Abstract
Endometriosis is a benign inflammatory onco-mimetic disease affecting 10–15% of women in the world. When it is refractory to medical treatments, surgery may be required. Usually, laparoscopy is the preferred approach, but robotic surgery has gained popularity in the last 15 years. This study aims to evaluate the safety and efficacy of robotic-assisted laparoscopic surgery (RAS) versus conventional laparoscopic surgery (LPS) in the treatment of endometriosis. This study adheres to PRISMA guidelines and is registered with PROSPERO. Studies reporting perioperative data comparing RAS and LPS surgery in patients with endometriosis querying PubMed, Google Scholar and ClinicalTrials.gov were included in the analysis. The Quality Assessment of Diagnostic Accuracy Studies 2 tool (QUADAS-2) was used for the quality assessment of the selected articles. Fourteen studies were identified, including 2709 patients with endometriosis stage I-IV for the meta-analysis. There were no significant differences between RAS and LPS in terms of intraoperative and postoperative complications, conversion rate and estimated blood loss. However, patients in the RAS group have a longer operative time (p < 0.0001) and longer hospital stay (p = 0.020) than those in the laparoscopic group. Robotic surgery is not inferior to laparoscopy in patients with endometriosis in terms of surgical outcomes; however, RAS requires longer operative times and longer hospital stay. The benefits of robotic surgery should be sought in the easiest potential integration of robotic platforms with new technologies. Prospective studies comparing laparoscopy to the new robotic systems are desirable for greater robustness of scientific evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis, is an “onco-mimetic” inflammatory disease influenced by estrogen, that impacts the 10–15% of women in their reproductive age [1]. It primarily presents in the pelvic region, manifesting as superficial peritoneal implants, ovarian endometriomas, or “deep” lesions extending beyond the peritoneal surface (> 5 mm), commonly found in areas like the uterosacral ligaments, rectouterine pouch, vagina, bowel, bladder, and ureters. Symptoms vary based on the location and may include dysmenorrhea, chronic pelvic pain, dyspareunia, infertility, and urinary and intestinal function impairment [2]. Surgical excision of lesions is considered recommended if hormonal treatments prove insufficient to manage the symptoms [3, 4], in case of bowel or ureteral stricture or in selective case of infertility [4]. Minimally invasive surgical (MIS) approaches have become predominant in the surgical management of the disease, with laparoscopy as a standard of care [4]. Despite its advantages, conventional laparoscopy has limitations such as 2-dimensional visualization, ergonomic limits, and a restricted range of instruments. Over the past decade, the viability, efficacy, and safety of robotic-assisted surgery (RAS) in addressing deep endometriosis has been reported, demonstrating its non-inferiority to laparoscopy [5]. Robotic systems offer enhanced depth perception, wrist articulation, and dexterity, particularly beneficial for complex cases or challenging anatomical locations like diaphragmatic endometriosis or sites involving the sacral plexus or ischial nerves [6, 7]. The use of robotic articulated instruments, equipped with clutching mechanisms that exceed the range of motion of the human wrist (> 360°), facilitates access to these areas. However, the lack of tactile feedback and the associated high costs of installation and maintenance present obstacles to the widespread adoption of RAS [8]. Despite established benefits in various surgical domains, the superiority of RAS over traditional laparoscopy in treating endometriosis remains unknown [9]. The aim of this meta-analysis is to compare the effectiveness and safety of these approaches in the surgical management of endometriosis.
Methods
The review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. Before data extraction, the review was registered with the International Prospective Register of Systematic Reviews (PROSPERO, Registration N° CRD CRD42023495700).
Eligibility criteria
According to the PICO [10] schema were selected articles focused on comparison between robotic assisted and laparoscopic surgery in deep endometriosis regarding at least one of the following parameters: (i) intraoperative complications (ii) postoperative complications (iii) operative time (iv) conversion rate (v) estimated blood loss (vi) hospital stay. Articles not reporting comparisons between the two surgical approaches were excluded. Only full-text studies were considered eligible for inclusion. Abstracts, reviews, meta-analyses, letters, case reports and editorials were excluded (Table 1).
Search strategy
The studies included for analysis were obtained querying the PubMed database, Google Scholar and ClinicalTrial.gov between September and November 2023, filtered only by English language and publication year (1980–2023). The search strategy is reported in the supplementary material (Online Supplementary A).
