Abstract
Abiotic stresses such as herbicides can affect plant growth and yield. Using herbicide-resistant plant growth-promoting bacteria is a new approach to mitigate these side effects. This study was conducted to evaluate the effect of three native plant growth-promoting bacteria isolated from the Medicago sativa rhizosphere, including Serratia rubidaea (A) and Pseudomonas putida (B), Serratia sp. (C) plus Synorhizobium meliloti (R) and their combinations (AB, AC, BC, ABC, AR, BR, CR, ABR, ACR, BCR, and ABCR) on microbial population, plant biomass, antioxidant enzymes (CAT, APX, and GPX) activities, and hydrogen peroxide and malondialdehyde contents at the presence and absence of imazethapyr herbicide. The results indicated that herbicide application decreased plant biomass but increased microbial population, antioxidant enzymes activities, and the concentrations of hydrogen peroxide and malondialdehyde of all inoculated and non-inoculated plants. Bacterial inoculation in most cases increased microbial population, plant biomass, and antioxidant activities. These increases were more evident under herbicide application. The highest increase in these attributes was achieved by AB, AR, and ABR inoculums in the presence and absence of the herbicide. The microbial population, plant biomass and antioxidant activities were decreased under BC, CR, BCR, and ABCR inoculations. It can be concluded that in addition to growth promotion, these bacteria increase resistance against herbicide stress by controlling free-radical induced oxidative stress and lipid peroxidation through antioxidant enzymes. These findings create new visions in biofertilizer preparation for reducing environmental stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
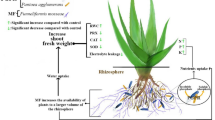
Medicago sativa L. (M. sativa) is high quality and valuable cattle forage and a critical element in crop rotation in sustainable agricultural systems (Yanes et al. 2012; Rad et al. 2020; Aghajanlou et al. 2021; Kumar et al. 2020; Moradi et al. 2021). The high quantitative productivity of this forage crop, with low fiber, high protein content, enriched with vitamins and minerals, high tolerance against drought and other environmental stresses, etc., are the most essential factors that grant special status to this forage crop (Putnam and Orloff 2014; Mastinu et al. 2021). The optimal productivity of this crop is very diverse due to M. sativa cultivars and environmental conditions like as low soil fertility, affliction with diseases, presence of weeds, etc., which decrease the yield (Hancock 2015; Ghorbani et al. 2008; Yousefi et al. 2020; Zangani et al. 2021). Pesticide application is the primary method for weed and disease control in plants. The over consumption of these chemical substances and their soil penetration property significantly reduces plant productivity in addition to their harmful environmental impacts, like as underground water pollution and extermination of beneficial soil microorganisms (Volenec et al. 2002; Lamb et al. 2006). Plant damage caused by herbicide application is the result of increased reactive oxygen species. These compounds lead to lipid peroxidation, cell membrane damage, and reactive oxygen production (Silva et al. 2013; Karimmojeni et al. 2021). The cell contents transude due to cell membrane damage and hence specific physiological processes like as photosynthesis will be disrupted (Kruse et al. 2006; Naservafaei et al. 2021). Plants activate their antioxidant defense systems to reduce damages caused by reactive oxygen species. This system involves low-weight molecular compounds like as ascorbic acid, glutathione and antioxidant enzymes, including glutathione peroxidase (GPX), ascorbate peroxidase (APX), catalase (CAT), etc. (Hussain et al. 2016; Gupta et al. 2020, 2021). The herbicide imazethapyr also known as Pursuit, is a selective systemic herbicide that has been and is being applied to control M. sativa weeds. Studies have revealed that plant-growth-promoting bacteria, colonized in M. sativa rhizosphere, improve productivity in different environmental conditions specially in stressed situations (Carrillo-Castañeda et al. 2002; Ramakrishna et al. 2019). These bacteria promote plant growth by providing nutrients, secretion of plant growth hormones, and many other mechanisms (Chiappero et al. 2019). Another function of plant-growth-promoting bacteria is decomposition of herbicide residues in soil (Akbar and Sultan 2016). Weeds and microbes destroyed by herbicides act as nutritional carbon and nitrogen sources for other soil microorganisms, leading to bacterial recovery and increasing their strength for crop-growth restoration (Lupwayi et al. 2010). Studies in recent years revealed that plant-growth-promoting bacteria are highly contributive in plant resistance against environmental stresses, like as herbicide application, by activating antioxidant mechanism (Khan et al. 2019; Vurukonda et al. 2016). Due to the emphasized role of biological fertilizers in sustainable agriculture in the past few decades, the aim of this study was to investigate the potential of native herbicide-resistant bacteria from M. sativa in prevention of yield reduction in alfalfa through promoting the activity of antioxidant enzymes under herbicide stress. Indeed, the majority of studies on PGPR have been focused on growth promotion potential of them under normal condition while rare studies have been concentrated on their role in mitigation of abiotic stresses in plants.
