Abstract
Doubled haploid (DH) technology in oat has not reached the same stage as in other cereals leading to its application in plant breeding. The objective of this investigation was to increase the effectiveness of Avena sativa L. haploid embryo germination obtained by the distant crosses with maize. Developed embryos (obtained from 22 genotypes) were transferred on five germination media: MS (Murashige and Skoog, Physiol Plant 15:473–497, 1962) with 3% sucrose, pH 5.8 (control medium), and 190-2 supplemented with 6 and 9% maltose. The pH of 190-2 was adjusted to 5.5 and 6.0. Of all tested genotypes, 591 haploid embryos were obtained, almost half of them (279) germinated. The rate of haploid embryo germination induced on 190-2 was 6.92%, while in MS it was 3.25%. The sugar and its concentration significantly affected the germination of haploid embryos. The highest percentage of haploid embryo germination (9.11%) and DH lines production (1.64%) was achieved on 190-2 with 9% maltose and pH 6.0. All DH lines are incorporated to breeding programs for the development of new cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oat (Avena sativa L.) is a cereal primarily used for its seeds, for human consumption as well as livestock fodder (Chaudhary et al. 2014). The development of new oat cultivars is mainly based on the several years of plant crossing leading to the high homozygosity and selection of individuals with desirable traits. The method to shorten even a few years of the above procedure is to obtain haploid plants and then doubled haploids (DH) by in vitro culture methods. Conventional methods used for new cultivars production depends on many years of inbred crossing and plant selection. However, by applying biotechnological methods such as generating in vitro DH, from which completely homozygous plants are derived, reduces this time significantly to a single generation. This intense time reduction is due to the fact that haploid-based methods produce homozygous lines in the first generation, as opposed to inbreeding. In addition, the DH lines are very helpful in a fast determining of the desired plants features and might be use for construction of mapping populations. It accelerates the breeding progress in this species, thus minimizing the impact of the environment and save workload, time and material costs. Accelerating the process of new cultivars production greatly increases the competitiveness of oat breeders on the European market. DH lines are highly homozygous and genetically stable, which guarantees that the subsequent populations will be identical in both genetic and phenotypic terms and that there is no risk of undesirable parental patterns occurring during long term inbred line selection.
DHs are commonly produced by androgenesis, gynogenesis, wide hybridization or interspecific crosses which result in chromosomes elimination (Ferrie et al. 2014). However, the low efficiencies of the available methods limit the use of haploids in oat breeding and research (Sidhu et al. 2006).
In Avena sativa L., DH lines were produced by in vitro microspore or anther culture (Kiviharju et al. 2005; Tanhuanpää et al. 2008; Ponitka and Ślusarkiewicz-Jarzina 2009; Sidhu and Davies 2009; Ferrie et al. 2014), and by distant crosses with maize (Zea mays L.) (Sidhu et al. 2006; Nowakowska et al. 2015; Warchoł et al. 2016; Skrzypek et al. 2016).
Using oat hybridization with maize, embryos are often aborted as a consequence of wide crossing. For this reason, it is necessary to remove the embryo from the mother plant and rescue the embryo in order for it to survive, because in other way it cannot develop. Usually, the rescue is carried out by direct transfer of the excised embryos to culture medium or indirectly by the culture of ovaries or ovules. The causes of the post-fertilization incompatibility include chromosome alterations, ploidy differences, absence or delayed degenerating endosperm development, or incompatibility between different chromosome sets. The culture medium substitutes the endosperm and supplies nutrients to the embryo. The rescue of immature embryos needs the more complex procedure than the mature embryos (Lulsdorf et al. 2014). Abortion of haploid embryos of oat is usually a consequence of failure of endosperm development in caryopses; thus they need to be cultured on the nutrient media in order to convert into plants. Development of oat haploid embryos was described so far on the following media: B5 (Gamborg et al. 1968) by Sidhu et al. (2006); 190-2 (Zhuang and Xu 1983) by Nowakowska et al. (2015) and Warchoł et al. (2016); MS (Murashige and Skoog 1962) by Rines and Dahleen (1990), or ½ MS (half strength MS) by Kynast et al. (2001); ½ LS (Linsmaier and Skoog 1965) by Ishii et al. (2013). Rescue medium also affects wheat haploid embryo germination. Variation in germination efficiency of this species has been extensively tested in relation to several culture media: MS (Murashige and Skoog 1962), ½ MS and B5 (Gamborg et al. 1968) (Dogramaci-Altuntepe and Jauhar 2001). The major elements influencing the haploid embryo germination are the type and concentration of carbohydrates supplemented in the media. Rines et al. (1997) described that the bilayer medium (Iglesias et al. 1994) used for germination of wheat embryos, increased twice number of haploid oat embryos that developed to haploid plants. In this protocol, embryos first were put on medium with 15% sucrose and then were placed on a standard medium with 3% sucrose. This system provided the high medium osmolarity which stimulated embryo development and then by the reduction of osmolarity due to the sucrose permeation permitting embryos to germinate. Five different sucrose concentrations (0, 2, 5, 8 and 10%) for rescuing wheat embryos were tested by Niu et al. (2014) who found that the maximum embryo germination rate was obtained at 5%. The most frequently used sucrose concentrations in the culture of oat haploid embryos were 2, 3 and 7% (Kynast and Riera-Lizarazu 2011; Ishii et al. 2013). In addition, maltose supplementation (6 and 9%) was used for oat haploid embryos instead of sucrose in the germination media (Marcińska et al. 2013; Nowakowska et al. 2015; Noga et al. 2016; Skrzypek et al. 2016; Warchoł et al. 2016).
