, Xue C, Chen Z, Mao Z (2023) Influence of SGLT2i and RAASi and their combination on risk of hyperkalemia in DKD: a network meta-analysis. Clin J Am Soc Nephrol. https://doi.org/10.2215/CJN.0000000000000205
Kovesdy CP (2014) Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol 10:653–662. https://doi.org/10.1038/nrneph.2014.168
Neuen BL, Oshima M, Perkovic V, Agarwal R, Arnott C, Bakris G, Cannon CP, Charytan DM, Edwards R, Gorriz JL, Jardine MJ, Levin A, Neal B, De Nicola L, Pollock C, Rosenthal N, Wheeler DC, Mahaffey KW, Heerspink HJL (2021) Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J 42:4891–4901. https://doi.org/10.1093/eurheartj/ehab497
Thomsen RW, Nicolaisen SK, Hasvold P, Garcia-Sanchez R, Pedersen L, Adelborg K, Egfjord M, Egstrup K, Sørensen HT (2018) Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc 7:e008912. https://doi.org/10.1161/JAHA.118.008912
Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, Egstrup K, Egfjord M, Sørensen HT (2018) Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrol Dial Transplant 33:1610–1620. https://doi.org/10.1093/ndt/gfx312
Rosati E, D’Ambrosio V, Baccaro R, Bargagli M, Ferraro PM (2022) Rates of hyperkalemia and management patterns in a tertiary outpatient renal clinic. Nephrol Dial Transplant 37:i349–i351
Luo J, Brunelli SM, Jensen DE, Yang A (2016) Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 11:90–100. https://doi.org/10.2215/CJN.01730215
Collins AJ, Pitt B, Reaven N, Funk S, McGaughey K, Wilson D, Bushinsky DA (2017) Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 46:213–221. https://doi.org/10.1159/000479802
Aldahl M, Jensen AC, Davidsen L, Eriksen MA, Møller Hansen S, Nielsen BJ, Krogager ML, Køber L, Torp-Pedersen C, Søgaard P (2017) Associations of serum potassium levels with mortality in chronic heart failure patients. Eur Heart J 38:2890–2896. https://doi.org/10.1093/eurheartj/ehx460
Furuland H, McEwan P, Evans M, Linde C, Ayoubkhani D, Bakhai A, Palaka E, Bennett H, Qin L (2018) Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol 19:211. https://doi.org/10.1186/s12882-018-1007-1
Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP (2012) Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract 120:c8-16. https://doi.org/10.1159/000329511
de Rooij ENM, de Fijter JW, Le Cessie S, Hoorn EJ, Jager KJ, Chesnaye NC, Evans M, Windahl K, Caskey FJ, Torino C, Szymczak M, Drechsler C, Wanner C, Dekker FW, Hoogeveen EK, Investigators ES (2023) Serum potassium and risk of death or kidney replacement therapy in older people with CKD stages 4–5: eight-year follow-up. Am J Kidney Dis. https://doi.org/10.1053/j.ajkd.2023.03.008
Provenzano M, De Francesco M, Iannazzo S, Garofalo C, Andreucci M, Genualdo R, Borrelli S, Minutolo R, Conte G, De Nicola L (2020) Cost-analysis of persistent hyperkalaemia in non-dialysis chronic kidney disease patients under nephrology care in Italy. Int J Clin Pract 74:e13475. https://doi.org/10.1111/ijcp.13475
Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM (2017) Serum potassium and short-term clinical outcomes among hemodialysis patients: impact of the long interdialytic interval. Am J Kidney Dis 70:21–29. https://doi.org/10.1053/j.ajkd.2016.10.024
Ferraro PM, Bolignano D, Aucella F, Brunori G, Gesualdo L, Limido A, Locatelli F, Nordio M, Postorino M, Pecoits-Filho R, Karaboyas A (2022) Hyperkalemia excursions and risk of mortality and hospitalizations in hemodialysis patients: results from DOPPS-Italy. J Nephrol 35:707–709. https://doi.org/10.1007/s40620-021-01209-5
Torlén K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R (2012) Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol 7:1272–1284. https://doi.org/10.2215/CJN.00960112
Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G (2006) Characteristics of sudden death in hemodialysis patients. Kidney Int 69:2268–2273. https://doi.org/10.1038/sj.ki.5000446
Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, Vincenti A (2009) Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant 24:2529–2536. https://doi.org/10.1093/ndt/gfp104
Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J (2015) Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 21:S212-220
Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Butler J (2019) Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol 73:2365–2383. https://doi.org/10.1016/j.jacc.2019.02.015
Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, Fonarow GC, Vaduganathan M, Coats AJS (2021) Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail 23:1499–1511. https://doi.org/10.1002/ejhf.2271
Kobayashi M, Voors AA, Ouwerkerk W, Duarte K, Girerd N, Rossignol P, Metra M, Lang CC, Ng LL, Filippatos G, Dickstein K, van Veldhuisen DJ, Zannad F, Ferreira JP (2021) Perceived risk profile and treatment optimization in heart failure: an analysis from BIOlogy Study to TAilored Treatment in chronic heart failure. Clin Cardiol 44:780–788. https://doi.org/10.1002/clc.23576
Riccio E, Capuano I, Buonanno P, Andreucci M, Provenzano M, Amicone M, Rizzo M, Pisani A (2022) RAAS inhibitor prescription and hyperkalemia event in patients with chronic kidney disease: a single-center retrospective study. Front Cardiovasc Med 9:824095. https://doi.org/10.3389/fcvm.2022.824095
Gulizia MM, Orso F, Mortara A, Lucci D, Aspromonte N, De Luca L, Di Tano G, Leonardi G, Navazio A, Pulignano G, Colivicchi F, Di Lenarda A, Oliva F (2022) BLITZ-HF: a nationwide initiative to evaluate and improve adherence to acute and chronic heart failure guidelines. Eur J Heart Fail 24:2078–2089. https://doi.org/10.1002/ejhf.2605
Leon SJ, Whitlock R, Rigatto C, Komenda P, Bohm C, Sucha E, Bota SE, Tuna M, Collister D, Sood M, Tangri N (2022) Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis 80:164-173.e161. https://doi.org/10.1053/j.ajkd.2022.01.002
Lisi F, Parisi G, Gioia MI, Amato L, Bellino MC, Grande D, Massari F, Caldarola P, Ciccone MM, Iacoviello M (2020) Mineralcorticoid receptor antagonist withdrawal for hyperkalemia and mortality in patients with heart failure. Cardiorenal Med 10:145–153. https://doi.org/10.1159/000505286
Vardeny O, Claggett B, Anand I, Rossignol P, Desai AS, Zannad F, Pitt B, Solomon SD, Randomized Aldactone Evaluation Study (RALES) Investigators (2014) Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail 7:573–579. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001104
Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA (2004) Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 351:543–551. https://doi.org/10.1056/NEJMoa040135
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW (2022) 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 79:e263–e421. https://doi.org/10.1016/j.jacc.2021.12.012
Burton JO, Coats AJS, Kovesdy CP, Palmer BF, Piña IL, Rosano G, Sood MM, Zieroth S (2022) An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum. Eur J Heart Fail 24:1467–1477. https://doi.org/10.1002/ejhf.2612
Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD, Teta D, Yee-Moon Wang A, Cuppari L (2020) KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 76:S1–S107. https://doi.org/10.1053/j.ajkd.2020.05.006
Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R, Participants C (2020) Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 97:42–61. https://doi.org/10.1016/j.kint.2019.09.018
Clegg DJ, Cody M, Palmer BF (2017) Challenges in treating cardiovascular disease: restricting sodium and managing hyperkalemia. Mayo Clin Proc 92:1248–1260. https://doi.org/10.1016/j.mayocp.2017.04.006
Saglimbene VM, Wong G, Ruospo M, Palmer SC, Garcia-Larsen V, Natale P, Teixeira-Pinto A, Campbell KL, Carrero JJ, Stenvinkel P, Gargano L, Murgo AM, Johnson DW, Tonelli M, Gelfman R, Celia E, Ecder T, Bernat AG, Del Castillo D, Timofte D, Török M, Bednarek-Skublewska A, Duława J, Stroumza P, Hoischen S, Hansis M, Fabricius E, Felaco P, Wollheim C, Hegbrant J, Craig JC, Strippoli GFM (2019) Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 14:250–260. https://doi.org/10.2215/cjn.08580718
Banerjee T, Carrero JJ, McCulloch C, Burrows NR, Siegel KR, Morgenstern H, Saran R, Powe NR (2021) Dietary factors and prevention: risk of end-stage kidney disease by fruit and vegetable consumption. Am J Nephrol 52:356–367. https://doi.org/10.1159/000514754
Morris A, Lycett D (2020) Experiences of the dietary management of serum potassium in chronic kidney disease: interviews with UK adults on maintenance hemodialysis. J Ren Nutr 30:556–560. https://doi.org/10.1053/j.jrn.2020.01.025
Natale P, Palmer SC, Ruospo M, Saglimbene VM, Strippoli GF (2020) Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst Rev 6:CD013165. https://doi.org/10.1002/14651858.CD013165.pub2
Nasir K, Ahmad A (2014) Treatment of hyperkalemia in patients with chronic kidney disease: a comparison of calcium polystyrene sulphonate and sodium polystyrene sulphonate. J Ayub Med Coll Abbottabad 26:455–458