Materials and methods
The cultivated M. sativa samples in Isfahan University of Technology (located at Lavark, Najaf-Abad, south-west of Isfahan Province, Iran) were harvested in winter. Samples were randomly collected from five points of field at three replicates from each point. The soil surrounding the roots was removed, and the bacterial isolation was done through preparing serial tenfold dilutions from the rhizosphere soil and subsequent culturing on Nutrient agar. The pure isolates were identified based on phenotypic and genotypic (16S rRNA sequencing) identification methods. These isolates were screened based on their plant growth promotion capabilities including nitrogen fixation, auxin production, phosphate, and potassium solubilization, lipase, protease, and cellulose enzymes production, and herbicide resistance. The activity level of these bacteria differed concerning the different characteristics and based on the biological activity of them the best isolates in terms of growth-promoting attributes were A, B, and C, respectively.
Pot experiment
This experiment was conducted in Chah-Anari field in Isfahan University of Technology (40 km south-west of Isfahan, 32º 32´ N 51º 23´ E) as a pot experiment. Hamedani M. sativa cultivar was cultured and subjected to 16 microbial treatments including non-inoculated (control), inoculation with A, B, C, and R (Synorhizobium meliloti) isolates, dual microbial inoculation, triple microbial inoculation, inoculation with four bacterial species, and two levels of herbicide including zero (control) and one liter per hectare as the usual herbicide dose in M. sativa fields. Imazethapyr (Bayer) was applied post-emergence at the 5–6 leaf stage. The herbicide was sprayed in such a way that all parts of the plants were washed and the soil surface was wet. This two-factor factorial experiment was run based on a randomized complete block design with five replications. Twenty seeds were sown in all pots; thinning was performed to have the same bush count in each pot before herbicide treatment and to have uniform germination with optimum density. M. sativa was planted on April 3, 2019, and harvested on June 11, 2019.
Inoculum preparation
To prepare bacterial inoculum, 100 μl of bacterial suspension (109 CFU/ml) was added to 20 ml nutrient broth, and the seeds’ primary inoculation took place just before cultivation. The secondary inoculation took place one month after cultivation, during the plants initial establishment. For this purpose, approximately 300 ml water was consumed for irrigation of 5-l pot; a proper measure to prevent water discharge. Then, according to the concentration of inoculum in the seed inoculation stage, 1500 μl of bacterial suspension (109 CFU/ml) was administered for 300 ml of irrigation water. For rapid bacterial penetration in the roots and preventing drought stress, the pots were irrigated before inoculation.
Biochemical parameters determination
Leaf samples were harvested 48 h after herbicide application and preserved at −80 °C before physiological measurement.
Hydrogen peroxide (H2O2)
The concentration of H2O2 was measured according to Velikova et al. (2000). Leaf tissue (200) mg was homogenized in 2 ml TCA solution (0.1% w:v) and the homogenate was centrifuged at 4 °C and 10,000 g for 10 min; 0.5 ml of the supernatant was mixed with 0.5 ml potassium phosphate buffer (0.1 M, pH 7.0) and 1 ml KI (1 M) and kept for 1 h at room temperature in a dark place. The adsorption was recorded at 390 nm (Chaichi et al. 2022).
Malondialdehyde (MDA)
Lipid peroxidation was measured according to the method described by Heath and Packer (1968) for malondialdehyde, and Meir et al. (1992) for aldehydes, with minor modifications (Yousefvand et al. 2022). Leaf tissue (200 mg) was homogenized in 2 ml TCA solution (0.1% w:v), and the homogenate was centrifuged at 10,000 rcf and 4 °C for 10 min; 0.5 ml of the supernatant was added to 1 ml of 20% TCA containing 0.5% (w:v) TBA. The reaction mixture was incubated at 95 °C for 30 min. Then the tubes were submerged in an ice bath to stop the reaction. The samples were centrifuged again at 10,000 rcf for 15 min. The lipid peroxidation was measured for MDA and the non-specific absorption samples at 532 and 600 nm, respectively. The absorption of the colored reaction was recorded at 455 nm for aldehydes. Red pigment (The MDA–TBA complex volume) was presented as μmol/g FW and determined by the 155 mM−1 cm1 extinction coefficient obtained through the following equation:
Determination of Antioxidant enzymes activity
Catalase (CAT), glutathione peroxidase (GPx), and ascorbate peroxidase (APX) activities of M. sativa were measured according to the methods described by (Chance and Maehly 1955; Herzog and Fahimi 1973; Kadkhodaie et al. 2013) with slight modifications. Fresh leaf tissue (0.1 g) was powdered by applying liquid nitrogen and homogenized in 1 ml Na-phosphate buffer (50 mM, pH 7) containing 2 mM a-dithiothreitol, 2 mM EDTA, 0.2% Triton X-100 and 50 mM Tris–HCl in a cold mortar. The sample mixture was centrifuged at 12,000 rpm and 4 °C for 30 min, and the supernatant was used for determining CAT, GPx, and APX enzymes activities.
Catalase enzyme activity (CAT)
This activity was assayed in a total volume of 3 ml of 50 mM Na-phosphate buffer (pH7.0) containing 4.51 μl of H2O2 (30%) and 50 μl enzyme extract (pH 7.8). The absorbance was recorded at 240 nm every 30 s for 2 min to measure H2O2 reduction. Specific catalase activity (enzyme unit) was expressed as the enzyme decomposed per milligram of protein (U mg−1 protein). The volume of the particular catalase activity was obtained through the following equation:
U: A unit of catalase activity is equal to the volume of the enzyme that catalysis the H2O2 to O2 and H2O2 in 1 min; ΔA: Absorbance differences in 240 nm in 1 min; TV: Total bulk of buffer and extract (3 ml); EV: Extract volume (0.05 ml); ε: Extinction coefficient for catalase (39.4 mM−1 cm−1); D: Dilution coefficient.
