Abstract
Biostimulants, including phytohormones, are of high agricultural importance. Thus far, several methods have been developed for phytohormone detection/determination in microalgal cultures. Most of them require expensive, state-of-the-art machinery which often has limited availability in developing, agricultural countries. The main goal of our study was to develop a cheap and straightforward method for brassinosteroid determination in microalgal cultures. We used a Klebsormidium strain whose genus members have reportedly high brassinosteroid content. Using brassinolide standard, we compared the response of four different rice cultivars in a rice lamina inclination bioassay (RLIA), and from these found the variety ‘Koshihikari’ the most suitable one. A dynamic response over a broad concentration range from 0.001 to 0.1 mg/L brassinolide concentration was observed. Attempts with commonly used mechanical methods for disrupting Klebsormidium cells resulted in only negligible brassinolide release, while methanolic extraction liberated almost all cellular brassinosteroids. To overcome the negative effect of methanol on rice lamina inclination, solid-phase extraction was applied to get rid of methanol from the assay. The estimated brassinolide concentration in Klebsormidium culture by RLIA was validated using ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS).
Similar content being viewed by others
Introduction
Plant hormones (phytohormones) are signal molecules, which, among other functions, play essential roles in plant growth regulation and plant responses to environmental stresses (Kapoore et al. 2021). Based on the (positive) impact of these chemically diverse biomolecules on various aspects of plant development, they are utilized in agriculture as biostimulants to improve crop quality and yield (Dhaubhadel et al. 1999; Hayat et al. 2012; Vriet et al. 2012; Ali 2019). Nowadays, as the demand for green agricultural practices is constantly growing, the importance of biostimulants has also been increasing.
Microalgal cultures have been used in agriculture for a very long time (Gonçalves 2021). Acutodesmus dimorphus, Calothrix elenkinii, Chlorella ellipsoidea, Chlorella infusionum, Chlorella vulgaris, Dunaliella salina, Scenedesmus quadricauda, and Spirulina maxima are the most commonly used microalgae as biostimulant (Ronga et al. 2019). Up to date, numerous studies reported the plant-growth promoting effects of microalgal cultures with special emphasis on their auxin, cytokinin and gibberellin content (Ördög et al. 2004; Jirásková et al. 2009; Hussain et al. 2010; Stirk et al. 2002, 2011, 2013b; Noble et al. 2014). In contrast, much less data are available on steroidal plant hormones, the so-called brassinostreoids (BRs) in microalgal cultures (Yokota 1987; Stirk et al. 2013a, 2014, 2018).
BRs encompass a relatively new class of phytohormones, essential for the proper regulation of multiple physiological processes related to plant growth, development, and stress tolerance (Hayat and Ahmad 2011). Due to these reasons, BRs are also of high agricultural interest. The first BR compound was isolated from the pollen of rapeseed, Brassica napus, which is reflected in its name, brassinolide (BL) (Grove et al. 1979). BRs currently include about 70 different polyhydroxylated sterol derivatives (Oklestkova et al. 2015), from which BL and castasterone (CS) widely occur in microalgae (Bajguz 2009; Bajguz and Piotrowska-Niczyporuk 2013; Stirk et al. 2013a; Kanwar et al. 2017). Regarding these two common steroid substances, BL is characterized not only by up to 60% higher cellular concentration but also shows about five-fold higher biological activities compared to CS (see Stirk et al. 2013a and Fujioka et al. 1995, respectively).
Cultures of Klebsormidium flaccidum have remarkably high BR content compared to other microalgal strains according to Stirk and co-workers (Stirk et al. 2013a). Hence, this microorganism can be considered as potential biostimulant as well as candidate for industrial-scale biotic BR production. K. flaccidum is an aeroterrestrial filamentous green microalga belonging to the Charophyta phylum. It is classified as one of the most abundant biological soil crust forming microalgae, due to their broad ecophysiological tolerance (Glaser et al. 2018).
The BR content of microalgal cultures is usually determined using sensitive bioanalytical methods such as ultra-high performance liquid chromatography coupled with tandem mass spectrometry UHPLC-MS/MS (Stirk et al. 2013a, 2014). Nonetheless, bioassays are also commonly used for determining the biological activity of BRs (Takatsuto et al. 1989; Joo et al. 2015; Roh et al. 2020).