Yu MY, Yeo JH, Park JS, Lee CH, Kim GH (2017) Long-term efficacy of oral calcium polystyrene sulfonate for hyperkalemia in CKD patients. PLoS One 12:e0173542. https://doi.org/10.1371/journal.pone.0173542
Lepage L, Dufour AC, Doiron J, Handfield K, Desforges K, Bell R, Vallée M, Savoie M, Perreault S, Laurin LP, Pichette V, Lafrance JP (2015) Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol 10:2136–2142. https://doi.org/10.2215/CJN.03640415
Laureati P, Xu Y, Trevisan M, Schalin L, Mariani I, Bellocco R, Sood MM, Barany P, Sjölander A, Evans M, Carrero JJ (2020) Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 35:1518–1526. https://doi.org/10.1093/ndt/gfz150
Noel JA, Bota SE, Petrcich W, Garg AX, Carrero JJ, Harel Z, Tangri N, Clark EG, Komenda P, Sood MM (2019) Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 179:1025–1033. https://doi.org/10.1001/jamainternmed.2019.0631
Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, Madsen D, Benton WW, Berman L, Buysse J (2016) Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther 21:456–465. https://doi.org/10.1177/1074248416629549
Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS (2014) Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One 9:e114686. https://doi.org/10.1371/journal.pone.0114686
European Medicines Agency (2022) Lokelma (soidum zirconium cyclosilicate): summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/lokelma-epar-product-information_en.pdf. Accessed 23 Oct 2022
Meaney CJ, Beccari MV, Yang Y, Zhao J (2017) Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 37:401–411. https://doi.org/10.1002/phar.1906
European Medicines Agency (2022) Veltassa (patiromer soirbitex calcium): summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/veltassa-epar-product-information_en.pdf. Accessed 23 Oct 2022
Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B (2019) Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 394:1540–1550. https://doi.org/10.1016/S0140-6736(19)32135-X
Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA, Investigators AMETHYST-DN (2015) Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA 314:151–161. https://doi.org/10.1001/jama.2015.7446
Buysse JM, Huang IZ, Pitt B (2012) PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol 8:17–28. https://doi.org/10.2217/fca.11.71
Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, Staroselskiy K, Vishnevskiy K, Lisovskaja V, Al-Shurbaji A, Guzman N, Bhandari S (2019) A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol 30:1723–1733. https://doi.org/10.1681/ASN.2019050450
Fishbane S, Ford M, Fukagawa M, McCafferty K, Rastogi A, Spinowitz B, Staroselskiy K, Vishnevskiy K, Lisovskaja V, Al-Shurbaji A, Guzman N, Bhandari S (2022) Potassium responses to sodium zirconium cyclosilicate in hyperkalemic hemodialysis patients: post-hoc analysis of DIALIZE. BMC Nephrol 23:59. https://doi.org/10.1186/s12882-021-02569-7
Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, Roger SD, Yang A, Lerma E, Singh B (2014) Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA 312:2223–2233. https://doi.org/10.1001/jama.2014.15688
Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B (2015) Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 372:222–231. https://doi.org/10.1056/NEJMoa1411487
Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ, Investigators PEARL-HF (2011) Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 32:820–828. https://doi.org/10.1093/eurheartj/ehq502
Roger SD, Spinowitz BS, Lerma EV, Singh B, Packham DK, Al-Shurbaji A, Kosiborod M (2019) Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol 50:473–480. https://doi.org/10.1159/000504078
Spinowitz BS, Fishbane S, Pergola PE, Roger SD, Lerma EV, Butler J, von Haehling S, Adler SH, Zhao J, Singh B, Lavin PT, McCullough PA, Kosiborod M, Packham DK, ZS-005 Study Investigators (2019) Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol 14:798–809. https://doi.org/10.2215/CJN.12651018
Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B, Investigators OPAL-HK (2015) Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372:211–221. https://doi.org/10.1056/NEJMoa1410853
Zannad F, Hsu BG, Maeda Y, Shin SK, Vishneva EM, Rensfeldt M, Eklund S, Zhao J (2020) Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail 7:54–64. https://doi.org/10.1002/ehf2.12561
Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ, Friede T, Fabien V, Kosiborod M, Metra M, Piña IL, Pinto F, Rossignol P, van der Meer P, Bahit C, Belohlavek J, Bohm M, Brugts JJ, Cleland JG, Ezekowitz J, Bayes-Genis A, Gotsman I, Goudev A, Khintibidze I, Lindenfeld J, Mentz RJ, Merkely B, Montes EC, Mullens W, Nicolau JC, Parkhomenko A, Ponikowski P, Seferovic PM, Senni M, Shlyakhto E, Cohen-Solal A, Szecsödy P, Jensen K, Dorigotti F, Weir MR, Pitt B (2022) Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J 43:4362–4373. https://doi.org/10.1093/eurheartj/ehac401
Roger SD, Lavin PT, Lerma EV, McCullough PA, Butler J, Spinowitz BS, von Haehling S, Kosiborod M, Zhao J, Fishbane S, Packham DK (2021) Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: comparative results from an open-label, phase 3 study. Nephrol Dial Transplant 36:137–150. https://doi.org/10.1093/ndt/gfz285
Hoy SM (2018) Sodium zirconium cyclosilicate: a review in hyperkalaemia. Drugs 78:1605–1613. https://doi.org/10.1007/s40265-018-0991-6
Official Journal of the Italian Republic. Number 216, 9 September 2021. https://www.gazzettaufficiale.it/do/atto/serie_generale/caricaPdf?cdimg=21A0528900100010110001&dgu=2021-09-09&art.dataPubblicazioneGazzetta=2021-09-09&art.codiceRedazionale=21A05289&art.num=1&art.tiposerie=SG. Accessed 31 July 2023
Provenzano M, Puchades MJ, Garofalo C, Jongs N, D’Marco L, Andreucci M, De Nicola L, Gorriz JL, Heerspink HJL (2022) Albuminuria-lowering effect of dapagliflozin, eplerenone, and their combination in patients with chronic kidney disease: a randomized crossover clinical trial. J Am Soc Nephrol 33:1569–1580. https://doi.org/10.1681/asn.2022020207
Bansal S, Pergola PE (2020) Current management of hyperkalemia in patients on dialysis. Kidney Int Rep 5:779–789. https://doi.org/10.1016/j.ekir.2020.02.1028
Kalantar-Zadeh K, Fouque D (2017) Nutritional management of chronic kidney disease. N Engl J Med 377:1765–1776. https://doi.org/10.1056/NEJMra1700312
De Nicola L, Garofalo C, Borrelli S, Minutolo R (2022) Recommendations on nutritional intake of potassium in CKD: it’s now time to be more flexible! Kidney Int 102:700–703. https://doi.org/10.1016/j.kint.2022.04.046
Acknowledgements
We would like to thank Catherine Rees who wrote the outline of this manuscript on behalf of Springer Healthcare Communications, and Mitali Choudhury, Ph.D., of Springer Healthcare Communications who wrote the first draft. This editorial support was funded by AstraZeneca.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This project was supported by AstraZeneca.
Author information
Authors and Affiliations
Contributions
Conceptualization: all the authors; writing—review and editing: all the authors. All the authors have approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
Luca De Nicola has received consultancy or lecture fees from Astellas, AstraZeneca, Novo Nordisk, and CSL Vifor. Pietro Manuel Ferraro has received research grants, consultancy fees, or other support from Allena Pharmaceuticals, Alnylam, Amgen, AstraZeneca, BioHealth Italia, Gilead, Otsuka Pharmaceuticals, Rocchetta, and CSL Vifor; and royalties as an author from UpToDate. Andrea Montagnani has received consultancy or lecture fees from BMS, Pfizer, Bayer, Boehringer Ingelheim, AstraZeneca, and CSL Vifor. Roberto Pontremoli has received consultancy or lecture fees from AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Novartis, Menarini, Bayer, Recordati International, Alfasigma, Novo Nordisk, and CSL Vifor. Giorgio Sesti has received speaker fees from Novo Nordisk, Eli Lilly, AstraZeneca, Teva, MSD, Sanofi, Daiichi Sankyo, Sobi, Janssen, and Servier. Francesco Dentali declares no conflicts of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luca De Nicola and Pietro Manuel Ferraro: on behalf of Società Italiana di Nefrologia (SIN).
Roberto Pontremoli and Giorgio Sesti: on behalf of Società Italiana di Medicina Interna (SIMI).
Andrea Montagnani and Francesco Dentali: on behalf of Federazione delle Associazioni dei Dirigenti Ospedalieri Internisti (FADOI).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Nicola, L., Ferraro, P.M., Montagnani, A. et al. Recommendations for the management of hyperkalemia in patients receiving renin–angiotensin–aldosterone system inhibitors. Intern Emerg Med 19, 295–306 (2024). https://doi.org/10.1007/s11739-023-03427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-023-03427-0