Abstract
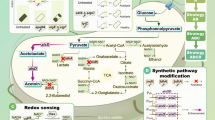
Acetoin is an important platform chemical, which has a wide range of applications in many industries. Halomonas bluephagenesis, a chassis for next generation of industrial biotechnology, has advantages of fast growth and high tolerance to organic acid salts and alkaline environment. Here, α-acetolactate synthase and α-acetolactate decarboxylase from Bacillus subtilis 168 were co-expressed in H. bluephagenesis to produce acetoin from pyruvate. After reaction condition optimization and further increase of α-acetolactate decarboxylase expression, acetoin production and yield were significantly enhanced to 223.4 mmol·L−1 and 0.491 mol·mol−1 from 125.4 mmol·L−1 and 0.333 mol·mol−1, respectively. Finally, the highest titer of 974.3 mmol·L−1 (85.84 g·L−1) of acetoin was accumulated from 2143.4 mmol·L−1 (188.6 g·L−1) of pyruvic acid within 8 h in fed-batch bioconversion under optimal reaction conditions. Moreover, the reusability of the cell catalysis was also tested, and the result illustrated that the whole-cell catalysis obtained 433.3, 440.2, 379.0, 442.8 and 339.4 mmol·L−1 (38.2, 38.8, 33.4, 39.0 and 29.9 g·L−1) acetoin in five repeated cycles under the same conditions. This work therefore provided an efficient H. bluephagenesis whole-cell catalysis with a broad development prospect in biosynthesis of acetoin.

Similar content being viewed by others
References
Xiao Z, Xu P. Acetoin metabolism in bacteria. Critical Reviews in Microbiology, 2007, 33(2): 127–140
Wang M, Fu J, Zhang X, Chen T. Metabolic engineering of Bacillus subtilis for enhanced production of acetoin. Biotechnology Letters, 2012, 34(10): 1877–1885
Xu H, Cui Y, Tian Y, Wang S, Zhu K, Zhou S, Huang Y, He Q, Han Y, Liu L, Li W, Zhu L, Jiang G, Liu J. A summary of producing acetoin by biological method. World Journal of Food Science and Technology, 2020, 4(4): 90–103
Sun J A, Zhang L Y, Rao B, Shen Y L, Wei D Z. Enhanced acetoin production by Serratia marcescens H32 with expression of a water-forming NADH oxidase. Bioresource Technology, 2012, 119: 94–98
He Y Z, Chen F X, Sun M J, Gao H F, Guo Z W, Lin H, Chen J B, Jin W S, Yang Y L, Zhang L Y, Yuan J. Efficient (3S)-acetoin and (2S,3S)-2,3-butanediol production from meso-2,3-butanediol using whole-cell biocatalysis. Molecules, 2018, 23(3): 691
Cui Z Z, Wang Z W, Zheng M Y, Chen T. Advances in biological production of acetoin: a comprehensive overview. Critical Reviews in Biotechnology, 2021, in press
Maina S, Prabhu A A, Vivek N, Vlysidis A, Koutinas A, Kumar V. Prospects on bio-based 2,3-butanediol and acetoin production: recent progress and advances. Biotechnology Advances, 2021, 54: 107783
Xu H, Jia S R, Liu J J. Development of a mutant strain of Bacillus subtilis showing enhanced production of acetoin. African Journal of Biotechnology, 2011, 10(5): 779–788
Bae S J, Kim S, Hahn J S. Efficient production of acetoin in Saccharomyces cerevisiae by disruption of 2,3-butanediol dehydrogenase and expression of NADH oxidase. Scientific Reports, 2016, 6(1): 1–8
Lu L X, Mao Y F, Kou M Y, Cui Z Z, Jin B, Chang Z S, Wang Z W, Ma H W, Chen T. Engineering central pathways for industrial-level (3R)-acetoin biosynthesis in Corynebacterium glutamicum. Microbial Cell Factories, 2020, 19(1): 102
Bae S J, Kim S, Park H J, Kim J, Jin H, Kim B G, Hahn J S. High-yield production of (R)-acetoin in Saccharomyces cerevisiae by deleting genes for NAD(P)H-dependent ketone reductases producing meso-2,3-butanediol and 2,3-dimethylglycerate. Metabolic Engineering, 2021, 66: 68–78
Almuharef I, Rahman M S, Qin W. Enzymatic conversion of glycerol to 2,3-butanediol and acetoin by Serratia proteamaculans SRWQ1. Waste and Biomass Valorization, 2018, 10(7): 1833–1844
