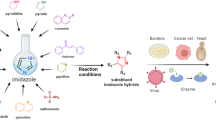
Abstract
Pyrimido[4,5-b]quinoline is a vital structural motif. The synthesis of pyrimido[4,5-b]quinolines has been a challenging topic in medicinal chemistry. A wide range of starting materials have been employed to achieve this nucleus such as quinoline derivatives and isatins. Multi-component one-pot synthestic approaches were employed either by using barbituric or thiobarbituric acid, amines and aldehydes or from 6-aminouracils, aldehydes and cyclohexanone derivatives. Recent synthetic strategies and many green chemistry techniques have improved pyrimido[4,5-b]quinolines synthesis over the last twenty years. Among the many reported biological activities of pyrimido[4,5-b]quinolines, anticancer activity attracted research attention over the past couple of decades. Many derivatives have shown promising anticancer activity on different cancer cell lines such as MCF-7, A549, K562 and others. They also demonstrated activity on different enzymes and receptors such as tyrosine kinases, tyrosyl-DNA Phosphodiesterase II and HDM2 ubiquitin ligase (E3) that promote apoptosis, repair DNA damage, and induce cell cycle arrest. This review critically examines the recent synthetic approaches employed for the synthesis of pyrimido[4,5-b]quinolines and explores their reported anticancer activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pyrimido[4,5-b]quinoline derivatives have attracted significant interest in the field of medicinal chemistry due to their diverse biological activities(El-Gazzar et al. 2009; Jayashree et al. 2010; Gouhar et al. 2017), including anticancer activity (Ghorab et al. 2009; Sachdeva et al. 2022; Ibrahim et al. 2023a). The early attempts to synthesize pyrimido[4,5-b]quinoline started in the 1970s, where the resemblance of them to the known biological co-factor flavins motivated the research efforts. Many pyrimido[4,5-b]quinoline derivatives are named relative to the flavin nucleus and called 5-deazaflavins (Dickens et al. 2013). Yoneda et al. demonstrated a synthetic approach based on the Pictet-Spengler reaction starting from 5-amino-6-arylpyrimidines and aldehydes or ketones resulted in a library of 5-deazaflavin derivatives, enabling further investigations into their biological activities and potential applications (Althuis et al. 1979; Yoneda et al. 1980a, b; Nagamatsu et al. 1984). Over the years, the synthesis of pyrimido[4,5-b]quinolines has developed and many synthetic strategies appeared and are linked to interesting biological activities (Singh Dolly et al. 1995; Kimachi et al. 1997; Ikeuchi et al. 2000; Sarhan et al. 2001). The last couple of decades have witnessed huge advances in the field of chemical synthesis regarding catalysis, ionic liquids, nanotechnology, green chemistry, and other techniques which raise the need for a study of the recent impact on the pyrimido[4,5-b]quinolines synthesis and biological activity. (Kumbhar et al. 2018; Reddy et al. 2018; Xu et al. 2018; Safari et al. 2020; Tabibi and Esmaeili 2023; Moosavi-Zare et al. 2023). Cancer is a major global health issue (Bray et al. 2018; Arnold et al. 2022). Due to the importance of pyrimido[4,5-b]quinoline nucleus as an anticancer agent, we are demonstrating in this review the research efforts of the synthesis and anticancer activity in the last twenty years.
Synthetic approaches for the preparation of pyrimido[4,5-b]quinoline derivatives
In this section, we are discussing different approaches in which pyrimido[4,5-b]quinolines have been synthesized over the scope period of our review.
Starting from quinoline derivatives
Starting from 2-aminoquinoline-3-carbonitrile derivatives
Cyclization of 2-aminoquinoline-3-carbonitriles afforded different variations of pyrimido[4,5-b]quinolines.
N-aryl-4-aminopyrimido[4,5-b]quinolines were obtained from 2-aminoquinoline-3-carbonitriles 1 by the reaction with N,N-dimethylformamide dimethyl acetal (DMF-DMA) to produce amidine derivatives 2, which were cyclized in the presence of aromatic amines in acetic acid to yield N-aryl-4-aminopyrimido[4,5-b]quinoline derivatives 3. Using this two-step approach with different primary aromatic amines achieved the target 3 (Boschelli et al. 2003; Ibrahim et al. 2023a, b) (Scheme 1). N-substituted-pyrimido[4,5-b]quinoline-4-amines 3 was also reported via a one-pot, three-component synthesis using 2-aminoquinoline-3-carbonitrile 1, DMF-DMA and aliphatic or aromatic primary amines under microwave irradiation catalyst-free (Xu et al. 2018).