Lulsdorf et al. (2014) reported that the culture of monocotyledonous plants is more simple than the dicocotyledonous plants culture, which requires a multistage procedure for embryos culture, as well as shoot and root development. Every stage needs a particular medium and growing conditions. Few reports described a simple way of oat haploid plant production on one regeneration medium (Sidhu et al. 2006; Ishii et al. 2013). A two-step procedure for haploid plant production has been also reported (Nowakowska et al. 2015; Warchoł et al. 2016), involving the passage of haploid plants to MS medium to improve the growth of shoots and roots.
Niu et al. (2014) tested a two-step procedure for wheat embryos culture, in which the excised embryos were placed on a 20-day-old seed endosperm and then cultured on MS medium. In spite of that this technique was very laborious, and was more effective for immature embryos’ development. The mature embryos were directly cultured on MS medium.
Modification of the medium components was the main route to develop protocols for embryo rescue that involved adaptations of the basal medium, sucrose concentration or vitamin and growth regulator content. Rescue of embryos became a helpful element of breeding programs which involved interspecies crossing, a reduction of time needed for next progeny production and preservation of genetic resources (Lulsdorf et al. 2014).
The objective of this investigation was to increase the effectiveness of Avena sativa L. haploid embryo germination obtained by the distant crosses with maize. The efficiency of haploid embryo germination was compared on five media differing in nutrient composition, sucrose concentration and pH. Furthermore, the development of haploids and doubled haploids was investigated.
Materials and methods
Plant material
Twenty-two oat (Avena sativa L.) genotypes (F1 progeny): DC09002 (Arab × Typhon), DC09006 (Bingo × Zuch), DC09012 (Breton × Krezus), DC09028 (Arkan × Bingo), DC09034 (CHD1430/02 × Bingo), DC09040 (Arden × Bingo), DC09105 (Naklan × Bingo), DC09120 (DCK98/16 × Skorpion), DC09121 (DCK98/16 × Ivory), DC09140 (Typhon × Flämingsprofi), DC09163 (Flämingsprofi × Breton), DC09166 (Flämingsprofi × Arab), DC09174 (Ivory × Breton), POB91 (Stork × Zolak), POB92 (Auron × Krezus), POB93 (Zuch × Krezus), STH201 (Expander × Skorpion), STH203 (Chimene × STH 85869(b)), STH204 (Chimene × STH85763(b)), STH205 (Bingo × Bajka), STH208 (Bingo × STH81957), STH228 (Chimene × Bingo) derived from Danko Plant Breeding Ltd., Małopolska Plant Breeding Ltd. HBP Polanowice and Strzelce Plant Breeding Ltd. were used in the study. A mixture of three genotypes of maize (Zea mays L. var. saccharata): MPC4, Dobosz and Wania obtained from Małopolska Plant Breeding Ltd. HBP Polanowice were used as pollen donors. The mixture of maize pollen was chosen according to Skrzypek et al. (2016). Oat and maize were grown in the glasshouse under natural (solar) light intensity (photosynthetic active radiation (PAR) was 800 µmol m− 2 s− 1) and 16-h light/8-h dark. Oat was cultivated at 21/17 °C day/night, while maize at 21–28/17 °C day/night. Every week plants were watered with Hoagland and Arnon medium (Hoagland and Arnon 1938).