Study selection
Rayyan software (Qatar Computing Research Institute, HBKU, Doha, Qatar) [11] was used independently by two authors (MP and AB) to screen titles and abstracts for eligibility. Manual searches were performed on pertinent resources and online links, and references of selected articles were examined. Duplicate entries were eliminated during the title/abstract review. For all relevant studies, the complete text was reviewed by both authors independently. Discordant assessments were resolved by consultation of a third author (MG).
Data collection
Data collection included: author, publication year, country, sample size, age, BMI, rASRM [12], stage previous surgery, intra- and postoperative reported data. We will provide our data for independent analysis by a selected team or for additional data analysis or for the reproducibility of this study in other centers if such is requested.
Assessment of risk of bias
The risk of bias was assessed independently by two reviewers (MP and AB) using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [13]. The risk of bias was assessed for the following domains: patient selection, index test, reference standard, and flow and timing. Discordant assessments were resolved by consultation of a third author (MG).
Analysis and data synthesis
Statistical analyses were performed using R statistical software (version 4.2.1) meta e metaplus statistical package of the software R was used. Risk Ratios (RRs) alongside their 95% confidence intervals (CIs) for intra-, postoperative complications and conversion rates data were extracted from the studies or calculated. To continue variables (operative time (min) OT, estimated blood loss (EBL) and hospitalization stay) SMD were calculated. A random-effects model was used to take the source of heterogeneity related to the clinical setting into account. To assess heterogeneity between studies, the Cochrane’s Q test and I2 index were used. p values of < 0.05 were considered as valid for heterogeneity tests. Pooled estimations and the related 95% CIs were evaluated using forest plots. A funnel plot was depicted for the detection of publication bias.
Results
Study selection and characteristics
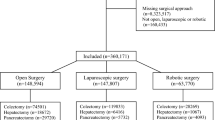
The initial search identified 340 articles. After removing duplicates and title/abstract screening, 79 manuscripts were assessed for eligibility. Of these, were excluded as they addressed a different outcome (51) or a different design (10) or were inaccessible (2) or in a language different than English (1). A list of excluded articles is provided in Online Supplementary B. Consequently, fourteen studies were included for data synthesis (Online Supplementary C) and one prospective trial was identified. The PRISMA flow diagram shows the complete review process from the original search to the final selection (Fig. 1). The Fourteen studies selected for the meta-analysis covered a total of 2709 patients. Of these twelve (85.7%) are retrospective and 2 prospective (14.3%).
Risk of bias of included studies
The quality assessment of the included studies is presented in Online Supplementary D. Most studies were at low risk of bias regarding patient selection, index test, and reference standard domains (8, 61,5%).
Five articles had an unclear risk of bias in the patient’s selection as they reported data on patients without differentiating the rASRM stage [5, 14,15,16,17] while one focused only on stage IV [18]. One was at an unclear risk of bias and applicability in patient selection due to the exclusion of women undergoing bladder ureteral or bowel resection [19].
Meta-analysis
Intra- and postoperative complications
Eight [5, 15,16,17, 20,21,22,23] studies assessed the intra-operative complications of RAS and LPS surgical procedures: the Risk Ratio (RR) of 1.638, 95% CI [0.552; 4.855] and p = 0.373, indicated no significant difference between RAS and LPS. The I2 was 23.3%, and test of heterogeneity suggested low statistical heterogeneity (Fig. 2).
Eleven [5, 15,16,17,18, 20,21,22,23,24,25] studies assessed the post-operative complication of RAS and LPS surgical procedures: the Risk Ratio (RR) of 0.952, 95% CI [0.776; 1.169] and p = 0.642, indicated no significant difference between RAS and LPS. The I2 was 0.0%, and test of heterogeneity suggested low statistical heterogeneity (Fig. 3).
Conversion rate
Four [5, 17, 21, 23] studies assessed the conversion rates of RAS and LPS surgical procedures: the Risk Ratio (RR) of 1.262, 95% CI [0.328; 4.846] and p = 0.734, indicated no significant difference between RAS and LPS. The I2 was 0.0%, and the test of heterogeneity suggested low statistical heterogeneity (Fig. 4).
Operative time
Eleven [5, 14, 15, 17, 20,21,22,23, 25,26,27] studies assessed the operative time of the two surgical procedures. The standardisation mean difference (SMD) of 0.54 (min), 95% CI [0.247; 0.842] and p < 0.0001, shows that the patients in the RAS group have a longer operative time than those of the laparoscopic group. The I2 was 83% and the Cochrane’s Q test significant results (p < 0.0001) suggested high statistical heterogeneity between studies (Fig. 5).