The major benefit of using such methods compared to sophisticated analytical approaches, typically requiring costly instrumentation and maintenance, is that bioassays provide easy and cheap alternatives for phytohormone content determination. Different bioassays have already been developed for various plant hormones. For instance, cytokinin- and auxin-like activities can be determined using a cucumber cotyledon bioassay (Fletcher et al. 1982; Zhao et al. 1992), the content of abscisic acids can be estimated using a wheat coleoptile straight growth bioassay (Zhao et al. 1992), while the concentration of BRs can be assessed using bean second internode (Mandava 1988) and rice lamina inclination bioassays (RLIA) (Han et al. 1997). The RLIA is the most commonly used biotest for BR detection due its specificity and sensitivity (Soto et al. 2021). No bioassay has yet been used to determine the BR content of microalgae.
The purpose of this work is to provide a cheap and straightforward alternative for determining the approximate level of BRs in microalgal cultures without employing expensive analytical instrumentation. Using BL, the most abundant and biologically most active BR, we exploited RLIA to give a rough estimation of the biologically active, total BR content of Klebsormidium cultures. The results were validated using UHPLC-MS/MS.
Materials and methods
Strain and culture conditions
In our work, Klebsormidium sp. BEA_IDA_0061B algae strain–deposited in the Spanish Bank of Algae (BEA)–was examined (Kutasi et al. 2018). This filamentous green alga was collected from a wet, artificially lit cave rock surface by the staff of Albitech Biotechnology Ltd. (Budapest, Hungary). The isolated algal sample was purified by consecutive plating on modified BBM (Bold Basal Medium) agar plate (Starr and Zeikus 1993). Taxonomic classification of axenic culture was performed using a standard sequencing protocol (based on nucleotide 816 of 18S rRNA) as well as based on macroscopic and microscopic markers.
Klebsormidium sp. BEA_IDA_0061B monocultures for the experiments were grown in BBM media in an aerated cylindrical photobioreactor (0.3 vvm) of a volume of 9 L at 22 °C. The applied light intensity was 20 μmol photon m−2 s−1 (36 W, natural white LED, V-TAC, Szeged, Hungary) during a 10-day cultivation period.
Rice cultivars
Three japonica rice varieties, ‘Arsenal’, ‘Hoshinoyume’, and ‘M 60’ were selected from the Rice Germplasm Collection of the Research Center for Irrigation and Water Management, Hungarian University of Agriculture and Life Sciences (Szarvas, Hungary). All these varieties grow efficiently under usual climate conditions in Hungary (Székely et al. 2022b) and were used to obtain comparative results with the generally used ‘Koshihikari’ cultivar. ‘Hoshinoyume’ was registered in Japan in 1996 (Shinbashi et al. 2003). The variety was derived from the crossing between ‘Akitakomachi’/‘Douhoku48’ and ‘Kirara397’. The growth duration of the variety is short (130–135 days), and it has good cold tolerance, but it is sensitive to blast disease. ‘M 60’ was registered in Hungary in 2002. The origin of the variety is similar to two important Hungarian rice varieties, ‘M 488’ and ‘M 225 (Simon-Kiss 2001). It was selected from the crossing between ‘Balilla’ and ‘H9 mutant’. It has short growth duration (128–134 days), intermediate cold tolerance and blast disease resistance. The Italian japonica rice variety, ‘Arsenal’ was registered in 2008 (Nghi et al. 2021). Growth duration of the variety is medium-long in Hungary (140–144 days) and short in Italy (130 days). It is tolerant to blast disease, but has a moderate cold tolerance (Székely et al. 2022a).
Rice lamina inclination bioassay
For brassinosteroid level determination, the method of Han et al. (1997) was modified as follows. The rice seeds used in the experiments were first soaked in tap water for 48 h and germinated on 1% agar in darkness at 30 °C. After 7 days of growth, rice plants with identical heights (cca 6.0–8.0 cm) were selected. From these, 4 cm long segments from the shoot tips, containing the second leaf lamina were cut. The excised segments were kept in distilled water for 24 h at 30 °C, and then the equally inclined ones (with a cca 10°–20° inclination degree) were selected. During the treatment, about 10 selected segments were placed in a Petri-dish filled with 20 mL of aquatic solution either with known or unknown BR content. After 48 h of incubation at 30 °C in darkness the angle (α) between the second leaf lamina and the stem was determined (Figs. 1, 2.) using the open source computer software ImageJ (https://imagej.nih.gov/ij/). After germination, all operations (except the measurement) were performed under dim red light to prevent any undesired inclination of the rice lamina.