Wachtmeister J, Rother D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Chemical biotechnology, 2016, 42: 169–177
Fukuda H, Hama S, Tamalampudi S, Noda H. Whole-cell biocatalysts for biodiesel fuel production. Trends in Biotechnology, 2008, 26(12): 668–673
Yamada-Onodera K, Yamamoto H, Kawahara N, Tani Y. Expression of the gene of glycerol dehydrogenase from Hansenula polymorpha Dl-1 in Escherichia coli for the production of chiral compounds. Acta Biotechnologica, 2002, 22(3–4): 355–362
Bao T, Zhang X, Rao Z, Zhao X J, Zhang R Z, Yang T W, Xu Z H, Yang S T. Efficient whole-cell biocatalyst for acetoin production with NAD+ regeneration system through Homologous co-expression of 2,3-butanediol dehydrogenase and NADH oxidase in engineered Bacillus subtilis. PLoS One, 2014, 9(7): e102951
Zhou X L, Zhou X, Zhang H Y, Cao R, Xu Y. Improving the performance of cell biocatalysis and the productivity of acetoin from 2,3-butanediol using a compressed oxygen supply. Process Biochemistry, 2018, 64: 46–50
Peng K, Guo D, Lou Q, Lu X, Cheng J, Qiao J, Lu L, Cai T, Liu Y, Jiang H. Synthesis of ligustrazine from acetaldehyde by a combined biological-chemical approach. ACS Synthetic Biology, 2020, 9(11): 2902–2908
Cui Z Z, Mao Y F, Zhao Y J, Zheng M Y, Wang Z W, Ma H W, Chen T. One-pot efficient biosynthesis of (3R)-acetoin from pyruvate by a two-enzyme cascade. Catalysis Science & Technology, 2020, 10(22): 7734–7744
Jia X, Liu Y, Han Y. A thermophilic cell-free cascade enzymatic reaction for acetoin synthesis from pyruvate. Scientific Reports, 2017, 7(1): 1–10
Pundir C S, Malik M, Chaudhary R. Quantification of pyruvate with special emphasis on biosensors: a review. Microchemical Journal, 2019, 146: 1102–1112
Chen G Q, Jiang X R. Next generation industrial biotechnology based on extremophilic bacteria. Current Opinion in Biotechnology, 2018, 50: 94–100
Tan D, Wu Q, Chen J C, Chen G Q. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates. Metabolic Engineering, 2014, 26: 34–47
Chen X, Yu L, Qiao G, Chen G Q. Reprogramming Halomonas for industrial production of chemicals. Journal of Industrial Microbiology & Biotechnology, 2018, 45(7): 545–554
Zhang X, Lin Y, Chen G Q. Halophiles as chassis for bioproduction. Advanced Biosystems, 2018, 2(11): 1800088
Yin J, Chen J C, Wu Q, Chen G Q. Halophiles, coming stars for industrial biotechnology. Biotechnology Advances, 2015, 33(7): 1433–1442
Ye J, Hu D, Che X, Jiang X, Li T, Chen J, Zhang H M, Chen G Q. Engineering of Halomonas bluephagenesis for low cost production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose. Metabolic Engineering, 2018, 47: 143–152
Ling C, Qiao G Q, Shuai B W, Olavarria K, Yin J, Xiang R J, Song K N, Shen Y H, Guo Y, Chen G Q. Engineering NADH/NAD(+) ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA). Metabolic Engineering, 2018, 49: 275–286
Chen Y, Chen X Y, Du H T, Zhang X, Ma Y M, Chen J C, Ye J W, Jiang X R, Chen G Q. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV). Metabolic Engineering, 2019, 54: 69–82
Zhao H, Yao Z, Chen X, Wang X, Chen G Q. Modelling of microbial polyhydroxyalkanoate surface binding protein PhaP for rational mutagenesis. Microbial Biotechnology, 2017, 10(6): 1400–1411
Ma H, Zhao Y, Huang W, Zhang L, Wu F, Ye J, Chen G Q. Rational flux-tuning of Halomonas bluephagenesis for coproduction of bioplastic PHB and ectoine. Nature Communications, 2020, 11(1): 1–12
Li T, Guo Y Y, Qiao G Q, Chen G Q. Microbial synthesis of 5-aminolevulinic acid and its coproduction with polyhydroxybutyrate. ACS Synthetic Biology, 2016, 5(11): 1264–1274
Jiang X R, Yan X, Yu L P, Liu X Y, Chen G Q. Hyperproduction of 3-hydroxypropionate by Halomonas bluephagenesis. Nature Communications, 2021, 12(1): 1–13