Ascorbate peroxide enzyme activity (APX)
The APX was measured in a total volume of 3 ml of 50 mM Na-phosphate buffer (pH 7.0), containing 4.51 μl of H2O2 (30%), 100 μl of 5 mM ascorbate and 50 μl of enzyme extract (pH 7.8). To evaluate APX, the absorbance was recorded through a spectrophotometer at 290 nm every 30 s for 2 min. The amount of specific ascorbate peroxide activity was calculated through the same equation applied for specific catalase activity, at ε = 2.8.
Glutathione peroxidase activity (GPx)
The GPX was evaluated in a total volume of 3 ml Na-phosphate buffer (50 mM, pH 7.0), containing 4.51 μl of H2O2 (30%), 3.35 μl Guiacol and 50 μl of enzyme extract (pH 7.8). The GPX was measured through recording the absorbance at 470 nm every 30 s for 2 min. The volume of specific peroxidase activity was calculated through the same equation applied for specific catalase activity, at ε = 26.6.
Protein content
Protein content was measured by consuming 3 ml of Bradford reagent (consisting of 50 ml ethanol (95%), 100 ml orthophosphoric acid, and 100 mg Coomassie brilliant blue in 1000 ml) and 100 μl of protein extract, mixed and incubated at room temperature for 30 min (Lazzari et al. 2011; Manca et al. 2013). The absorbance was recorded at 595 nm. Bovine serum albumin (BSA) was used as a standard reagent (Bradford 1976) (Lazzari et al. 2012; Sanna et al. 2009).
Microbial population
The M. sativa plants were harvested from the soil 20 days after herbicide application, and the microbial population in the plant rhizosphere was counted. In this process, the soil surrounding the plant root was removed by gentle shaking, and the rhizosphere soil on the root was suspended in saline buffer. The obtained suspension was used to prepare 10–1 till 10–5 dilutions, and 0.1 ml of each dilution was streak cultured on nutrient agar. After 24 h incubation at 28 °C, the formed colonies were counted.
Shoot dry matter.
To measure the plants' dry weight in each pot, the shoots were dried at 72 °C oven for 48 h and their weights were measured.
Statistical analysis
The obtained data were subjected to statistical analysis (ANOVA) by SAS statistical software (version 9.4), and the means were compared by applying the least significant difference (LSD) at a 0.05 probability level.
Results
Shoot dry matter
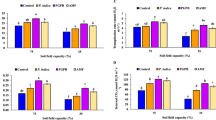
The dry shoot matter was affected (at 5% level) by the main and the interaction effects of herbicide and microbial treatments (Table 1). Biomass production decreased by herbicide application in both inoculated and non-inoculated plants (Fig. 1). Microbial inoculation in most cases increased the dry shoot matter under with and without herbicide treatments. The highest increases were found in case of ABR, Ab and AR, respectively (Fig. 2).
Hydrogen peroxide
H2O2 content at 1% level was significantly affected by the main effects and the interaction effects of herbicide and microbial treatments (Table 1). Herbicide application increased the H2O2 content in all inoculated and non-inoculated plants (Table 2). The H2O2 content in most cases was decreased by microbial treatments. The highest decreases in H2O2 content were observed by AB and ABR, respectively (Table 3).
Malondialdehyde
MDA content at 1% level was significantly affected by the main effects and the interaction effects of herbicide and microbial treatments (Table 1). The herbicide application increased malondialdehyde content in the sprayed plants, indicating a degree of damage to the plant tissues caused (Table 2). The MDA content in most cases was decreased by microbial treatments. The highest decreases in MDA content were observed by ABR and AB, respectively (Table 3).
Antioxidant enzyme activity
The results obtained from ANOVA revealed that the main effects of the herbicide and microbial treatments were (at a 1% level) on the activities of CAT, APX, and GPX enzymes. The interaction effects of herbicide and microbial treatment were only significant (at 1% level) in CAT and APX activities (Table1). Herbicide application, increased the activities of CAT, APX, and GPX (Table 2). The microbial inoculation, in most cases, increased the activities of all three antioxidant enzymes under both the applied and non-applied herbicide conditions (Table 3). The highest increases in CAT were observed by AB, ABR, and AR, respectively. Also, the highest increases in GPX and APX were observed by AB, ABR, AR, ACR, and AC, respectively (Table 3).
Microbial population
The main and the interaction effects of herbicide and microbial treatments were significant (at 5% level) on microbial population (Table 1). Herbicide application increased the microbial population under all inoculated and non-inoculated treatments except under ABCR inoculation (Fig. 3). Microbial population in most cases increased following inoculation, under both herbicide treatments. The maximum increases in microbial population were observed by ABR and AB, respectively (Fig. 4).
Discussion
Biomass production was decreased as a consequent of herbicide application in both inoculated and non-inoculated plants. According to Bastiaans et al., field application of herbicide parallel to removing the weeds slows the crop growth (Bastiaans et al. 2008). Some herbicides have similar composition to plastoquinone, which can interfere with electron transfer process of photosynthesis and imposing oxidative stress on the plant (Zimdahl 2007). The oxidative stress leads to cell membrane lysis through membrane lipids peroxidation (Abate et al. 2021; Mahdavi et al. 2020); therefore, the plant has to consume part of its energy to produce antioxidant compounds to alleviate the oxidative stress related to herbicide. These consequences reduce plant growth and yield (Zimdahl 2007).