Chemicals
The BL standard and 3-(dansylamino)phenylboronic acid used in the study were obtained from the Merck Group (Darmstadt, Germany). All other chemicals used were of analytical grade. During the experiments, we used 1 mg BL/mL methanolic stock solution of BL.
Sample preparation
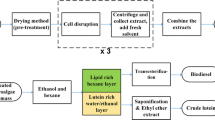
18 L of Klebsormidium sp. BEA_IDA_0061B (hereafter Klebsormidium) cultures were used during the sample preparation (Fig. 2). The algae cultures were centrifuged (4500 rpm, 10 min) and the supernatant was discarded. The pellet was extracted with fourfold volumes of ice-cold 80% methanol at 5 °C overnight. After incubation, the extraction mixture was centrifuged (4500 rpm, 10 min), and the separated supernatant was stored in a refrigerator. Then, extraction and spinning down were repeated. The combined supernatant was purified using an EVOLUTE ABN™ (610-0010-H, Biotage, Uppsala, Sweden) solid-phase extraction (SPE) column. The eluate obtained was evaporated to dryness with a rotary vacuum evaporator (Rotavapor R-114, Büchi Flawil, Switzerland) and was finally dissolved in water. The final volume was 20 mL. Prior to analytical measurements the samples were derivatized with 3-(dansylamino)phenylboronic acid acc. to (Gamoh et. al 1990).
UHPLC-MS/MS analysis
UHPLC-MS/MS analysis was performed using an analytical HPLC with high-resolution Orbitrap mass spectrometry: a Dionex Ultimate3000 UHPLC system (TCC-3000RS column thermostat, HPG-3400RS pump, SRD-3400 solventrack degasser, WPS-3000TRS autosampler) connected to an Orbitrap Q Exactive Focus Mass Spectrometer equipped with an electrospray ionization (ESI) source (Thermo Fisher Scientific, Waltham, MA, USA). Column: Kinetex C18 column (75 × 3 mm; 2.6 μm) (Phenomenex,Torrance, CA, USA). Eluents: eluent A, 0.1% v/v formic acid, eluent B, acetonitrile: 0.1% v/v formic acid (80:20, v/v). Gradient program: 0.0 min, 80% B; 5.0 min, 100% B (linear gradient); 10.0 min, 100% B (isocratic); flow rate: 0.3 mL/min; column temperature: 25 °C; injected volume: 1.0–10.0 μL. The ESI source was operated in the positive mode and operation parameters were optimized automatically using the built-in software. Working parameters: spray voltage, 3500 V ( +); capillary temperature 256 °C; sheath-, auxiliary-, and spare-gases (N2): 47.50, 11.25, and 2.25 arbitrary units, respectively. MS/MS (MS2) scans were acquired at a resolution of 70,000 using a collision energy of 50 eV; precursor ion: m/z 815, product ion: 174 (ion intensity of this product ion was used in the quantitation).
Statistical analyses
Multiway ANOVA was performed to describe whether the observed rice lamina inclination is related to the applied BR contents and/or rice type. Tukey’s post hoc tests were conducted to compare the pairwise differences among the tested cultivars. Welch tests were used to examine the differences between the degree of rice lamina inclination at different BR concentrations. All statistical analyses were performed in the 4.2.1 version of R statistical software (R Core Team 2021) using the vegan package (Oksanen et al. 2022).