Haploid plant production
The development of oat caryopses without endosperms (emasculation, pollination and auxin treatment) was described in detail by Warchoł et al. (2016). The enlarged ovaries were isolated 21 days after florets pollination, disinfected in 70% ethanol (60 s.), 2.5% calcium hypochlorite (7 min.), 0.1% mercuric chloride (60 s.) and in sterile distilled water (3×). Then, each embryo was transferred to 60 mm × 15 mm Petri dishes with 190-2 medium (Zhuang and Xu 1983), enriched in 6 or 9% maltose, solidified with 0.6% agar (Sigma-Aldrich). The media pH was 5.5 or 6.0 prior to autoclaving at Microjet autoclave (Enbio Technology Ltd., Poland). Culture on MS medium (Murashige and Skoog 1962) with 3% sucrose solidified with 0.6% agar and pH 5.8 was used as a control. Approximately 100 haploid embryos were placed on each medium. Haploid embryos germinated at 21 ± 2 °C, at the 16 h of light at the intensity 60 µmol m− 2 s− 1. Haploid seedlings were grown on MS medium. Haploid plants were transferred from Petri dishes to containers with perlite and then to the soil with sand (3:1). Plants’ acclimation took place in a glasshouse (conditions as described above).
Chromosome doubling
Haploids’ roots were immersed in a 0.1% colchicine with 4% dimethyl sulfoxide (DMSO), 0.025 g dm− 3 gibberellic acid (GA3) and 20 µl of Tween 20. Plants were left for 7.5 h at 25 °C and 80–100 µmol m− 2 s− 1 light intensity and roots were rinsed with water for 48 h. Next, plants were placed in 3 dm− 3 pots with soil and sand (3:1). DNA content was estimated before and after colchicine usage by a MACS Quant flow cytometer (Miltenyi Biotec GmbH, Germany), with aircooled laser (488 nm) and MACSQuantifyTM software, as described by Warchoł et al. (2016).
Statistical analysis
Data analysis was done using analysis of variance (ANOVA), Duncan’s test and principal component analysis (PCA) were implemented in the STATISTICA 10.0 (Stat-Soft, Inc., USA). Significant differences between treatments were marked at p ≤ 0.05.
Results
Analysis of variance showed significant differences in the efficiency of haploid embryo germination relative to the kind of medium, the type and concentration of sugar and pH of the media used in the experiment (Table 1). There were no significant differences among genotypes in embryo germination. All examined factors did not have a significant impact on the number of produced DH lines (data not shown).
The effect of oat genotype on the efficiency of oat doubled haploid production
Five thousand eight hundred and fifty-seven oat florets from 22 genotypes were emasculated and pollinated with maize pollen (Table 2). Twenty-one days after pollination, 591 haploid embryos (Fig. 1a) were found in all tested genotypes (Table 2). The amount of haploid embryos varied among genotypes from 19 to 42. The highest amount of haploid embryos was developed by the DC09028, whereas the lowest by the DC09105. Almost half of haploid embryos (279) germinated (Fig. 1b).
In vitro culture of haploid embryos resulted in the development of 132 haploid plants (Fig. 1c; Table 2). Conversion of haploid embryos into plants ranged from 1 to 13 haploid plants per genotype. Nearly two-thirds of haploid plants did not survive the process of acclimatization to natural conditions. Chromosome doubling using colchicine was a critical step of the whole procedure. Fifty-four plants survived this treatment, and 48 DH lines were obtained from them (Fig. 1d; Table 2). The highest amount of DH lines was developed from genotypes DC09002 and STH203 (6 and 5, respectively). The highest survival rate was recorded for genotypes DC09002, DC09166 and STH203. Only two genotypes (DC09120 and STH201) did not develop DH lines. In total, the DH lines produced 4,878 seeds, and the most productive genotypes were DC09040, POB92 and DC09166 (263, 244 and 209 seeds per DH line, respectively).