Estimated blood loss
Nine [5, 14, 15, 17, 20,21,22,23, 25] studies assessed the estimated blood loss of RAS and LPS surgical procedures: the standardisation mean difference (SMD) of 0.028, 95% CI [− 0.080; 0.136] and p = 0.616, indicated no significant difference between RAS and LPS. The I2 was 1.8%, and the test of heterogeneity suggested low statistical heterogeneity (Fig. 6).
Length of hospital stay
Seven [17, 20,21,22,23, 25, 26] studies assessed hospitalization stay of RAS vs LPS surgical procedures: the standardisation mean difference (SMD) of 0.135, 95% CI [0.022; 0.262] and p = 0.020, indicated a significant difference between RAS and LPS. The I2 was 26.7%, and the test of heterogeneity suggested low statistical heterogeneity (Fig. 7).
Discussion
The results of this meta-analysis show the absence of significant differences between the robotic-assisted surgery and the standard laparoscopic approach for endometriosis surgery in terms of intraoperative and postoperative complications, conversion rate and estimated blood loss. However, patients in the RAS group have a longer operative time (p < 0.0001) and longer hospital stay (p = 0.020) than those in the laparoscopic group.
These results confirm what was previously reported in the metanalysis of Restaino et al. comprising 5 articles on the same topic, with no statistical differences for operative outcomes and a longer OT reported for RAS with a weighted mean difference of 0.54 (p < 0.00001) [9]. Therefore, discrepancies are reported in the literature regarding OT in RAS procedures for endometriosis. A longer operating time (MD = 28.09 min, CI 11.59–44.59) and an increased average time of use of the operating room (MD = 51.39 min, CI 15.07–87.72;) is also shown by Csirzó et al. in their recent article [28]. However, Magrina et al. [21] after adjusting their findings for age, blood loss, and number of procedures per patient, showed that RAS approach resulted in 16.2% shorter OT than LPS.
A recent prospective multicentre randomized trial (LAROSE trial) enrolling 73 patients with suspicion of pelvic endometriosis, showed a similar OT between RAS and LPS (mean ± SD, 107 ± 48 min vs. 102 ± 63 min) when adjusted to the stage of disease [5]. According to the latter, the study of Raimondo et al. [23] showed no significant difference between the two groups regarding OT.
Among the factors contributing to the extension of the time required to perform robotic surgery is the docking of the platform. However, these times are directly proportional to the team’s experience and decrease with the learning curve [29]. Regarding the longer hospital stay this could be attributable to a bias in the worst health conditions of patients who are candidates for robotic surgery than for LPS (i.e. obesity) [30].
In addition, after two decades of the Da Vinci® surgical robotic system (Intuitive Surgical, California, USA) as the sole protagonist in the field of RAS, the introduction of new robotic platforms on the marketplace with different features (i.e. open consoles, independent bed-side units) may highlight new evidence. The feasibility of surgical interventions for endometriosis using the new HUGO™ RAS (Medtronic, Minneapolis, USA) [31, 32] has already been demonstrated while for other new platforms as the Versius (CMR robotics, UK) system studies are ongoing [33]. Robotic single-site surgery for managing endometriosis was carried out by Huang et al. In 12% of cases, an extra port was introduced to facilitate greater precision of instruments and to address a broader surgical field, particularly in instances involving more complex locations [34]. Despite the growing global adoption of robotic surgery and the increased expertise among surgeons, there is currently insufficient evidence to establish the superiority of robotic surgery over standard laparoscopy in endometriosis surgery. The limited reimbursement for robotic procedures and the extended operative time remains significant concerns, particularly when juxtaposed with the absence of discernible differences in perioperative outcomes. It is important to assess the benefits of the development of robotic surgery beyond the comparison of specific outcomes. As the range of available platforms continues to expand, it becomes imperative to precisely delineate the potential advantages and constraints associated with different systems. The crucial task is not solely to choose the most suitable platform for an individual surgeon, but also to pinpoint the optimal system tailored to the specific requirements of single patients or procedures [35].