Results
Selection of a suitable rice cultivar for rice lamina inclination bioassay
First, we compared the usability of four rice cultivars, all available and successfully cultivated in Hungary (see Materials and Methods), including the globally cultivated rice variety Koshihikari, in RLIA, by treating them with a BL standard at a final concentration of either 0.1 or 1 mg/L. According to multiway ANOVA, rice lamina inclination was significantly affected by the BR content (p < 0.001), as well as by the rice type (p < 0.001). Each tested rice cultivar responded positively (i.e., showed a much larger degree of inclination compared to the corresponding control) for the BL treatment, yet, to various extent. The response of the cultivars ‘Koshihikari’, ‘M 60’, and ‘Arsenal’ was much more pronounced than that of the cultivar ‘Hoshinoyume’ even at 1.0 mg/L BL concentration (Table 1). As the cultivar ‘M 60’ apparently showed the maximal possible degree of inclination (i.e., 180°) even at the expected highest BL level in concentrated Klebsormidium cultures, we also excluded this variety from further studies. From the remaining two cultivars, the variety ‘Koshihikari’ provided much more reproducible results and showed remarkably higher seed germination efficiency (data not shown). Putting these all together, we have chosen the cultivar ‘Koshihikari’ for further studies, including the detailed examination of the BL concentration dependence of the lamina inclination.
Determination of the sensitivity of RLIA
The inclination response of the rice cultivar ‘Koshihikari’ to BL treatment was examined over a broad concentration range, i.e., from 0.0001 to 0.1 mg/L (Fig. 3). Although some hormonal response (a 7° increase in the degree of declination) was already triggered by a BL concentration of as low as 0.0001 mg/L, statistical analysis showed this change insignificant (p > 0.05). In contrast, from 0.001 mg/L BL concentrations, the presence of BL in the assay induced significant increase in the degree of rice lamina inclination (p < 0.05). Applying 0.001, 0.01 and 0.1 mg/L BL in RLIA resulted in 17°, 50°, and 78° degree enhancement of the degree of rice lamina inclination, respectively (Fig. 3).
BL concentration dependence of the inclination degree of rice lamina isolated from the rice cultivar “Koshihikari” (n = 9). For comparison, the inclination degree of untreated controls, as well as of rice lamina treated with intact Klebsormidium culture (Kb) is also shown. Letters above the columns refer the results of the Welch tests (groups with the same letters are not different, whereas groups with different letters differ significantly)
Estimation of the BL content in Klebsormidium using RLIA
Using intact (i.e., unbroken) Klebsormidium cells in RLIA did not induce any increase in the degree of rice lamina inclination, which suggests that brassinosteroid content of the Klebsormidium cultures are mainly localized intracellular (Fig. 3). Based on this observation, to liberate the BR content of the Klebsormidium cells, we tested various cell disruption as well as hormone extraction protocols. The former included various (relatively mild) attempts to break the cell wall, i.e., by sonication, shearing with a hand blender, and autoclaving; however, none of these efforts yielded any positive results (data not shown). Regarding chemical methods, extraction using methanol as organic solvent provided the highest apparent extraction rate. However, mutual application of BL standards and methanol revealed that this solvent has a negative effect on rice lamina inclination, which has to be taken into account at analyzing the data. As shown in Fig. 4, presence of 5% methanol in the assay, which is the expected methanol percentage after the extraction procedure, results in an about 34% mean decrease in the degree of rice lamina inclination (p < 0.001 in the control, p = 0.048 at 0.001 mg/L, p < 0.001 at 0.01 mg/L).
To overcome this problem, we subsequently applied a methanol-to-water solvent exchange using SPE. Using this protocol, yet it overall still results in some decrease in the degree of lamina inclination, the inclination angle was much higher in the absence of methanol compared to the case when methanol was present. Hence, we applied this approach (i.e., solvent exchange) to estimate BR content of Klebsormidium. Prior to the preparation of microalgal samples, we carefully tested our sample preparation method using BL standard in a preliminary experiment. In that experiment, we used BL dissolved in a mixture of 95% water and 5% methanol with a final concentration of 0.05 mg L−1. Initially, we divided this solution into two parts. The first part was tested directly in RLIA, while the second part was purified with SPE. The obtained eluate was evaporated to dryness with a rotary vacuum evaporator and was dissolved in pure water and eventually tested also in RLIA. No significant difference was observed in rice lamina inclination using BL standards prepared under either way.