Principal component and biplot analysis
Biplot analysis identified superior oat genotypes depending on the regeneration abilities, as the genotypes were simultaneously compared for all the parameters (Fig. 2a). Biplot analysis shows correlation for all the studied parameters. Acute angle between the measured parameters means that positive correlation was observed. The first two principal components (PCA) accounted for 89.26% of the total variation of the data set. PCA revealed that the first PC explained 73.95% of the variation with developed haploid embryos, germinated haploid embryos, haploid plants on MS medium, haploid plants in perlite, haploid plants after colchicine treatment and doubled haploid lines. All above-mentioned features were calculated per 100 emasculated florets. The second PC explained 15.31% of the total variability. The PCA indicated that parameters describing regeneration abilities could discriminate between the oat genotypes (Fig. 2b). The genotypes with lower PC1 and PC2 (III quadrant) developed the most haploid embryos per emasculated florets. Embryos from these genotypes were also better in conversion into plants. The genotypes with lower PC1 and higher PC2 (II quadrant) were better in terms of acclimation for natural conditions, chromosome doubling and finally DH lines production. The genotypes grouped in the I and IV quadrants had the lowest DH plant production. These parameters allowed to separate and identify genotypes according to their regeneration abilities. The PCA as well as analysis of variance did not indicate the differences in regeneration abilities due to the genetic background of studied genotypes.
Biplot based on first two principal component axes (PC 1 and PC 2) for parameters determining oat regeneration abilities calculated per 100 of emasculated florets (EF): DHE/EF—developed haploid embryos, GHE/EF—germinated haploid embryos, HP on MS/EF—haploid plants on MS medium, HP in perlite/EF—haploid plants in perlite, HP after colchicine/EF—haploid plants after colchicine treatment, DH lines/EF—doubled haploid lines of 22 oat genotypes (a) and distribution of 22 oat genotypes based on the first two components obtained from principal component analysis (b); I quadrant—oat genotypes numbered: STH228 (Chimene × Bingo), STH205 (Bingo × Bajka), STH208 (Bingo × STH81957), POB91 (Stork × Zolak), POB92 (Auron × Krezus), DC09140 (Typhon × Flämingsprofi), DC09105 (Naklan × Bingo); II quadrant: STH203 (Chimene × STH 85869b), DC09002 (Arab × Typhon), DC09163 (Flämingsprofi × Breton), DC09034 (CHD1430/02 × Bingo), DC09174 (Ivory × Breton), DC09166 (Flämingsprofi × Arab); III quadrant: DC09006 (Bingo × Zuch), DC09040 (Arden × Bingo), DC09012 (Breton × Krezus), DC09028 (Arkan × Bingo), POB93 (Zuch × Krezus); IV quadrant: STH204 (Chimene × STH85763(b)), STH201 (Expander × Skorpion), DC09121 (DCK98/16 × Ivory), DC09120 (DCK98/16 × Skorpion)
The effect of media, sugars and pH on haploid embryo germination and DH line production
The average haploid embryo germination induced on MS medium was 3.25%, while in the case of 190-2 medium, it was 6.92% (Fig. 3a). Despite the observed difference, the percentage of DH lines production on these media did not differ statistically (MS—0.96%; 190-2—1.26%). As previously mentioned, the type of sugar and its concentration in media significantly affected the haploid embryos germination (Fig. 3b). The highest number of haploid embryos germinated on medium with 9% maltose (7.95%), followed by media with 6% maltose (5.98%), and 3% sucrose (3.25%). Approximately 7.61% of haploid embryos germinated on 190-2 medium with pH adjusted to 6.0, 6.31% on the same medium with pH 5.5 and 3.25% on the control MS medium (Fig. 3c).
The percentage of germinated haploid embryo and DH lines per germinated embryos obtained after pollination by maize, depending on the type of medium (for 190-2 medium bars represents average of maltose concentrations and pH levels) (a), sugar (for both concentrations of maltose bars represents average of pH levels) (b) and pH (for both pH levels bars represents average of maltose concentrations) (c). Mean ± SE. Significant differences according to Tukey test at the 0.05 probability level (separately for germinated embryos/emasculated florets and DH lines/emasculated florets) are marked with different letters; ns not significant
Haploid embryos of only seven genotypes of 22 tested germinated on the control medium (MS with 3% sucrose, pH 5.8) (Table 3). The highest percentage of haploid embryos that germinated were from genotypes DC09174 (22.2%), POB92 (16.9%) and STH205 (13.9%). Haploid embryos of STH205 did not germinate on 190-2 medium with 6% maltose and pH 5.5. The highest percentage of germinated embryos were recorded for genotypes DC09174 (13.9%), POB92 (11.5%) and DC09006 (10.1%) on the 190-2 medium with 6% maltose and 5.5 pH. Elevating the pH of this medium to 6.0 caused an increase of germination in 10 genotypes. The highest increase was observed for STH205 (from 0 to 11.3%), whereas embryos from two genotypes (DC09140 and POB93) did not germinate at all.