Herbicide application increased the hydrogen peroxide content and consequently led to increase in malondialdehyde in treated plants which indicates a degree of damage to the plant tissues. In the study conducted on the treatment of Salvia officinalis by five herbicides, the concentrations of hydrogen peroxide and malondialdehyde were increased in the herbicide-treated plants (Teimouri Jervekani et al. 2018). More assessments run on the changes of fatty acid profile and antioxidant systems in a Nostoc muscorum exposed to bentazon indicated that bentazon led to increase in reactive oxygen radicals, oxidative stress, and programmed cell death, which ultimately increased the malondialdehyde content of the plant (Galhano et al. 2011) (Figs. 5 and 6).
Application of herbicide was led to increase in microbial population of root rhizosphere in all conditions except under the ABCR treatment. Pozo et al. found that consuming 2–20 kg per hectare of alachlor increased the total population of bacteria and fungi (Pozo et al. 1994). Similarly, study of short-term effects of napropamide on the activity and structure of soil microbial population revealed that the microbial population increased following herbicide application as previously reported (Cycoń et al. 2013). It is assumed that pesticide-resistant microorganisms can metabolize herbicide as carbon and nitrogen sources and form dominant population in their habitat. Moreover, the biomass of sensitive and dead microorganisms act as nutrient resources for these population. Similar to our research, atrazine degrading bacteria were isolated from previously treated field with this herbicide and were able decompose atrazine as carbon and nitrogen sources, leading to an increase in their ability to recover the microbial population in the presence of herbicides (Radosevich et al. 1995).
Bacterial treatments in most cases increased plant biomass and microbial population in root rhizosphere; while, less reductions were found consequent to herbicide application in inoculated plants. The maximum increase in microbial population and plant biomass, in both herbicide and non-herbicide treatments, were obtained following inoculation with A bacterium accompanied with B and R but not C bacterium. Auxin accelerates symbiotic interaction between rhizobium and legume through signaling and leading to improvement in nitrogen fixation, which can eventually stimulate the plant growth (Lin et al. 2020). The plant biomass and microbial population was decreased following BC, CR, BCR, and ABCR bacterial treatments. This may indicate that inoculum C had antagonistic effects, especially when accompanied with inoculums B and R. Bacterium C can produce hydrogen cyanide, a gas that negatively affects root metabolism and is a very strong inhibitor for many metalloenzymes. The microbial population of rhizosphere can be negatively affected due to increase in hydrogen cyanide production.
Some researchers have found that PGPB strains can increase growth and modulate negative effects of herbicides on plant growth. PGPB-related enhanced growth and increased secondary metabolites and antioxidant potential have been reported in Cannabis sativa (Pagnani et al. 2018; Kumar et al. 2019). According to Ahemad and Khan, although consuming Quizalafop-p-Ethyl and Clodinafop herbicides led to decrease in chickpea growth, the plants inoculated with Mesorhizobium sp. MRC4 had more yields (Ahemad and Khan 2009). Ma et al. assessed the improvement in Brassica juncea growth and nickel tolerance by nickel resistant-plant-growth-promoting bacteria (Ma et al. 2009). Their results revealed that Psychrobacter sp. SRA2 significantly increased the fresh and dry biomass of subjected plants. Shahid and Khan found that due to inoculating chickpea with Burkholderia cepacia, a phosphate solubilizing and glyphosate-resistant bacterium, the concentrations of chlorophyll b, a, and carotenoid pigments were increased, as well as the negative effects of glyphosate on chickpea were decreased (Shahid and Khan 2018). In Rego and colleagues study, two rhizobacteria, including Pseudomonas fluorescens BRM-32111 and Burkholderia pyrrocinia BRM-32113, were isolated from rice rhizosphere, and their role on mitigation of toxic allelochemicals and crop yield were assessed in field experiment in the presence of allelopathic rice residues (Rêgo et al. 2018). They found that rice inoculation with these bacteria can significantly reduce the yield drop rate related to toxic allelochemicals. Pandey et al. assessed the potential of metal toxicity amelioration and growth-promoting abilities of Ochrobactrum sp., Bacillus sp. and Bacillus sp.; cadmium, lead and arsenic resistant species, respectively, while applied on rice. Following inoculation of metal-treated rice with these bacterial species, the toxic effect of metal was reduced, and the overall biomass and root/shoot ratio increased (Pandey et al. 2013). Rizvi and Khan reported that the presence of a metal tolerant nitrogen fixing Azotobacter chroococcum in the soil polluted with Cu and Pb, caused growth and yield enhancement of maize (Rizvi and Khan 2018).