The degree of rice lamina inclinations as a function of BL concentrations is shown in Fig. 5. This reveals a semi-logarithmic dependence of the degree of lamina inclination vs. BL concentration over a concentration range covering three orders of magnitude from 0.001 to 0.1 mg/L BL. Above the latter concentration, the increase in BL concentration did not increase the hormonal response much further, most likely due to steric reasons (notice that 180° is the maximal possible inclination). To validate the usability of this approach for estimating the BR content of Klebsormidium cultures, next we determined the BL content of some independent Klebsormidium cultures by mass spectrometry and compared these data to those obtained with RLIA. The mean BL concentration in dense extracts prepared from ordinary Klebsormidium cultures (for details, see Materials and Methods) was 14.05 ± 1.03 μg/L (n = 4). Probing the same extracts using RLIA resulted in a mean lamina inclination of 117° ± 26°. Plotting these values in Fig. 5 revealed a good agreement between the BR levels estimated either by UHPLC-MS/MS or RLIA.
Discussion
RLIA is amongst the most commonly used bioassays for estimating the biologically active, total BR content in plant materials and extracts, or simply to probe the absence or presence (above a certain threshold) of BRs in biological samples. For example, such assays were used for screening biological activity of purified extracts from floating macroalga Hydrodictyon reticulatum (Yokota et al. 1987), sunflower (Takatsuto et al. 1989), and Pinus sylvestris (Kim et al. 1990). In spite of its potential benefits, RLIA has not been used thus far for estimating/determining brassinosteroid content in microalgal cultures. Rather, such determinations were performed using UHPLC-MS/MS coupled with isotope dilution method (Stirk et al. 2014, 2018). Although that method can provide quantitative data with high accuracy, it could also have several constraints, i.e., needs expensive instrumentation and chemical agents, as well as highly skilled/qualified personnel. Our developed method proposes a cheap and simple alternative for BR determination in microalgal cultures. To our knowledge, this was the first attempt aiming to use RLIA to probe BR content of microalgae.
An essential part of our study was to select a suitable rice cultivar for RLIA. The major criteria at choosing an appropriate variety were: (i) efficient growth under Central European climate conditions; preferably widely cultivated and easily available there, (ii) good germination efficiency under laboratory conditions, and (iii) dynamic, well reproducible RLIA response over a broad, biologically relevant BR concentration range. For these tests we used BL standard since its relative sensitivity is five-fold higher in RLIA than CS, the second most biologically active BR (Fujioka et al. 1995). From the four tested rice cultivars, the cultivar “Koshihikari” fit these criteria the most. This rice variety showed a log-linear response in RLIA over the 0.001–0.1 mg/L BL concentration range. This range perfectly overlaps with the reported BR content found in microalgal extracts (Stirk et al. 2013a).
One major constraint of applying RLIA at BR determination in microalgae is their thick cell wall which makes cell disruption, and, in turn, release of cellular BR content difficult. We tried several conventional cell disruption methods, but all of these attempts were unsuccessful. As an alternative of applying mechanical cell disruption methods to make BRs available for RLIA, we also tried extracting steroid hormones from Klebsormidium by organic solvents, which approach is also commonly used at exploring hydrophobic biomolecules. From the tested organic solvents, methanol yielded the most effective BR extraction; however, it also resulted in a remarkable decrease in the degree of rice lamina inclination. This problem was overcome by methanol-to-water solvent exchange using SPE.
Importantly, the applicability of the method had to be validated. This was performed by simultaneous BR content determination in Klebsormidium cultures via RLIA and UHPLC-MS/MS. In contrast to the experiments with BL standard, whose concentration could have been varied over a very broad scale (i.e., over several orders of magnitude), the BR content of this microalga scatter over a narrow range. The analytically determined mean concentration in the extracts (with 900-fold concentrated BL levels) was 14.05 ± 1.03 μg/L BL, concurred with a 117° ± 26° degree of rice lamina inclination using RLIA (Fig. 5). This latter corresponds to 8.46 ± 1.88 μg/L BL, yet, the difference between these two concentrations is statistically insignificant. These BL concentrations are equivalent to 15.60 ± 1.14 and 9.40 ± 2.09 ng/L BL concentrations in Klebsormidium cultures. The statistically insignificant difference between the cellular BL concentrations determined by UHPLC-MS/MS (selective for BL) and RLIA (selective for all hormonally active substances) confirms both the high relative abundance of BL in Klebsormidium extracts as well as its high hormonal activity (see Stirk et al. 2013a and Fujioka et al. 1995). Conversely, it also shows that the contribution of other hormonally active substances present in the extracts to rice lamina inclination is small. These estimated BL levels in the tested Klebsormidium strain were significantly below the concentration reported for Klebsormidium flaccidum (Stirk 2013a). To find the reason for this difference in BL levels in distinct strains and optimization of Klebsormidium culturing e.g. for biotechnological purposes were beyond the scope of this work. Nevertheless, the applicability of RLIA over a broad concentration range enabled estimating the BR level even in the studied strain with relatively low BR content. Remarkably, the developed method offers a promising tool for screening microalgal strains from low to high BR content.
