Abstract
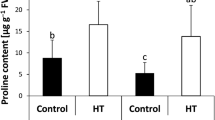
Followed a heat acclimation pretreatment, seedlings of Freesia hybrida ‘Shangnong Jinghuanghou’ were exposed to heat stress at 38°C for 6 h treatment and then recovered at 22°C for 72 h to study the impact of heat acclimation (30°C) on thermotolerance under heat stress. The results showed that the pretreated seedlings performed better under heat stress than control. Heat acclimation could slow down the decrease of chlorophyll contents under heat stress and recover better. Higher levels of soluble sugar and proline and slight lower level of soluble protein were observed in pretreated seedlings. After recovery, similar levels of proline and soluble protein were maintained in all seedlings. However, a higher level of soluble sugar was maintained in pretreated seedlings. MDA content and EL showed a stable level in pretreated seedlings while a significant increase in control, followed by a significant decrease after recovery. Significant different responses of SOD, POD, CAT, and APX activities were observed in pretreated seedlings and control. Heat acclimation led to higher activities of these enzymes and a significant response of antioxidant enzyme activities occurred in a time-dependent manner under heat stress. Exposure to high temperature caused a significant increase in SOD and APX activity, and much higher levels in SOD and APX activity were observed in pretreated seedlings compared to control during heat stress. A slight difference in change pattern of POD and CAT activity was presented. The highest activities of POD and CAT were observed at 4 and 6 h of heat stress in pretreated seedlings and control, respectively. After 72 h recovery, the activities of all tested enzymes decreased to similar levels in all seedlings.



Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- Chl:
-
Chlorophyll
- EL:
-
Electrolytic leakage
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- O ·−2 :
-
Superoxide radical
- POD:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008) A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mungbean (Vigna radiata L. Wilczek). Environ Exp Bot 62:153–159
Berghoef J, Zevenbergen AP (1990) The effect of air and soil temperature on assimilate partitioning and flower bud initiation of freesia. Acta Hortic 266:169–176
Blokhina O, Virolainen E, Gagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot (Lond) 91:179–194
Camejo D, Jiménez A, Alarcón JJ, Torres W, Gómez JM, Sevilla F (2006) Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct Plant Biol 33:177–187
Chaitanya KV, Sundar D, Masilamani S, Ramachandra RA (2002) Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul 36:175–180
Chen JX, Wang XF (2006) Plant physiology experimental guide. Higher Education Press, Beijing, pp 24–25, 55–56
Davis DG, Swanson HR (2001) Activity of stress-related enzymes in the perennial weed leafy spurge (Euphorbia esula L.). Environ Exp Bot 46:95–108
De Hertogh AA, Le Nard M (1993) The physiology of flower bulbs: a comprehensive treatise on the physiology and utilization of ornamental flowering bulbous and tuberous plants. Freesia 21:285–296, Elsevier, London
Farooq M, Basra SMA, Wahid A, Cheema ZA, Cheema MA, Khaliq A (2008) Physiological role of exogenously applied glycinebetaine in improving drought tolerance of fine grain aromatic rice (Oryza sativa L.). J Agron Crop Sci 194:325–333
Foyer CH, Lelandai M, Kunert KJ (1994a) Photooxidative stress in plants. Physiol Plant 92:696–717
Foyer C, Descourvieres P, Kunert KJ (1994b) Protection against oxygen radicals: an important defense mechanism studied in transgenic plant. Plant Cell Environ 17:507–523
Gong M, Li YJ, Dai X, Tian M, Li ZG (1997) Involvement of calcium and calmodulin in the acquisition of heat-shock-induced thermotolerance in maize seedlings. J Plant Physiol 150:615–621
Gong M, Knight MR, Trewavas AJ (1998) Heat-shock-induced changes of intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol 116:429–437
Guo YP, Zhou HF, Zhang LC (2006) Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hort 108:260–267
Hao JJ, Zhang KL, Yu Y (2006) Experimental technique of plant physiology. Chemical Industry Press, Beijing (in chinese)
Hatice G, Atilla E (2003) Some physiological changes in strawberry (Fragaria × ananassa ‘Camarosa’) plants under heat stress. J Hort Sci Biotech 78:894–898
Horton P, Ruban AV, Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47:655–684
Houghton JT, Ding Y, Griggs DJ, Noguer M, Linden PJ, Xiaosu D (2001) Climate change 2001: the scientific basis contribution of working group first to third assessment report of the intergovernmental panel on climate change. Cambridge University Press, UK
Howarth CJ (2005) Genetic improvements of tolerance to high temperature. In: Ashraf M, Harris PJC (eds) Abiotic stresses: plant resistance through breeding and molecular approaches. Howarth Press Inc., New York
Jain M, Mathur G, Konl S, Sarin NB (2001) Ameliorative effects of praline on salt stress lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep 20:463–468
Jiang YW, Huang BG (2001) Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J Exp Bot 52:341–349
Kavi Kishore PB, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Levitt J (1980) Responses of plants to environmental stresses: water, radiation, salt and other stresses, 2nd edn. Academic Press, New York
Li HS (2000) Experimental principles and technique of plant physiology and biochemistry. Higher Education Press, Beijing (in chinese)
Panchuk II, Volkov RA, Schoffl F (2002) Heat stress and heat shock transcription factor dependent expression and activity of ascorbate peroxidase. Plant Physiol 129:838–853
Qin WY, Lin YX (1995) Freesia research. Shanghai Science and Technology Press, Shanghai, pp 1–12
Sairam RK, Tyagi A (2004) Physiology and molecular biology of salinity stress tolerance in plants. Curr Sci 86:407–421
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638
Scandalios JG, Acevedo A, Ruzsa S (2000) Catalase gene expression in response to chronic high temperature stress in maize. Plant Sci 156:103–110
Sreenivasulu N, Sopory SK, Kavi-Kishor PB (2007) Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 388:1–13
Sun W, Montagu MV, Verbruggen N (2002) Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta 1577:1–9
Wahid A, Close TJ (2007) Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol Plant 51:104–109
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang L (2007) Freesia. In: Anderson NO (ed) Flower breeding and genetics. Springer, pp 665–693
Wang LJ, Li SH (2006) Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul 48:137–144
Xu S, Li JL, Zhang XQ, Wei H, Cui LJ (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplast in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Ye LA, Gao HY, Zou Q (2000) Responses of antioxidant systems and xanthophylls cycle in Phaseolus vulgaris to combined stress of high irradiance and high temperature. Photosynthetica 38:205–210
Yin H, Chen QM, Yi MF (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul 54:45–54
Zabalza A, Gálvez L, Marino D, Royuela M, Arrese-Igor C, González EM (2008) The application of ascorbate or its immediate precursor, galactono-1, 4-lactone, does not affect the response of nitrogen-fixing pea nodules to water stress. J Plant Physiol 165:805–812
Zhang XZ (1992) Crop physiology research methods. China Agriculture Press, Beijing, pp 148–149
Zhu JC, Mawjuda Y (1998) The relationship between the thermal stability and chloroplast in plant. J Xinjiang Normal Univ (Nat Sci Ed) 17(1):47–52
Acknowledgments
The work was supported by National Natural Science Foundation of China (No: 30872061).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Hajduch.
Rights and permissions
About this article
Cite this article
Yuan, Y., Qian, H., Yu, Y. et al. Thermotolerance and antioxidant response induced by heat acclimation in Freesia seedlings. Acta Physiol Plant 33, 1001–1009 (2011). https://doi.org/10.1007/s11738-010-0633-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0633-4