Maltose at 9% in the 190-2 medium supported germination of haploid embryos of all tested genotypes. When pH was adjusted to 5.5, the germination rate ranged from 0.7 to 17.6%. Higher pH (6.0) resulted in the germination rate between 3.4–17.6%. The increase of medium pH caused better germination of 14 genotypes.
Overall, the most effective germination of haploid embryos was observed on 190-2 medium with 9% maltose and pH 6.0 (9.11%). The efficiency of germination was lower approximately by 2–3% on other media with maltose. The lowest germination was observed on MS with sucrose, which reduced the germination by 6% compared with the most effective medium (190-2 with 9% maltose, pH 6.0).
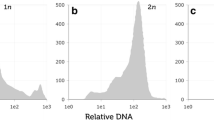
Although media affected haploid embryo germination, it did not influence further development of plants. The media used for germination showed no subsequent impact on further steps of DH line production. Embryos from only 2 genotypes (DC09166 and DC09174), which germinated on MS medium, converted to DH plants. On 190-2 medium variants, only haploid embryos from the STH201 genotype did not produce DH lines. The average DH line production on the two 190-2 media ranged from 0.93 to 1.64%. These results show that acclimatization and chromosome doubling are the critical steps of this procedure, which limit the number of obtained DH lines (see Table 3). The ploidy of the plants treated with colchicine solution was confronted with diploid oat (Supplementary Fig. 1). Cytometric measurements show that the applied procedure doubled chromosome number of obtained plants.
Discussion
Embryo in vitro cultures are the method used more than fifty years to help in developing the embryos obtained after alien pollination (Bridgen 1994). The culture of immature embryos is difficult due to the early growth stages of the embryos isolation and complex nutrient medium requirements. The development of haploid embryos can only be achieved through embryo culture. Since the isolation of small embryos often leads to their mechanical injury, in vitro cultures are preferred with the embryos at more mature stages, when they are more likely to germinate (Bridgen 1994).
The crucial phase of embryo culture is the choice of right medium. It is problematic to propose one medium with an exact plant hormone for rescue all of the immature embryos (Mishra and Goswami 2014). Numerous mineral salts were used for embryo in vitro culture regardless of their role in the embryo development (Bhojwani and Razdan 1983). Gamborg’s B5 medium (Gamborg et al. 1968), MS medium (Murashige and Skoog 1962) and 190-2 medium (Zhuang and Xu 1983), with some changes, are broadly applied in embryo recue method. Since early 1990s, oat haploid embryos obtained by pollination with maize were cultured on MS with sucrose (Rines et al. 1997; Kynast et al. 2001). In our investigations, it was found that the culture of oat haploid embryos on 190-2 doubled their germination compared with MS. 190-2 medium which promoted better germination of oat haploid embryos is poorer in macronutrients, mainly nitrogen, potassium, magnesium and calcium comparing to MS medium. First of all, 190-2 has lower nitrogen content than MS and different ratio of ammonium and nitrate ions. 190-2 contains 12.9 mmol/dm3 total nitrogen (18:82, ammonium ions: nitrate ions), whereas MS contains 60.0 mmol/dm3 total nitrogen (34:66, ammonium ions: nitrate ions). 190-2 has also less than MS micronutrients such as iodide, boron, manganese, zinc and lack of molybdenum, copper and cobalt. Mordhorst and Lörz (1993) approved lower nitrogen necessities in barley androgenesis and high plants recovery when nitrogen in medium was low 20–35 mmol/dm3. Similar observation was done by Immonen and Anttila (2000) in rye androgenesis where the highest induction of embryo like structures was on 190-2 medium with a low nitrogen content.
In our experimental system, sugar type was the key factor that determined the germination of haploid embryos. According to Bogunia and Przywara (1999), chemical nature of sugar added to the in vitro culture is critical in the modulation of morphogenetic reactions. It has been found that the development of rescued immature embryos is attributable to the type of carbohydrate and its concentration. Sugars are commonly used as the main source of carbon and energy in the media, but they are also significant in keeping appropriate osmotic potential.