Naturally, the proposed method also has some limitations, which include, as was shown above, that (i) RLIA is unable to determine cellular BR concentration as precisely as analytical methods, (ii) it is arguably more time consuming, and (iii) it cannot distinguish the responses of different RLIA active compounds. This latter includes not only BRs (i.e., BL, CS, etc.) but also auxins [e.g., indole-3-acetic acid (IAA)]. IAA, can induce some rice lamina inclination compared to BRs, however, in the examined concentration range, the effect of IAA on RLIA is negligible (Wada et. al 1984; Han et. al 1997). Overall, the listed shortcomings are well compensated by beneficial features of the project, i.e., low cost, simplicity, and low infrastructural requirements, which features are of high relevance in some, e.g., agricultural, applications.
Conclusion
This work explored the use of a rice lamina inclination bioassay for determining brassinosteroid content in microalgal cultures. Our results suggest that brassinosteroid levels can fairly be estimated by the applied method which was validated using UHPLC-MS/MS. Although its accuracy is more behind that of such state-of-the-art analytical methods, RLIA provides a cheap and straightforward alternative for brassinosteroid quantification.
Data availability
We described/shared all necessary details within this paper.
References
Ali B (2019) Brassinosteroids: the promising plant growth regulators in horticulture. In: Hayat S, Yusuf M, Bhardwaj R, Bajguz A (eds) Brassinosteroids: plant growth and development. Springer, Singapore, pp 349–365
Bajguz A (2009) Isolation and characterization of brassinosteroids from algal cultures of Chlorella vulgaris Beijerinck (Trebouxiophyceae). J Plant Physiol 166:1946–1949. https://doi.org/10.1016/j.jplph.2009.05.003
Bajguz A, Piotrowska-Niczyporuk A (2013) Synergistic effect of auxins and brassinosteroids on the growth and regulation of metabolite content in the green alga Chlorella vulgaris (Trebouxiophyceae). Plant Physiol Biochem 71:290–297. https://doi.org/10.1016/j.plaphy.2013.08.003
Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P (1999) Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol 40:333–342. https://doi.org/10.1023/A:1006283015582
Fletcher RA, Kallidumbil V, Steele P (1982) An improved bioassay for cytokinins using cucumber cotyledons. Plant Physiol 69:675–677. https://doi.org/10.1104/pp.69.3.675
Fujioka S, Sakurai A, Inoue T et al (1995) Biological activities of biosynthetically-related congeners of brassinolide. Biosci Biotechnol Biochem 59:1973–1975. https://doi.org/10.1271/bbb.59.1973
Gamoh K, Okamoto N, Takatsuto S, Tejima I (1990) Determination of traces of natural brassinosteroids as dansylaminophenylboronates by liquid chromatography with fluorimetric detection. Anal Chim Acta 228:101–105. https://doi.org/10.1016/S0003-2670(00)80484-5
Glaser K, Baumann K, Leinweber P et al (2018) Algal richness in BSCs in forests under different management intensity with some implications for P cycling. Biogeosciences 15:4181–4192. https://doi.org/10.5194/bg-15-4181-2018
Gonçalves AL (2021) The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating and biopesticide roles. Appl Sci 11:1–21. https://doi.org/10.3390/app11020871
Grove MD, Spencer GF, Rohwedder WK et al (1979) Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 281:216–217. https://doi.org/10.1038/281216a0