Another finding was that in most cases bacterial treatments increased the activity of antioxidant enzymes under both herbicide-treated and -untreated conditions. The maximum increases with regard to plant biomass production and antioxidant enzyme activities were observed in the plants inoculated with AB, AR, and ABR, which highlights the contribution of antioxidant enzyme activation in promoting plant growth by bacterial inoculation. This correlation became evident through the simultaneous reductions in plant biomass and enzyme activities under BC, CR, BCR, and ABCR inoculums. The significant correlations between plant biomass and antioxidant enzyme, lipid peroxidation and microbial population under both herbicide treatments suggest the higher incidence of oxidative stress under herbicide application which greatly stimulated antioxidant enzyme activation to reduce herbicide stress in plants. The higher increase in CAT, APX, and GPX activity induced by the most beneficial bacterial inoculations (AB, AR, and ABR) may suggest high contribution of these enzymes in plant biomass improvement through inoculation under herbicide application. This is confirmed with high negative correlations between antioxidant enzymes activity and free radical production and lipid peroxidation (Tables 4 and 5).
In the study of Shaid and Khanon, the productivity of glyphosate exposed chickpea, the chickpea was inoculated with glyphosate-tolerant phosphate solubilizing Burkholderia cepacia and the antioxidant enzymes of the herbicide-treated plant was measured (Shahid and Khan 2018). In the study of Pramanik et al the role of cadmium-resistant PGPR strain named as Enterobacter aerogenes MCC 3092 in reduction of cadmium toxicity in rice was evaluated and it was found that superoxide dismutase and catalase activity in inoculated plants with Enterobacter aerogenes was higher than the non-inoculated seedlings (Pramanik et al. 2018). Islam et al. assessed the effectiveness of resistant Pseudomonas aeruginosa on wheat productivity in soils with high concentrations of zinc. Their results revealed that total biomass, antioxidant enzyme activity (SOD, POD, and CAT), and antioxidant compounds, including ascorbic acid and phenolic compounds, increased in inoculated plants (Islam et al. 2014).
Under herbicide stress, the plant with the more potent antioxidant system will be subjected to less lipid peroxidation with free radicals which leading to low MDA production. The high antioxidant activity in the pesticide-resistant plants can indicate that these plants can maintain their growth and productivity at a normal level by neutralizing the harmful oxidants and preventing severe damage to membrane. The results of this study are consistent with several similar studies. Assessing the effect of herbicide-treated soil on the antioxidant system of sweet corn vegetative organs, revealed that the herbicide application increases the activities of catalase, superoxide dismutase, and peroxidase in the leaves and roots (Grigoryuk et al. 2016). Mohsin et al (2020) pretreated wheat seedlings with 2,4-D to assess the effect of this toxin on the improvement of the plant tolerance against oxidative stress caused by salinity and tolerance to methylglyoxal toxicity by activating the antioxidant defense system, ion homeostasis, and the glyoxalase system. They found that the activity level of antioxidant enzymes, ascorbic acid, and glutathione content, were increased in the 2,4-D-treated seedlings (Mohsin et al. 2020).
This fact that microbial population has a positive correlation with plant biomass and antioxidant enzyme activities and has negative correlation with free radical production and lipid peroxidation (Tables 4 and 5), indicates the contribution of rhizosphere microbial population in alleviating oxidative stress through potentiating antioxidant enzyme activities in plants. The highest decrease in hydrogen peroxide and MDA contents and the highest increase in antioxidant enzymes’ activities were observed in the plants inoculated with AB, AR, and ABR, especially under herbicide application. The hydrogen peroxide and MDA contents were high under BC, CR, BCR, and ABCR inoculations, while antioxidant enzymes activities were low under the same bacterial treatments. The results revealed enzymatic antioxidant defense system’s activation in inoculated plants to control lipid peroxidation and oxidative stress induced by herbicide application. In general, increases in microbial population, plant biomass, and antioxidant enzymes activities, and consequently decreases in hydrogen peroxide and MDA concentrations due to bacterial inoculation, were higher under herbicide conditions.
Pandey et al. used three different bacterial isolates (a cadmium-resistant Ochrobactrum sp., a lead resistant Bacillus sp., and an arsenic resistant Bacillus sp.) as inoculum for metal-treated rice (Pandey et al. 2013). They found that the increased of MDA level in rice roots, due to metal stress, was decreased following bacterial inoculation. Inoculation of metal tolerant Azotobacter chroococcum in Cu and Pb contaminated corn field, caused decreasing in MDA levels (Rizvi and Khan 2018).
Excessive consumption of herbicides is one of the most important problems in conventional agriculture, affecting soil fertility negatively and cause considerable reduction in the crop yield. Most of the microbial inoculations obtained from the M. sativa rhizosphere can increase the crop dry weight, enhance the antioxidant system against the herbicide stresses and decrease the damage caused by herbicide. It can be concluded that there exists a direct relation between the population of the bacterial treatment in the soil and the antioxidant activity and the defensive power of the plant against the herbicide. This phenomenon can prevent the drastic drop in the crop yield by decreasing the hydrogen peroxide contents; thus, an inhibitory function in the peroxidation of membrane lipids. As these plant growth-promoting bacteria introduced and used in this study, their ability to enhance plant stability against abiotic stresses may make them as appropriate candidates for biofertilizers in farmlands.
Author contribution statement
MM, MZ, and HK conceived the study; MZ, HK, and HM performed biochemical, anatomical and morphological analysis, and helped interpret results. MM, MZ, and HK provided plant care, performed histological analysis and helped interpret results. MZ, HK, and AM interpreted results and wrote original draft of the manuscript; HK and AM performed statistical analysis, review and editing of the manuscript.
Data availability
The data will be made available on reasonable request.