Sucrose concentration and the osmotic potential in the regeneration media have a key effect on the germination and conversion of embryos (Mishra and Goswami 2014). A high accumulation of sugar in medium is beneficial for the development of rescued immature embryos, but it inhibits the development of mature embryos (Bogunia and Przywara 1999). Low sucrose concentration (2–3%) is frequently used for mature embryo regeneration, while higher (8–12%), similar to the osmotic potential in embryo sac, is required for immature embryo development. Usually, the older the embryos, the medium with lower osmotic potential is necessary. The high osmolarity prevents precocious germination and prevents the cells at the division stage from entering the elongation stage. The effect of sucrose on the development of embryo or embryo-like structures (ELS) has been examined since the late 1960s, when Nitsch and Nitsch (1969) demonstrated successful regeneration of tobacco via androgenesis. Sucrose is added to the medium at 2–3% and mature embryos are able to grow normally on a semi-solid medium with mineral salts and 2.5–5% sucrose, though an increase in sucrose content can be positive for morphogenesis by inhibiting the propagation of somatic tissues (Sopory and Munshi 1996). At 12%, sucrose promotes the induction of ELS from cultured anther, and at a lower (3%) concentration it favors further multiplication of ELS and their regeneration (Srivastava and Chaturvedi 2011). Some plants need 12–13% sucrose for the development of haploid ELS via androgenesis (Bogunia and Przywara 1999). According to Mishra and Goswami (2014), the kind and level of sugar added to the media for haploid induction varies among methods and species. A high sucrose concentration (7%) was shown to be helpful in the formation of oat haploid embryos (Rines and Dahleen 1990), whereas in the summer squash (Cucurbita pepo L.) 9% sucrose was deleterious for the embryo development (Shalaby 2007). According to Thorpe et al. (2008), the use of high sucrose concentrations has been frequently described in studies on androgenesis, when the media supplementation with 5–20% sucrose stimulated the microspores to the embryo formation. Osmotic potential initiates the embryo formation, but high carbohydrate content is not needed for further growth.
In our experiment, the germination of oat haploid embryos was associated with the type of sugar and its concentration in the media. Fewer haploid embryos germinated on medium with 3% sucrose, compared with 9% maltose. Maltose is not only taken up more slowly than sucrose, but its hydrolysis is also longer. The favorable effect of maltose over sucrose as a carbohydrate source was discussed in the production of certain cereal haploids by Małuszyński et al. (2003). In rye, 6% maltose was most effective for regeneration of haploids in anther culture (Immonen and Anttila 2000), while 9% maltose in triticale (Żur et al. 2014). Karsai et al. (1994) reported that maltose increased callus proliferation and plant formation in androgenesis of triticale and wheat. Maltose also increased regeneration of indica and japonica rice (Biswas and Zapata 1993; Jain et al. 1997) and germination of asparagus embryos (Kunitake et al. 1997).
The media pH might regulate the differentiation in plant tissue culture, similarly to substances modifying cellular pH (Thorpe et al. 2008). The media pH is changed throughout culture, but the initial pH should be 5.5–6.0. In media, the negative effects of unsuitable pH are mostly connected with ion and nutrient accessibility. Most plants’ tissue cultures accept pH ca. 4.0–7.2. The best effects are achieved in a little acidic environment. The typical pH implemented by numerous micropropagation methods on various media was 5.6, but regulations to 3.5 and 7.1 were also attempted. The culture of isolated zygotic embryos should have a medium pH not greater than 5.2 (Thorpe et al. 2008). In the current study, oat haploid embryos developed better on medium with pH 6.0 than on medium with pH 5.5; however, there was the relation between the kind of sugar and medium pH.
According to Rines (2003) the quite low rate of embryo development joined with often low frequencies of less embryo germination and conversion into plants causes difficulty to perform research of satisfactory statistical evaluation of conditions influencing efficiency of plant regeneration. As the efficiency of this method is still low, it should be taken into consideration that even some genotypes formed haploid embryos which germinate, but not always develop into vigorous plants.
In conclusion, the efficiency of oat haploid embryo recovery and conversion depends mostly on the type of medium as well as sugar and its concentration, and ranged from 3.25 to 9.11%. In our research 591 haploid embryos were formed from 22 genotypes. Finally, we produced 48 fertile DH plants producing in all 4,878 seeds. All DH lines are tested for new cultivars by polish breeding companies. According to our knowledge, this is the first study describing the influence of media, sugar and pH on oat haploid embryo germination and conversion into plants.
Author contribution statement
MW, ES designed experiment, analyzed the data and wrote the manuscript; MW, ES, IC-M, KD, AN, IM carried out the experiment. AN carried out statistical analysis. All these authors have read and approved the final manuscript.