Han K-S, Ko K-W, Nam S-J et al (1997) Optimization of a rice lamina inclination assay for detection of brassinosteroids: I. effect of phytohormones on the inclination activity. J Plant Biol 40:240–244. https://doi.org/10.1007/bf03030454
Hayat S, Ahmad A (2011) Brassinosteroids: a class of plant hormone. Springer, Dordrecht
Hayat S, Alyemeni MN, Hasan SA (2012) Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J Biol Sci 19:325–335. https://doi.org/10.1016/j.sjbs.2012.03.005
Hussain A, Krischke M, Roitsch T, Hasnain S (2010) Rapid determination of cytokinins and auxin in cyanobacteria. Curr Microbiol 61:361–369. https://doi.org/10.1007/s00284-010-9620-7
Jirásková D, Poulíčková A, Novák O et al (2009) High-throughput screening technology for monitoring phytohormone production in microalgae. J Phycol 45:108–118. https://doi.org/10.1111/j.1529-8817.2008.00615.x
Joo SH, Jang MS, Kim MK et al (2015) Biosynthetic relationship between C28-brassinosteroids and C29-brassinosteroids in rice (Oryza sativa) seedlings. Phytochem 111:84–90. https://doi.org/10.1016/j.phytochem.2014.11.006
Kanwar MK, Bajguz A, Zhou J, Bhardwaj R (2017) Analysis of brassinosteroids in plants. J Plant Growth Regul 36:1002–1030. https://doi.org/10.1007/s00344-017-9732-4
Kapoore RV, Wood EE, Llewellyn CA (2021) Algae biostimulants: a critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol Adv 49:107754
Kim SK, Abe H, Little CHA, Pharis RP (1990) Identification of two brassinosteroids from the Cambial region of scots pine (Pinus silverstris) by gas chromatography-mass spectrometry, after detection using a dwarf rice lamina inclination bioassay. Plant Physiol 94:1709–1713. https://doi.org/10.1104/pp.94.4.1709
Kutasi J., Futó P., Bencsik A., Greipel E. (2018) Algae inoculate. Hungarian Patent No. HU1800183A2
Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol 39:23–52. https://doi.org/10.1146/annurev.pp.39.060188.000323
Nghi KN, Tagliani A, Mariotti L et al (2021) Auxin is required for the long coleoptile trait in japonica rice under submergence. New Phytol 229:85–93. https://doi.org/10.1111/nph.16781
Noble A, Kisiala A, Galer A et al (2014) Euglena gracilis (Euglenophyceae) produces abscisic acid and cytokinins and responds to their exogenous application singly and in combination with other growth regulators. Eur J Phycol 49:244–254. https://doi.org/10.1080/09670262.2014.911353
Oklestkova J, Rárová L, Kvasnica M, Strnad M (2015) Brassinosteroids: synthesis and biological activities. Phytochem Rev 14:1053–1072. https://doi.org/10.1007/s11101-015-9446-9
Oksanen J, Simpson GL, Blanchet FG, et al (2022) vegan: Community Ecology Package
Ördög V, Stirk WA, Van Staden J et al (2004) Endogenous cytokinins in three genera of microalgae from the chlorophyta. J Phycol 40:88–95. https://doi.org/10.1046/j.1529-8817.2004.03046.x
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Roh J, Moon J, Youn JH et al (2020) Establishment of biosynthetic pathways to generate castasterone as the biologically active brassinosteroid in Brachypodium distachyon. J Agric Food Chem 68:3912–3923. https://doi.org/10.1021/acs.jafc.9b07963
Ronga D, Biazzi E, Parati K et al (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy 9:192. https://doi.org/10.3390/agronomy9040192
Shinbashi N, Maeda H, Kunihiro Y, Tanno H (2003) A new rice variety “Hoshinoyume.” Bull Hokkaido Prefect Agric Exp Station 1–12.