The current challenge lies in the training of surgeons and the development of the operating room of the future. In the era of digital surgery, robotic platforms serve as computer interfaces capable of integrating various real-time data analysis modalities. This enables advanced systems to provide augmented surgical vision through augmented reality (AR), improved surgical decisions using artificial intelligence (AI), and enhanced surgical manoeuvres through the advancement of robotic instruments [36]. The incorporation of preoperative planning, utilizing 3D acquisition of radiological images, coupled with the utilization of deep learning (DL) algorithms to analyze surgical phases, forms an ideal toolkit for enhancing robotic surgery [37]. This holistic approach aims to reduce intraoperative complications and optimize surgical outcomes by minimizing surgical discrepancies. The operating room is transitioning into a control center akin to an airport control tower, capable of processing 2D/3D inputs derived from preoperative images, environmental and laparoscopic cameras, and patient physiological signals. It then relays outputs to robotic platforms, offering real-time information on the surgeon’s screen during intraoperative processes, such as remaining operating time or the patient’s clinical situation. Image-guided surgery, particularly intraoperative ultrasound, is gaining prominence in robotic surgery [38, 39]. The integration of drop-in ultrasound probes, easily manipulated by robotic graspers, allows access to challenging anatomical spaces [40]. Intraoperative ultrasound, with images projected onto the surgeon’s screen via platforms like Intuitive Surgical’s TilePro, proves beneficial for achieving surgical radicality in endometriosis [41]. Moreover, robotic systems prove beneficial for educational purposes, providing simulators that can democratize training opportunities, even for non-expert surgeons [42].
In this context, the recent published IDEAL Robotics Colloquium proposes recommendations for evaluation during development, comparative study and clinical monitoring of surgical robots—providing practical guidelines for developers, clinicians, patients and healthcare systems [43].
This paper represents the most recent analysis of the current literature on the comparison of RAS and laparoscopy in patients with endometriosis. The inclusion of 5 papers published in the last 24 months, as well as the methodological accuracy and the assessment of the risk of bias are undoubtedly strengths of the work. However, the retrospective nature of most of the included articles and the adoption in all papers of the Da Vinci platform as the only robotic system analysed represent a limitation of this research. Only one prospective trial was found ongoing (NCT05179109) with the aim to examine whether robot-assisted laparoscopy is superior compared to conventional laparoscopy as regards to patient outcome at 6, 12 and 24 months postoperatively, measured by questionnaires concerning the pain symptoms and disease-related quality-of-life. Future studies, including experience with new robotic platforms and comparisons between these, will be needed to better understand the benefits of RAS over conventional laparoscopy.
Conclusion
In conclusion, robotic surgery is not inferior to laparoscopy in patients with endometriosis in terms of surgical outcomes; however, RAS require longer operative times and longer hospital stays. The benefits of robotic surgery should be sought in the easiest potential integration of robotic platforms with new technologies. Furthermore, prospective studies comparing laparoscopy to the new robotic systems are desirable for greater robustness of scientific evidence.
Data availability
All data generated or analyzed in this review are included in the manuscript and its figures/tables. Further enquiries can be directed to the corresponding author.
References
Giudice LC (2010) Clinical practice. Endometr N Engl J Med 362(25):2389–2398
Ianieri MM, Raimondo D, Rosati A, Cocchi L, Trozzi R, Maletta M et al (2022) Impact of nerve-sparing posterolateral parametrial excision for deep infiltrating endometriosis on postoperative bowel, urinary, and sexual function. Int J Gynaecol Obstet 159(1):152–159
Ianieri MM, Buca DIP, Panaccio P, Cieri M, Francomano F, Liberati M (2017) Retroperitoneal endometriosis in postmenopausal woman causing deep vein thrombosis: case report and review of the literature. Clin Exp Obstet Gynecol 44(1):148–150
Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B et al (2014) ESHRE guideline: management of women with endometriosis. Hum Reprod 29(3):400–412
Soto E, Luu TH, Liu X, Magrina JF, Wasson MN, Einarsson JI et al (2017) Laparoscopy vs. robotic surgery for endometriosis (LAROSE): a multicenter, randomized, controlled trial. Fertil Steril 107(4):996-1002.e3
Roman H, Dennis T, Grigoriadis G, Merlot B (2022) Robotic management of diaphragmatic endometriosis in 10 steps. J Minim Invasive Gynecol 29(6):707–708
Ianieri MM, Nardone ADC, Pavone M, Benvenga G, Pafundi MP, Campolo F et al (2023) Are ureterolysis for deep endometriosis really all the same? an anatomical classification proposal for ureterolysis: a single-center experience. Int J Gynaecol Obstet 162:1010–1019
Pavone M, Marescaux J, Seeliger B (2023) Current status of robotic abdominopelvic surgery. Show-Chwan Med J 22(3):467473. https://doi.org/10.30185/scmj.202307/pp.0003
Restaino S, Mereu L, Finelli A, Spina MR, Marini G, Catena U et al (2020) Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: a systematic review and meta-analysis. J Robot Surg 14(5):687–694
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5(1):210
American Society for Reproductive Medicine (1997) Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil Steril 67(5):817–821
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Nezhat C, Lewis M, Kotikela S, Veeraswamy A, Saadat L, Hajhosseini B et al (2010) Robotic versus standard laparoscopy for the treatment of endometriosis. Fertil Steril 94(7):2758–2760
Dulemba JF, Pelzel C, Hubert HB (2013) Retrospective analysis of robot-assisted versus standard laparoscopy in the treatment of pelvic pain indicative of endometriosis. J Robot Surg 7(2):163–169
Hiltunen J, Eloranta ML, Lindgren A, Keski-Nisula L, Anttila M, Sallinen H (2021) Robotic-assisted laparoscopy is a feasible method for resection of deep infiltrating endometriosis, especially in the rectosigmoid area. J Int Med Res 49(8):3000605211032788
Ferrier C, Le Gac M, Kolanska K, Boudy AS, Dabi Y, Touboul C et al (2022) Comparison of robot-assisted and conventional laparoscopy for colorectal surgery for endometriosis: a prospective cohort study. Int J Med Robot 18(3):e2382
Legendri S, Carbonnel M, Feki A, Moawad G, Aubry G, Vallée A, et al. Improvement of Post-Operative Quality of Life in Patients 2 Years after Minimally Invasive Surgery for Pain and Deep Infiltrating Endometriosis. J Clin Med [Internet]. 2022 [cited 10AD Jan 1];11(20). https://pubmed.ncbi.nlm.nih.gov/36294462/. Accessed 12 Dec 2023
Nezhat CR, Stevens A, Balassiano E, Soliemannjad R (2015) Robotic-assisted laparoscopy vs conventional laparoscopy for the treatment of advanced stage endometriosis. J Minim Invasive Gynecol 22(1):40–44
Nezhat FR, Sirota I (2014) Perioperative outcomes of robotic assisted laparoscopic surgery versus conventional laparoscopy surgery for advanced-stage endometriosis. JSLS 18(4):e2014.00094
Magrina JF, Espada M, Kho RM, Cetta R, Chang YHH, Magtibay PM (2015) Surgical excision of advanced endometriosis: perioperative outcomes and impacting factors. J Minim Invasive Gynecol 22(6):944–950
Le Gac M, Ferrier C, Touboul C, Owen C, Arfi A, Boudy AS et al (2020) Comparison of robotic versus conventional laparoscopy for the treatment of colorectal endometriosis: pilot study of an expert center. J Gynecol Obstet Hum Reprod 49:101885
Raimondo D, Alboni C, Orsini B, Aru AC, Farulla A, Maletta M et al (2021) Comparison of perioperative outcomes between standard laparoscopic and robot-assisted approach in patients with rectosigmoid endometriosis. Acta Obstet Gynecol Scand 100(9):1740–1746
Volodarsky-Perel A, Merlot B, Denost Q, Dennis T, Chanavaz-Lacheray I, Roman H (2023) Robotic-assisted versus conventional laparoscopic approach in patients with large rectal endometriotic nodule: the evaluation of safety and complications. Colorectal Dis 25(11):2233–2242
Verrelli L, Merlot B, Chanavaz-Lacheray I, Braund S, D’Ancona G, Kade S, et al. Robotic surgery for severe endometriosis: a preliminary comparative study of cost estimation. J Minim Invasive Gynecol [Internet]. 2023 [cited 11AD Jan 1]. https://pubmed.ncbi.nlm.nih.gov/37935331/. Accessed 12 Dec 2023
Crestani A, Bibaoune A, Le Gac M, Dabi Y, Kolanska K, Ferrier C, et al. Changes in hospital consumption of opioid and non-opioid analgesics after colorectal endometriosis surgery. J Robot Surg [Internet]. 2023 [cited 8AD Jan 1]. https://pubmed.ncbi.nlm.nih.gov/37606871/. Accessed 12 Dec 2023