References
Abate G, Zhang LL, Pucci M, Morbini G, Mac Sweeney E, Maccarinelli G, Ribaudo G, Gianoncelli A, Uberti D, Memo M, Lucini L, Mastinu A (2021) Phytochemical analysis and anti-inflammatory activity of different ethanolic phyto-extracts of Artemisia annua L. Biomolecules. https://doi.org/10.3390/Biom11070975
Aghajanlou F, Mirdavoudi H, Shojaee M, Mac Sweeney E, Mastinu A, Moradi P (2021) Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae. https://doi.org/10.3390/horticulturae7040067
Ahemad M, Khan MS (2009) Toxicity assessment of herbicides quizalafop-p-ethyl and clodinafop towards Rhizobium pea symbiosis. Bull Environ Contam Toxicol 82(6):761–766. https://doi.org/10.1007/s00128-009-9692-x
Akbar S, Sultan S (2016) Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz J Microbiol 47(3):563–570. https://doi.org/10.1016/j.bjm.2016.04.009
Bastiaans L, Paolini R, Baumann DT (2008) Focus on ecological weed management: what is hindering adoption? Weed Res 48(6):481–491. https://doi.org/10.1111/j.1365-3180.2008.00662.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Carrillo-Castañeda G, Juárez Muños J, Peralta-Videa JR, Gomez E, Tiemannb KJ, Duarte-Gardea M, Gardea-Torresdey JL (2002) Alfalfa growth promotion by bacteria grown under iron limiting conditions. Adv Environ Res 6(3):391–399. https://doi.org/10.1016/s1093-0191(02)00054-0
Chaichi M, Nemati A, Dadrasi A, Heydari M, Hassanisaadi M, Yousefi AR, Baldwin TC, Mastinu A (2022) Germination of Triticum aestivum L.: effects of soil–seed interaction on the growth of seedlings. Soil Systems. 6(2):37. https://doi.org/10.3390/soilsystems6020037
Chance B, Maehly AC (1955). [136]Assay of Catalases and Peroxidases. https://doi.org/10.1016/s0076-6879(55)02300-8
Chiappero J, Cappellari LdR, Sosa Alderete LG, Palermo TB, Banchio E (2019) Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod 139:111553. https://doi.org/10.1016/j.indcrop.2019.111553
Cycoń M, Wójcik M, Borymski S, Piotrowska-Seget Z (2013) Short-term effects of the herbicide napropamide on the activity and structure of the soil microbial community assessed by the multi-approach analysis. Appl Soil Ecol 66:8–18. https://doi.org/10.1016/j.apsoil.2013.01.014
Galhano V, Santos H, Oliveira MM, Gomes-Laranjo J, Peixoto F (2011) Changes in fatty acid profile and antioxidant systems in a Nostoc muscorum strain exposed to the herbicide bentazon. Process Biochem 46(11):2152–2162. https://doi.org/10.1016/j.procbio.2011.08.015
Ghorbani R, Wilcockson S, Koocheki A, Leifert C (2008) Soil management for sustainable crop disease control: a review. Environ Chem Lett 6(3):149–162. https://doi.org/10.1007/s10311-008-0147-0
Grigoryuk IP, Lykholat UV, Rossykhina-Galycha GS, Khromykh NO, Serga OI (2016) Effect of soil herbicides on the antioxidant system of maize vegetative organs during ontogenesis. Annals of Agrarian Science 14(2):95–98. https://doi.org/10.1016/j.aasci.2016.05.008
Gupta AK, Rather MA, Jha AK, Shashank A, Singhal S, Sharma M, Pathak U, Sharma D, Mastinu A (2020) Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. flowers: new Sources of bioactive compounds. Plants-Basel. https://doi.org/10.3390/Plants9101329
Gupta AK, Dhua S, Sahu PP, Abate G, Mishra P, Mastinu A (2021) Variation in phytochemical, antioxidant and volatile composition of pomelo fruit (Citrus grandis (L.) Osbeck) during seasonal growth and development. Plants-Basel. https://doi.org/10.3390/Plants10091941
Hancock JG (2015) Fungal and bacterial diseases of north American forage crops. Pasture and Forage Crop Pathol. https://doi.org/10.2134/1996.pastureforagecroppathol.c9
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Herzog V, Fahimi HD (1973) A new sensitive colorimetric assay for peroxidase using 3,3′-diaminobenzidine as hydrogen donor. Anal Biochem 55(2):554–562. https://doi.org/10.1016/0003-2697(73)90144-9
Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N (2016) Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev 2016:1–9. https://doi.org/10.1155/2016/7432797
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 104:285–293. https://doi.org/10.1016/j.ecoenv.2014.03.008
Kadkhodaie A, Zahedi M, Razmjoo J, Pessarakli M (2013) Changes in some anti-oxidative enzymes and physiological indices among sesame genotypes (Sesamum indicum L.) in response to soil water deficits under field conditions. Acta Physiol Plant 36(3):641–650. https://doi.org/10.1007/s11738-013-1442-3
Karimmojeni H, Rahimian H, Alizadeh H, Yousefi AR, Gonzalez-Andujar JL, Mac Sweeney E, Mastinu A (2021) Competitive ability effects of Datura stramonium L. and Xanthium strumarium L. on the development of maize (Zea mays) Seeds. Plants-Basel. https://doi.org/10.3390/Plants10091922