Synthesis of derivatives 3 was not reported using chlorination followed by nucleophilic substitution with amines. Instead, N-aryl-5,10-dihydropyrimido[4,5-b]quinolin-4-amines 5 were synthesized from the corresponding 5,10-dihydropyrimido[4,5-b]quinolin-4(1H)-ones 4, followed by chlorination with phosphorous oxychloride then nucleophilic substitution with appropriate aniline (Boschelli et al. 2003; Cui et al. 2017) (Scheme 2).
Cyclization of 2-amino-7,7-dimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile derivatives 6 using formic acid, formamide phenyl isothiocyanates, benzoyl chloride, and diethyl oxalate produced different varieties of substituted pyrimido[4,5-b]quinoline structures 7–11 (Abdel-Gawad et al. 2005) (Scheme 3).
Cyclization of 6 with different aromatic aldehydes in acetic acid produced 2-phenyl-2,3,7,8,9,10-hexahydropyrimido[4,5-b]quinoline-4,6(1H,5H)-dione 16 (Ghorab et al. 2011) (Scheme 4).
2-Amino-3-cyano-8-methyl-4-aryl-5,6,7,8-tetrahydroquinoline-3-carbonitrile derivatives 13 were another start point for the cyclization reactions via as formic acid, formamide, acetic anhydride, phenyl isothiocyanate, urea, thiourea and triethyl orthoformate followed by treatment with hydrazine hydrate to obtain pyrimido[4,5-b]quinoline derivatives 15–21 (Faidallah and Rostom 2013; Faidallah et al. 2014) ( Scheme 5).
Sulfonamides derivatives of pyrimido[4,5-b]quinoline 23 were synthesized starting from 2-aminoquinoline-3-carbonitrile derivatives 22 and sulfonamide isothiocyanates in DMF via intramolecular cyclization. Treatment of 24 with triethyl orthoformate in the presence of acetic anhydride followed by refluxing with different sulfanilamides in pyridine achieved pyrimido[4,5-b]quinoline derivatives 25 (Ghorab et al. 2009) (Scheme 6).
The target pyrimido[4,5-b]quinones 27, 28, 31 and 32 were synthesized were obtained from the 2-amino-1,4,5,6,7,8-hexahydro-4-arylquinoline-3-carbonitriles 26 using different cyclizing agents. Firstly, using ammonium thiocyanate in refluxing glacial acetic acid to afford the thiourea derivatives 27. 2-(Cyanomethyl)pyrimido[4,5-b]quinoline derivatives 28 were obtained by heating the ortho-aminonitriles with excess ethyl cyanoacetate. Hydrolysis of 25 to the corresponding ortho-aminoamide derivatives 29 presented a good intermediate for further cyclization with triethyl ortho-esters and different benzylideneanilines 30 to obtain pyrimido[4,5-b]quinolines 32 and 31, respectively (El-Gohary 2013) (Scheme 7).
In 2016, oxalyl chloride was used as the cyclization agent on 2-amino-1-cyclohexyl-4-(3,4-dimethoxyphenyl)-1,4,5,6,7,8-hexahydroquinoline-3-carbonitrile 33 to produce the corresponding pyrimido[4,5-b]quinoline-2-carbonyl chloride 34 which was subsequently used as a starting material for the synthesis of more pyrimido[4,5-b]quinoline derivatives (Gouhar et al. 2017) (Scheme 8).
Starting from 2-chloroquinoline-3-carbonitriles
In 2012, Singh et al. employed Vilsmeier-Haack cyclization to obtain pyrimido[4,5-b]quinoline-4-ones 36 in a two-step synthesis starting from 2-chloroquinoline-3-carbonitriles 35 which were subjected to amination reaction in water followed by the cyclization using Vilsmeier reagent (DMF/POCl3) at 60 °C (Singh et al. 2012). 2-Chloroquinoline-3-carbonitriles 35 was also cyclized with guanidine hydrochloride in presence of t-BuOK catalyst to obtain 2-amino-3H-pyrimido[4,5-b]quinolin-4-ones 38. The reaction could be extended to other 1,3-nucleophiles such as urea, thiourea and other guanidine derivatives (Chandra et al. 2011) (Scheme 9).