Hodneland E, Dybvik JA, Wagner-Larsen KS, Šoltészová V, Munthe-Kaas AZ, Fasmer KE et al (2021) Automated segmentation of endometrial cancer on MR images using deep learning. Sci Rep 8(11):179
Csirzó Á, Kovács DP, Szabó A, Fehérvári P, Jankó Á, Hegyi P et al (2023) Robot-assisted laparoscopy does not have demonstrable advantages over conventional laparoscopy in endometriosis surgery: a systematic review and meta-analysis. Surg Endosc 38:529–539
Panico G, Mastrovito S, Campagna G, Monterossi G, Costantini B, Gioè A et al (2023) Robotic docking time with the Hugo™ RAS system in gynecologic surgery: a procedure independent learning curve using the cumulative summation analysis (CUSUM). J Robot Surg 17(5):2547–2554
Alboni C, Mattos LC, Marca AL, Raimondo D, Casadio P, Seracchioli R et al (2023) Robotic surgery and deep infiltrating endometriosis treatment: the state of art. CEOG 50(1):13
Pavone M, Seeliger B, Alesi MV, Goglia M, Marescaux J, Scambia G et al (2023) Initial experience of robotically assisted endometriosis surgery with a novel robotic system: first case series in a tertiary care center. Updates Surg. https://doi.org/10.1007/s13304-023-01724-z
Pavone M, Goglia M, Campolo F, Scambia G, Ianieri MM (2023) En-block butterfly excision of posterior compartment deep endometriosis: the first experience with the new surgical robot Hugo™ RAS. Facts Views Vis Obgyn 15(4):359–362
Sighinolfi MC, De Maria M, Meneghetti J, Felline M, Ceretti AP, Mosillo L et al (2024) The use of versius CMR for pelvic surgery: a multicentric analysis of surgical setup and early outcomes. World J Urol 42(1):31
Huang Y, Duan K, Koythong T, Patil NM, Fan D, Liu J et al (2022) Application of robotic single-site surgery with optional additional port for endometriosis: a single institution’s experience. J Robotic Surg 16(1):127–135
Fanfani F, Restaino S, Ercoli A, Chiantera V, Fagotti A, Gallotta V et al (2016) Robotic versus laparoscopic surgery in gynecology: which should we use? Minerva Ginecol 68(4):423–430
Lecointre L, Verde J, Goffin L, Venkatasamy A, Seeliger B, Lodi M et al (2022) Robotically assisted augmented reality system for identification of targeted lymph nodes in laparoscopic gynecological surgery: a first step toward the identification of sentinel node. Surg Endosc 36(12):9224–9233
Seeliger B, Diana M, Ruurda JP, Konstantinidis KM, Marescaux J, Swanström LL (2019) Enabling single-site laparoscopy: the SPORT platform. Surg Endosc 33(11):3696–3703
Mascagni P, Padoy N (2021) OR black box and surgical control tower: recording and streaming data and analytics to improve surgical care. J Visc Surg 158(3S):S18-25
Pavone M, Spiridon IA, Lecointre L, Seeliger B, Scambia G, Venkatasamy A et al (2023) Full-field optical coherence tomography imaging for intraoperative microscopic extemporaneous lymph node assessment. Int J Gynecol Cancer. https://doi.org/10.1136/ijgc-2023-005050
Guerra F, Amore Bonapasta S, Annecchiarico M, Bongiolatti S, Coratti A (2015) Robot-integrated intraoperative ultrasound: initial experience with hepatic malignancies. Minim Invasive Ther Allied Technol 24(6):345–349
Otani K, Kiyomatsu T, Ishimaru K, Kataoka A, Hayashi Y, Gohda Y (2023) Usefulness of real-time navigation using intraoperative ultrasonography for rectal cancer resection. Asian J Endosc Surg 16(4):819–821
Simmonds C, Brentnall M, Lenihan J (2021) Evaluation of a novel universal robotic surgery virtual reality simulation proficiency index that will allow comparisons of users across any virtual reality simulation curriculum. Surg Endosc 35(10):5867–5875
Marcus HJ, Ramirez PT, Khan DZ, Layard Horsfall H, Hanrahan JG, Williams SC et al (2024) The IDEAL framework for surgical robotics: development, comparative evaluation and long-term monitoring. Nat Med 30(1):61–75
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Contributions
Study design: MP, MMI; Literature search: MP, AB, MG; Data extraction: MP, AB; Data synthesis: MP, AB, SR, LCT, DR; Manuscript drafting: MP, MG; Statistical analysis: AC; Critical revision of the manuscript: CA, JM, FF, GS. All Authors approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no relevant conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavone, M., Baroni, A., Campolo, F. et al. Robotic assisted versus laparoscopic surgery for deep endometriosis: a meta-analysis of current evidence. J Robotic Surg 18, 212 (2024). https://doi.org/10.1007/s11701-024-01954-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01954-2