Simon-Kiss I (2001) Six decades of rice cultivation and varietal improvement in Hungary. Hungarian Agric Res 10:4–7
Soto N, Ferrer K, Díaz K et al (2021) Synthesis and biological activity of new brassinosteroid analogs of type 24-Nor-5β-cholane and 23-benzoate function in the side chain. Int J Mol Sci 22:4808. https://doi.org/10.3390/ijms22094808
Starr RC, Zeikus JA (1993) UTEX-The culture collection of algae at the University of Texas at Austin. J Phycol 29:1–106. https://doi.org/10.1111/j.0022-3646.1993.00001.x
Stirk WA, Bálint P, Tarkowská D et al (2013a) Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol Biochem 70:348–353. https://doi.org/10.1016/j.plaphy.2013.05.037
Stirk WA, Bálint P, Tarkowská D et al (2018) Endogenous brassinosteroids in microalgae exposed to salt and low temperature stress. Eur J Phycol 53:273–279. https://doi.org/10.1080/09670262.2018.1441447
Stirk WA, Ördög V, Novák O et al (2013b) Auxin and cytokinin relationships in 24 microalgal strains1. J Phycol 49:459–467. https://doi.org/10.1111/jpy.12061
Stirk WA, Ördög V, Van Staden J, Jäger K (2002) Cytokinin- and auxin-like activity in Cyanophyta and microalgae. J Appl Phycol 14:215–221. https://doi.org/10.1023/A:1019928425569
Stirk WA, Tarkowská D, Turečová V et al (2014) Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J Appl Phycol 26:561–567. https://doi.org/10.1007/s10811-013-0062-z
Stirk WA, van Staden J, Novák O et al (2011) Changes in endogenous cytokinin concentrations in Chlorella (Chlorophyceae) in relation to light and the cell cycle. J Phycol 47:291–301. https://doi.org/10.1111/j.1529-8817.2010.00952.x
Székely Á, Szalóki T, Lantos C et al (2022a) Data of selected set of rice accessions at the germination stage under cold stress. Data Br 41:107929. https://doi.org/10.1016/j.dib.2022.107929
Székely Á, Szalóki T, Pauk J et al (2022b) Salinity tolerance characteristics of marginally located rice varieties in the Northernmost rice-growing Area in Europe. Agronomy 12:652. https://doi.org/10.3390/agronomy12030652
Takatsuto S, Yokota T, Omote K et al (1989) Identification of Brassinolide, Castasterone and Norcastasterone (Brassinone) in Sunflower (Helianthus annuus L.) Pollen. Agr Biol Chem 53:2177–2180. https://doi.org/10.1080/00021369.1989.10869607
Vriet C, Russinova E, Reuzeau C (2012) Boosting crop yields with plant steroids. Plant Cell 24:842–857. https://doi.org/10.1105/tpc.111.094912
Wada K, Marumo S, Abe H, Morishita T et al (1984) A Rice lamina inclination Test-A micro-quantitative bioassay for brassinosteroids. Agr Biol Chem 48:719–726. https://doi.org/10.1080/00021369.1984.10866208
Yokota T, Kim SK, Fukui Y et al (1987) Brassinosteroids and sterols from a green alga, Hydrodictyon reticulatum: Configuration at C-24. Phytochem 26:503–506. https://doi.org/10.1016/S0031-9422(00)81442-9
Zhao ZR, Wu ZL, Huang GQ, Li GR (1992) An improved disk bioassay for determining activities of plant growth regulators. J Plant Growth Regul 11:209–213. https://doi.org/10.1007/BF02115479
Acknowledgements
This research was financially supported by the National Research, Development and Innovation Office of Hungary, NKFIH (awards K 140351 and RRF-2.3.1-21-2022-00014 to G.B., and MKI-2018-00034 to P.F., J.K., M.F., and N.M.), and by the ÚNKP-22-3 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund (to P.F.).
Funding
Open access funding provided by HUN-REN Balaton Limnological Research Institute.
Author information
Authors and Affiliations
Contributions
PF, MF, NM conducted the laboratory experiments. GB, PF, MJ participated in the elaboration of the manuscript. JK, PF participated in experimental design. EL, GB acquired the funding, supervised all the work. JK, EL critically revised the manuscript. GB, PF, JK analyzed the data. EL and PF were responsible for the statistical analyses. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by J. Kovacik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Futó, P., Kutasi, J., Lengyel, E. et al. Straightforward method for brassinosteroid detection in microalgae. Acta Physiol Plant 46, 25 (2024). https://doi.org/10.1007/s11738-024-03649-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03649-5