References
Bhojwani SS, Razdan MK (1983) Plant Tissue Culture: Theory and Practice. Elsevier Science Publishers B.V., Amsterdam
Biswas GCG, Zapata FJ (1993) High-frequency plant regeneration from protoplasts of indica rice (Oryza sativa L.) using maltose. J Plant Physiol 141:470–475
Bogunia H, Przywara L (1999) Rola cukrowców w roślinnych kulturach in vitro. Wiadomości Botaniczne 43(1/2):25–36 (in Polish)
Bridgen MP (1994) A review of plant embryo culture. Hort Science 29(11):1243–1246
Chaudhary HK, Kaila V, Rather SA, Tayeng T (2014) Distant hybridization and doubled-haploidy breeding In: Pratap A, Kumar J (eds) Alien gene transfer in crop plants, innovations, methods and risk assessment, vol 1. Springer, New York, pp 143–164
Dogramaci-Altuntepe M, Jauhar PP (2001) Production of durum wheat substitution haploids from durum x maize crosses and their cytological characterization. Genome 44:137–142
Ferrie AMR, Irmen KI, Beattie AD, Rossnagel BG (2014) Isolated microspore culture of oat (Avena sativa L.) for the production of doubled haploids: effect of pre-culture and post-culture conditions. Plant Cell Tiss Organ Cult 116:89–96
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Hoagland DR, Arnon DI (1938) A water culture method for growing plants without soil. Circ Univ Calif, Agric Exp Stn No. 347
Iglesias VA, Gisel A, Potrykus I, Sautter C (1994) In vitro germination of wheat proembryos to fertile plants. Plant Cell Rep 13:377–380
Immonen S, Anttila H (2000) Media composition and anther plating for production of androgenetic green plants from cultivated rye (Secale cereale L.). J Plant Physiol 156:204–210
Ishii T, Tanaka H, Eltayeb AE, Tsujimoto H (2013) Wide hybridization between oat and pearl millet belonging to different subfamilies of Poaceae. Plant Reprod 26:25–32
Jain RK, Davey MR, Cocking EC, Wu R (1997) Carbohydrate and osmotic requirements for high-frequency plant regeneration from protoplast-derived colonies of indica and japonica rice varieties. J Exp Bot 48:751–758
Karsai I, Bedo Z, Hayes PM (1994) Effect of induction medium pH and maltose concentration on in vitro androgenesis of hexaploid winter triticale and wheat. Plant Cell Tissue Organ Cult 39:49–53
Kiviharju E, Moisander S, Laurila J (2005) Improved green plant regeneration rates from oat anther culture and the agronomic performance of some DH lines. Plant Cell Tiss Org Cult 81:1–9
Kunitake H, Nakashima T, Mori K, Tanaka M (1997) Normalization of asparagus somatic embryogenesis using a maltose-containing medium. J Plant Physiol 150:458–461
Kynast RG, Riera-Lizarazu O (2011) Development and use of oat-maize chromosome and radiation hybrids. In: Birchler JS (ed) Plant chromosome engineering: methods and protocols, Methods in Molecular Biology, vol 701. Humana Press, New York, pp 259–284
Kynast RG, Riera-Lizarazu O, Vales MI, Okagaki RJ, Maquieira SB, Gang C, Ananiev EV, Odland WE, Russell ChD, Stec AO, Livingston SM, Zaia HA, Rines HW, Philips RL (2001) A complete set of maize individual chromosome additions to the oat genome. Plant Physiol 25:1216–1227
Linsmaier EM, Skoog F (1965) Organic growth factor requirement of tobacco tissue cultures. Physiol Plantarum 18:100–127
Lulsdorf MM, Ferrie A, Slater SMH, Yuan HY (2014) Methods and role of embryo rescue technique in alien gene transfer. In: Pratap A, Kumar J (eds) Alien gene transfer in crop plants, innovations, methods and risk assessment vol 1, Springer, New York, pp 77–103
Małuszyński M, Kasha KJ, Forster BP, Szarejko I (2003) Doubled haploid production in crop plants—a manual. Kluwer, Dordrecht/Boston/London, p 428
Marcińska I, Nowakowska A, Skrzypek E, Czyczyło-Mysza I (2013) Production of double haploids in oat (Avena sativa L.) by pollination with maize (Zea mays L.). Cent Eur J Biol 8:306–313
Mishra VK, Goswami R (2014) Haploid production in higher plant. Int J Chem Biol Sci 1(1):25–45
Mordhorst AP, Lörz H (1993) Embryogenesis and development of isolated barley (Hordeum vulgare L.) microspores are influenced by the amount and composition of nitrogen sources in culture media. J Plant Physiol 142:485–492