In 2018, 2-arylpyrimido[4,5-b]quinoline-4-ones were achieved using a Cu-catalyzed cascade reaction between 2-chloroquinoline-3-carbonitriles and benzyl amines in the presence of sodium hydroxide and atmospheric oxygen. The sequential reaction proceeded via Ullmann-coupling and hydrolysis of the nitrile group to amide succeeded be a nucleophilic addition of amide nitrogen onto iminium carbon and finally air oxidation (Singh et al. 2018) (Scheme 10).
Starting from 2-aminoquinoline-3-carboxamide
In 2012, 2-aminoquinoline-3-carboxamide was used to obtain 2-spiro-2,3-dihydropyrimido[4,5-b]quinolin-4(1H)-ones 45 via the reaction with cyclic ketones in the presence of a zinc chloride catalyst (Rane et al. 2012) (Scheme 11).
In 2015, a mixture of 2-phenylpyrimido[4,5-b]quinolin-4(3H)-one 46 and 2-phenyl-2,3-dihydropyrimido[4,5-b]quinolin-4(1H)-one 47a were synthesized by the reaction of 2-aminoquinoline-3-carboxamide and benzaldehyde in different conditions. The presence of Cu(OAc)2 favored the formation of the unsaturated derivative 46 (Zhang et al. 2015) (Scheme 12).
In 2023, similar approach was employed by reacting 2-aminoquinoline-3-carbonitrile 43 with different aldehydes in a mixture of acetic acid and sodium acetate (Ibrahim et al. 2023a) (Scheme 13).
Starting from 2-aminoquinoline-3-carbaldehyde
Pyrimido[4,5-b]quinoline derivatives 49 were synthesized starting from 2-aminoquinoline-3-carbaldehyde 48 via Friedländer condensation in different conditions. The reaction proceeded in ethanolic KOH under reflux for 3 h, neat with heating at 175–180 °C, reflux in high-boiling solvent such as diphenyl ether for 2–3 h, in aq. HCl as strong acid at 90 °C for 6 h, in the presence of SnCl2. 2H2O as a Lewis acid and via the use of a catalytic amount of HCl under microwave irradiation. The reaction in ethanolic KOH produced the best yield (81–85%) and resulted in fewer side-products (Shelar et al. 2012; Maleki et al. 2015; Maleki and Mofrad 2017) (Scheme 14).
Starting from 2-chloro-formylquinolines
In 2003, Kumar et al. developed a one-pot synthesis using substituted 2-chloro-3-formylquinolines 50 and guanidine nitrate to obtain 2-aminopyrimido[4,5-b]quinoline derivatives 51. This approach benefited from the availability of 2-chloro-3-formylquinolines (Kumar et al. 2003) (Scheme 15).
In 2008, the 2-chloroquinoline-3-carbaldehyde 50a was cyclized with urea or thiourea under microwave irradiation, with anhydrous potassium carbonate as a catalyst to produce pyrimido[4,5-b]quinolin-2-ol/thiol 52 and 53. The spectral data proved the predominance of the ol form by the presence of the OH and SH peaks in the IR spectra and a singlet peak at 10.5 (OH) and 11.3 (SH) ppm in 1H NMR (Prakash Naik et al. 2008) (Scheme 16).
In 2011, a series of fused pyrimidoquinolines 56 were synthesized in a good yield ranging from 64 to 75% by the reaction of 3-arylaminoisoxazol-5(2H)-ones 55 with derivatives of 2-chloroquinoline-3-carbaldehyde 50 via reflux in toluene (Marjani et al. 2011) (Scheme 17).
One-pot synthesis using aldehydes, amines and barbituric or thiobarbituric acid
2-aminonaphthalene reacted with different aldehydes and barbituric or thiobarbituric acid in one-pot condensation to produce 12-aryl-7,8,9,10,11,12-hexahydrobenzo[f]pyrimido[4,5-b]quinoline-9,11-diones 57 in different reaction conditions and substitution patterns throughout the years.
In 2007, N. G. Kozlov and L. I. Basalaeva started the synthesis using boiling butanol as the solvent for 30–40 min (Kozlov and Basalaeva 2007; Kozlov et al. 2007). The reaction yield was affected by the substituent on the aldehyde. Electron-withdrawing groups at para-position showed the highest yields ranging from 55 to 70%. The same substituents in the meta-position showed smaller yields. Electron-donating substituents hindered the reaction and had yields as low as 30% and 37%, the same substituents on the meta-position did not exert the same hindrance to form the corresponding pyrimido[4,5-b]quinolines (Kozlov and Basalaeva 2007) (Scheme 18).