Khan N, Bano A, Rahman MA, Guo J, Kang Z, Babar MA (2019) Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci Rep. https://doi.org/10.1038/s41598-019-38702-8
Kruse ND, Vidal RA, Dalmaz C, Trezzi MM, Siqueira I (2006) Estresse oxidativo em girassol (Helianthus annuus) indica sinergismo para a mistura dos herbicidas metribuzin e clomazone. Planta Daninha 24(2):379–390. https://doi.org/10.1590/s0100-83582006000200023
Kumar A, Premoli M, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Memo M, Mastinu A (2019) Cannabimimetic plants: are they new cannabinoidergic modulators? Planta 249(6):1681–1694. https://doi.org/10.1007/s00425-019-03138-x
Kumar A, Memo M, Mastinu A (2020) Plant behaviour: an evolutionary response to the environment? Plant Biol 22(6):961–970. https://doi.org/10.1111/plb.13149
Lamb JFS, Sheaffer CC, Rhodes LH, Sulc RM, Undersander DJ, Brummer EC (2006) Five Decades of Alfalfa Cultivar Improvement: Impact on Forage Yield, Persistence, and Nutritive Value. Crop Sci 46(2):902–909. https://doi.org/10.2135/cropsci2005.08-0236
Lazzari P, Sanna A, Mastinu A, Cabasino S, Manca I, Pani L (2011) Weight loss induced by rimonabant is associated with an altered leptin expression and hypothalamic leptin signaling in diet-induced obese mice. Behav Brain Res 217(2):432–438. https://doi.org/10.1016/j.bbr.2010.11.022
Lazzari P, Pau A, Tambaro S, Asproni B, Ruiu S, Pinna G, Mastinu A, M. Curzu M, Reali R, Emilio Heiner Bottazzi M, A. Pinna G, Murineddu G, (2012) Synthesis and Pharmacological Evaluation of Novel 4-Alkyl-5-thien-2’-yl Pyrazole Carboxamides. Cent Nerv Syst Agents Med Chem 12(4):254–276. https://doi.org/10.2174/187152412803760636
Lin I, Frank M, Reid D (2020) No home without hormones: how plant hormones control legume nodule organogenesis. Plant Commun 1:100104. https://doi.org/10.1016/j.xplc.2020.100104
Lupwayi NZ, Brandt SA, Harker KN, O’Donovan JT, Clayton GW, Turkington TK (2010) Contrasting soil microbial responses to fertilizers and herbicides in a canola–barley rotation. Soil Biol Biochem 42(11):1997–2004. https://doi.org/10.1016/j.soilbio.2010.07.024
Ma Y, Rajkumar M, Freitas H (2009) Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J Hazard Mater 166(2–3):1154–1161. https://doi.org/10.1016/j.jhazmat.2008.12.018
Mahdavi A, Moradi P, Mastinu A (2020) Variation in terpene profiles of Thymus vulgaris in water deficit stress response. Molecules. https://doi.org/10.3390/Molecules25051091
Manca I, Mastinu A, Olimpieri F, Falzoi M, Sani M, Ruiu S, Loriga G, Volonterio A, Tambaro S, Bottazzi MEH, Zanda M, Pinna GA, Lazzari P (2013) Novel pyrazole derivatives as neutral CB 1 antagonists with significant activity towards food intake. Eur J Med Chem 62:256–269. https://doi.org/10.1016/j.ejmech.2012.12.056
Mastinu A, Bonini SA, Premoli M, Maccarinelli G, Mac Sweeney E, Zhang LL, Lucini L, Memo M (2021) Protective effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced inflammation and motor alteration in mice. Molecules. https://doi.org/10.3390/Molecules26030570
Meir S, Rubin L, Zauberman G, Fuchs Y (1992) Changes in fluorescent lipid peroxidation products of room-ripened and vine-ripened tomato fruits in relation to other ripening parameters. Postharvest Biol Technol 2(2):125–135. https://doi.org/10.1016/0925-5214(92)90015-h
Mohsin SM, Hasanuzzaman M, Parvin K, Fujita M (2020) Pretreatment of wheat (Triticum aestivum L.) seedlings with 2,4-D improves tolerance to salinity-induced oxidative stress and methylglyoxal toxicity by modulating ion homeostasis, antioxidant defenses, and glyoxalase systems. Plant Physiol Biochem 152:221–231. https://doi.org/10.1016/j.plaphy.2020.04.035
Moradi P, Aghajanloo F, Moosavi A, Monfared HH, Khalafi J, Taghiloo M, Khoshzaman T, Shojaee M, Mastinu A (2021) Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustain-Basel. https://doi.org/10.3390/Su13147874
Naservafaei S, Sohrabi Y, Moradi P, Mac Sweeney E, Mastinu A (2021) Biological response of Lallemantia iberica to brassinolide treatment under different watering conditions. Plants-Basel. https://doi.org/10.3390/Plants10030496
Pagnani G, Pellegrini M, Galieni A, D’Egidio S, Matteucci F, Ricci A, Stagnari F, Sergi M, Lo Sterzo C, Pisante M, Del Gallo M (2018) Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: an alternative fertilization strategy to improve plant growth and quality characteristics. Ind Crops Prod 123:75–83. https://doi.org/10.1016/j.indcrop.2018.06.033
Pandey S, Ghosh PK, Ghosh S, De TK, Maiti TK (2013) Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J of Microbiol 51(1):11–17. https://doi.org/10.1007/s12275-013-2330-7