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Niu Z, Jiang A, Abu Hammad W, Oladzadabbasaadi A, Xu SS, Mergoum M, Elias EM (2014) Review of doubled haploid production in durum and common wheat through wheat x maize hybridization. Plant Breed 133:313–320
Noga A, Skrzypek E, Warchoł M, Czyczyło-Mysza I, Dziurka K, Marcińska I, Juzoń K, Warzecha T, Sutkowska A, Nita Z, Werwińska K (2016) Conversion of oat (Avena sativa L.) haploid embryos into plants in relation to embryo developmental stage and regeneration media. In Vitro Cell Dev Biol Plant 52:590–597
Nowakowska A, Skrzypek E, Marcińska I, Czyczyło-Mysza I, Dziurka K, Juzoń K, Cyganek K, Warchoł M (2015) Application of chosen factors in the wide crossing method for the production of oat doubled haploids. Open Life Sci 10:112–118
Ponitka A, Ślusarkiewicz-Jarzina A (2009) Regeneration of oat androgenic plants in relation to induction media and culture condition of embryo-like structures. Acta Soc Bot Pol 78(3):209–213
Rines HW, Dahleen LS (1990) Haploid oat plants produced by application of maize pollen to emasculated oat florets. Crop Sci 30:1073–1078
Rines HW, Riera-Lizerazu O, Nunez VM, Davis DW, Phillips RL (1997) Oat haploids from anther culture and from wide hybridizations. In: Jain SM, Sopory SK, Veilleux RE (eds) In vitro haploid production in higher plants, vol 4. Kluwer Acad Publishers, Dordrecht, pp 205–221
Shalaby TA (2007) Factors affecting haploid induction through in vitro gynogenesis in summer squash (Cucurbita pepo L.). Sci Hortic 115:1–6
Sidhu PK, Davies PA (2009) Regeneration of fertile green plants from oat isolated microspore culture. Plant Cel Rep 28:571–577
Sidhu PK, Howes NK, Aung T, Zwer PK, Davies PA (2006) Factors affecting haploid production following oat x maize hybridization. Plant Breed 125:1–6
Skrzypek E, Warchoł M, Czyczyło-Mysza I, Marcińska I, Nowakowska A, Dziurka K, Juzoń K, Noga (A 2016) The effect of light intensity on the production of oat (Avena sativa L.) doubled haploids through oat x maize crosses. Cer Res Comm 44/3:490–500
Sopory S, Munshi M (1996) Anther culture. In: Mohan JM et al (eds) In vitro haploid production in higher plants, vol 1. Kluwer, Dordrecht, pp 145–176
Srivastava P, Chaturvedi R (2011) Increased production of azadirachtin from an improved method of androgenic cultures of a medicinal tree Azadirachta indica A. Juss. Plant Signal Behav 6(7):974–981
Tanhuanpää P, Kalendar R, Schulman AH, Kiviharju E (2008) The first doubled haploid linkage map for cultivated oat. Genome 51:560–569
Thorpe T, Stasolla C,. Yeung EC, de Klerk G-J, Roberts A, George EF (2008) The components of plant tissue culture media II: organic additions, osmotic and pH effects, and support systems. In: George EF, Hall MA De Klerk GJ (eds) Plant propagation by tissue culture 3rd Edition, Volume 1. The Background. Springer, Dordrecht, pp 115–175
Warchoł M, Skrzypek E, Nowakowska A, Marcińska I, Czyczyło-Mysza I, Dziurka K, Juzoń K, Cyganek K (2016) The effect of auxin and genotype on the production of Avena sativa L. doubled haploid lines. Plant Growth Regul 78:155–156
Zhuang JJ, Xu J (1983) Increasing differentiation frequencies in wheat pollen callus. In: Hu H, Vega MR (eds) Cell and tissue culture techniques for cereal crop improvement. Science Press, Beijing, p 431
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Płażek A (2014) Antioxidant activity and ROS tolerance in triticale (x Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tiss Organ Cult 119:79–94
Acknowledgements
The research was financed by the Ministry of Agriculture and Rural Development, grant no HORhn- 801-4/12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Peng.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Warchoł, M., Czyczyło-Mysza, I., Marcińska, I. et al. The effect of genotype, media composition, pH and sugar concentrations on oat (Avena sativa L.) doubled haploid production through oat × maize crosses. Acta Physiol Plant 40, 93 (2018). https://doi.org/10.1007/s11738-018-2669-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2669-9