Derivatives 57 were achieved employing green synthesis conditions of aqueous medium catalyzed by iodine. The method had yields ranging from 82 to 91% better than the previously reported synthesis (Kozlov and Basalaeva 2007; Wang et al. 2009). In 2010, the same reaction proceeded in an ionic liquid of [bmim] BF4 at 90 °C with similar good yields (Guo and Yu 2010) (Scheme 18).
The formation of 57 was reported to have better yields in a mixture of acetic acid and water as the solvent, catalyst-free. The reaction starts with a Knoevenagel condensation of aldehyde to the C5 of barbituric acid or thiobarbituric acid followed by Michael addition and finally cyclization (Kumbhar et al. 2018). The following is the proposed mechanism (Scheme 19).
By the year 2012, mixture of substituted anilines, aldehydes, and barbituric or thiobarbituric acid has become a common way for synthesis of 5-arylpyrimido[4,5-b]quinoline-2,4(1H,3H)-dione derivatives 58 (Aknin et al. 2010) (Scheme 20).
The reaction conditions varied over the years. Green approaches using L-proline as a catalyst and water as the solvent (Khalafi-Nezhad et al. 2012) or using 1,4-diaza-bicyclo[2.2.2]octane (DABCO) as a catalyst in water. By microwave assistance, the reaction time was shortened to under a minute using aromatic, heterocyclic and polycyclic aldehydes (Mosslemin et al. 2014) (Scheme 20).
In 2017, low transition temperature mixtures (LTTMs) and deep eutectic solvents (DESs) as environmentally safer solvent options were used for the synthesis of 5-substituted-5,10-dihydropyrimido[4,5-b]quinoline-2,4(1H,3H)-diones 58 from the one-pot three-component condensation of p-chloroaniline, different aromatic aldehydes and barbituric acid (Mohire et al. 2017) (Scheme 20).
The reaction was reported in eco-friendly conditions of mixed solvent of water and glycerol under UV irradiation catalyst-free (Nongthombam et al. 2018) and supramolecular catalyst β-cyclodextrin in water (Reddy et al. 2018). Also, glutathione immobilized on superparamagnetic iron-oxide nanoparticles with ultrasound as the catalyst and water as a solvent. The magnetic nanoparticles are sustainable and reusable (Nongthombam and Nongkhlaw 2018). Aliphatic aldehydes were successfully employed in the protocol (Aknin et al. 2010) (Scheme 20).
By replacing the aromatic aldehyde with cyclohexanone at the same conditions, spiropyrimido[4,5-b]quinoline-2,4(1H,3H)-diones 59 were produced(Khalafi-Nezhad et al. 2012) (Scheme 21).
One-pot synthesis using 6-aminouracil derivatives, aldehydes and cyclohexanone derivatives:
Reaction of 6-amino-2-methylthiouracil, 6-aminouracil or 6-amino-1,3-dimethyluracil with equimolar amounts of different cyclic ketones or cyclic 1,3-diketones and the appropriate aromatic aldehydes in one-pot synthesis resulted in pyrimido[4,5-b]quinoline derivatives 60–64 (Hassan et al. 2007; Foroughi Kaldareh et al. 2020) (Scheme 22).
In 2007, El-Gazzar et al. synthesized 5-aryl-2-thioxo-2,3,6,7,8,9-hexahydropyrimido[4,5-b]quinoline-4(1H)–ones 66 (60–70%) and non-oxidized forms 5-aryl-2-thioxo-2,3,5,6,7,8,9,10-octahydropyrimido[4,5-b]quinolin-4(1H)-ones 65 (30–40%) (El-Gazzar et al. 2007) (Scheme 23).
In 2008, a series of 5-aryl-7,8,9,10-tetrahydropyrimido[4,5-b]quinoline 2,4,6-triones 63 were prepared in high yields in ionic liquid conditions of 1-n-butyl-3-methylimidazolium bromide ([bmim]Br) at 95 °C. The electronic nature of the aryl moiety of the aromatic aldehyde, i.e., bearing electron-withdrawing or electron-donating groups or being heterocyclic, appeared to have no significant effect on the reaction. Aliphatic aldehydes, on the other hand, failed to produce the cyclic form and stopped at uncyclized derivative 67 Interestingly, when using phenylacetaldehyde, 6,7,8,9-tetrahydropyrimido[4,5-b]quinoline-2,4,6-trione 68 was obtained in 67% yield with the loss of toluene moiety (Ji et al. 2008) (Scheme 24).