Pozo C, Salmeron V, Rodelas B, Martinez-Toledo MV, Gonzalez-Lopez J (1994) Effects of the herbicide alachlor on soil microbial activities. Ecotoxicology 3(1):4–10. https://doi.org/10.1007/bf00121384
Pramanik K, Mitra S, Sarkar A, Maiti TK (2018) Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J Hazard Mater 351:317–329. https://doi.org/10.1016/j.jhazmat.2018.03.009
Putnam DH, Orloff SB (2014). Forage Crops. https://doi.org/10.1016/b978-0-444-52512-3.00142-x
Rad SV, Valadabadi SAR, Pouryousef M, Saifzadeh S, Zakrin HR, Mastinu A (2020) Quantitative and qualitative evaluation of Sorghum bicolor L. under intercropping with legumes and different weed control methods. Horticulturae. https://doi.org/10.3390/horticulturae6040078
Radosevich M, Traina SJ, Hao YL, Tuovinen OH (1995) Degradation and mineralization of atrazine by a soil bacterial isolate. Appl Environ Microbiol 61(1):297–302. https://doi.org/10.1128/aem.61.1.297-302.1995
Ramakrishna W, Yadav R, Li K (2019) Plant growth promoting bacteria in agriculture: two sides of a coin. Appl Soil Ecol 138:10–18. https://doi.org/10.1016/j.apsoil.2019.02.019
Rêgo MCF, Cardoso AF, da C Ferreira T, de Filippi MCC, Batista TFV, Viana RG, da Silva GB, (2018) The role of rhizobacteria in rice plants: Growth and mitigation of toxicity. J Integr Agric 17(12):2636–2647. https://doi.org/10.1016/s2095-3119(18)62039-8
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20. https://doi.org/10.1016/j.ecoenv.2018.03.063
Sanna D, Sanna A, Mara L, Pilichi S, Mastinu A, Chessa F, Pani L, Dattena M (2009) Oct4 Expression in in vitro produced sheep Blastocysts and Embryonic stem-like cells. Cell Biol Int. https://doi.org/10.1042/cbi20090008
Shahid M, Khan MS (2018) Glyphosate induced toxicity to chickpea plants and stress alleviation by herbicide tolerant phosphate solubilizing Burkholderia cepacia PSBB1 carrying multifarious plant growth promoting activities. 3 Biotech. https://doi.org/10.1007/s13205-018-1145-y
Silva IPDFE, Junior JFDAS, Putti FF, Latorre DDO, Schimidt AP, Ludwig R (2013) Herbicidas Inibidores Do Fotossistema Ii – Parte Ii. Revista Brasileira De Engenharia De Biossistemas 7(1):12–22. https://doi.org/10.18011/bioeng2013v7n1p12-22
Teimouri Jervekani M, Karimmojeni H, Razmjo J, Tseng T-M (2018) Common sage (Salvia officinalis L.) tolerance to herbicides. Ind Crops Prod 121:46–53. https://doi.org/10.1016/j.indcrop.2018.04.082
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151(1):59–66. https://doi.org/10.1016/s0168-9452(99)00197-1
Volenec JJ, Cunningham SM, Haagenson DM, Berg WK, Joern BC, Wiersma DW (2002) Physiological genetics of alfalfa improvement: past failures, future prospects. Field Crop Res 75(2–3):97–110. https://doi.org/10.1016/s0378-4290(02)00020-5
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24. https://doi.org/10.1016/j.micres.2015.12.003
Yanes ML, De La Fuente L, Altier N, Arias A (2012) Characterization of native fluorescent Pseudomonas isolates associated with alfalfa roots in Uruguayan agroecosystems. Biol Control 63(3):287–295. https://doi.org/10.1016/j.biocontrol.2012.08.006
Yousefi AR, Rashidi S, Moradi P, Mastinu A (2020) Germination and seedling growth responses of Zygophyllum fabago Salsola kali L. and Atriplex canescens to PEG-induced drought stress. Environments. https://doi.org/10.3390/environments7120107
Yousefvand P, Sohrabi Y, Heidari G, Weisany W, Mastinu A (2022) Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 27(10):3083. https://doi.org/10.3390/molecules27103083
Zangani E, Afsahi K, Shekari F, Mac Sweeney E, Mastinu A (2021) Nitrogen and phosphorus addition to soil improves seed yield, foliar stomatal conductance, and the photosynthetic response of rapeseed (Brassica napus L.). Agric-Basel. https://doi.org/10.3390/agriculture11060483
Zimdahl RL (2007) Fundamentals of weed science, 3rd edn. Elsevier/Academic Press, Amsterdam, Oxford
Acknowledgements
This study was supported by a grant from research council of Isfahan University of Technology and University of Brescia.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest:
I would like to declare on behalf of my co-authors that there is no conflict of interest exits in the submission of this manuscript which is approved by all authors.
Additional information
Communicated by C. L. Cespedes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motamedi, M., Zahedi, M., Karimmojeni, H. et al. Effect of rhizosphere bacteria on antioxidant enzymes and some biochemical characteristics of Medicago sativa L. subjected to herbicide stress. Acta Physiol Plant 44, 84 (2022). https://doi.org/10.1007/s11738-022-03423-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-022-03423-5