Du H, Zhao Y, Wu F, Ouyang P, Chen J, Jiang X, Ye J, Chen G Q. Engineering Halomonas bluephagenesis for L-threonine production. Metabolic Engineering, 2020, 60: 119–127
Zhang J, Jin B, Hong K, Lv Y, Wang Z, Chen T. Cell catalysis of citrate to itaconate by engineered Halomonas bluephagenesis. ACS Synthetic Biology, 2021, 10(11): 3017–3027
Zhang J, Zhang X, Mao Y, Jin B, Guo Y, Wang Z, Chen T. Substrate profiling and tolerance testing of Halomonas TD01 suggest its potential application in sustainable manufacturing of chemicals. Journal of Biotechnology, 2020, 316: 1–5
Zhao H, Zhang H M, Chen X, Li T, Wu Q, Ouyang Q, Chen G Q. Novel T7-like expression systems used for Halomonas. Metabolic Engineering, 2017, 39: 128–140
Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology, 1983, 1(9): 784–791
Tan D, Xue Y S, Aibaidula G, Chen G Q. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01. Bioresource Technology, 2011, 102(17): 8130–8136
Qin Q, Ling C, Zhao Y, Yang T, Yin J, Guo Y, Chen G Q. CRISPR/Cas9 editing genome of extremophile Halomonas spp. Metabolic Engineering, 2018, 47: 219–229
Silva-Rocha R, Martinez-Garcia E, Calles B, Chavarria M, Arce-Rodriguez A, de Las Heras A, Paez-Espino A D, Durante-Rodriguez G, Kim J, Nikel P I, Platero R, de Lorenzo V. The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Research, 2013, 41(D1): D666–D675
Wang X, Cai P P, Chen K Q, Ouyang P K. Efficient production of 5-aminovalerate from L-lysine by engineered Escherichia coli whole-cell biocatalysts. Journal of Molecular Catalysis B: Enzymatic, 2016, 134: 115–121
Li J X, Huang Y Y, Chen X R, Du Q S, Meng J Z, Xie N Z, Huang R B. Enhanced production of optical (S)-acetoin by a recombinant Escherichia coli whole-cell biocatalyst with NADH regeneration. RSC Advances, 2018, 8(53): 30512–30519
Guo Z, Zhao X, He Y, Yang T, Gao H, Li G, Chen F, Sun M, Lee J K, Zhang L. Efficient (3R)-acetoin production from meso-2,3-Butanediol using a new whole-cell biocatalyst with co-expression of meso-2,3-butanediol dehydrogenase, NADH oxidase, and Vitreoscilla Hemoglobin. Journal of Microbiology and Biotechnology, 2017, 27(1): 92–100
Cui Z Z, Zhao Y J, Mao Y F, Shi T, Lu L X, Ma H W, Wang Z W, Chen T. In vitro biosynthesis of optically pure D-(-)-acetoin from meso-2,3-butanediol using 2,3-butanediol dehydrogenase and NADH oxidase. Journal of Chemical Technology and Biotechnology, 2019, 94(8): 2547–2554
Zajkoska P, Rebros M, Rosenberg M. Biocatalysis with immobilized Escherichia coli. Applied Microbiology and Biotechnology, 2013, 97(4): 1441–1455
Sun J, Rao B, Zhang L, Shen Y, Wei D. Extraction of acetoin from fermentation broth using an acetone/phosphate aqueous two-phase system. Chemical Engineering Communications, 2012, 199(11): 1492–1503
Dai J, Guan W, Ma L, Xiu Z. Salting-out extraction of acetoin from fermentation broth using ethyl acetate and K2HPO4. Separation and Purification Technology, 2017, 184: 275–279
Becker J, Lange A, Fabarius J, Wittmann C. Top value platform chemicals: bio-based production of organic acids. Current Opinion in Biotechnology, 2015, 36: 168–175
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0900 200) and the National Natural Science Foundation of China (Grant No. NSFC-21621004). We thank Prof. Guo-Qiang Chen from Tsinghua University for generously providing experimental materials.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zheng, M., Cui, Z., Zhang, J. et al. Efficient acetoin production from pyruvate by engineered Halomonas bluephagenesis whole-cell biocatalysis. Front. Chem. Sci. Eng. 17, 425–436 (2023). https://doi.org/10.1007/s11705-022-2229-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-022-2229-0