The reaction of 6-amino-2-methylthiouracil and 6-amino-1,3-dimethyluracil with cyclic 1,3-diketones and the appropriate aromatic aldehydes was reported again in the presence of water as the solvent and indium trichloride as the catalyst (Khurana et al. 2012) (Scheme 25) and in ethanol under ultrasonic irradiation at 50 °C which reduced the reaction time to 1.5–3 min and improved the yields to 94–99%(Jourshari et al. 2012) (Scheme 26).
2,6-diaminopyrimidin-4(3H)-one, dimedone or cyclohexa-1,3-dione and different aromatic aldehydes were the building blocks for 2-amino-5-arylpyrimido[4,5-b]quinoline-4,5-dione derivatives 69. The reaction was reported in an aqueous medium in the presence of triethyl benzyl ammonium chloride with good yields and environmentally friendly conditions (Shi et al. 2009). The advances in the field of nanoparticle catalysts improved the synthesis, and ZrO2 nanoparticles were used as the catalyst in ethylene glycol at 120 °C. This green synthesis protocol resulted in excellent yields (90–98%) and reaction times (8–60 min) (Mokhtary and Mirfarjood Langroudi 2014; Mamaghani et al. 2017; Foroughi Kaldareh et al. 2020; Gholami et al. 2020) (Scheme 27).
A one-pot reaction of aromatic aldehydes, 2-hydroxy-1,4-naphthoquinone and 6-aminouracil derivatives in ionic liquid [bmim]BF4 at 80 °C was developed to produce 5-aryl-5,12-dihydrobenzo[g]pyrimido[4,5-b]quinoline-2,4,6,11(1H,3H)-tetraone derivatives 70 (Du et al. 2012). The reaction was reported again in acetic acid/water mixture under microwave conditions (Mokhtary and Mirfarjood Langroudi 2014; Bharti et al. 2017) and by using magnetic nanocomposite, containing 12-phosphotungstic acid functionalized chitosan@NiCo2O4 nanoparticles (PWA/CS/NiCo2O4), (Safari et al. 2020). In 2022, metal–organic framework (MOF) based on Zr metal, [Zr-UiO-66- PDC-SO3H]FeCl4, is used as a porous catalyst at 100 °C with good to excellent yields (Jalili et al. 2022) (Scheme 28).
Reaction of 5-chlorouracil and amines
Synthesis of 10-arylpyrimido[4,5-b]quinoline-2,4(3H,10H)-diones 70 or 5-deazaflavins was achieved by two-step convergent synthesis. The first step is the reaction between 6-chlooruracil with the primary amine to produce the 6-substituted-aminouracil 70. The reported primary amines were at first aromatic, and then the procedure was extended to the use of more complex aromatic amines and aliphatic ones. The second step is the reaction of the 5-amino derivatives 71 with 2-halo benzaldehydes in DMF (Wilson et al. 2007; Raoof et al. 2013; Kankanala et al. 2019). The position of the substituents is controlled by the pattern of the substitution in the aniline moiety (Wilson et al. 2007). The 6-chlorodeazaflavins were synthesized using 2- chloro-6-fluorobenzaldehyde, because the cyclocondensations reaction is enhanced more in case of ortho-fluoro group than the ortho-chloro (Dickens et al. 2013) (Scheme 29).
In another approach to achieve 5-deazaflavins, ribosylated amines 73 was reacted with 6-chloruracil with a catalyst of malononitrile in methanol to produce the substituted 6-aminouracil derivative which underwent cyclization using Vilsmeier reagent (POCl3/DMF) to obtain the ribosylated 5-deazaflavin derivative 74 (Carlson and Kiessling 2004) (Scheme 30).
Starting from isatins to produce spiropyrimido[4,5-b]quinoline at C-5
In 2008, Dabiri et al. studied the reaction between 6-aminouracils with isatins. Two subsequent molecules of 6-aminouracil reacted with isatin followed by internal cyclization to produce the spiroderivatives 75. This approach was extended to 6-amino-2-(methylthio)pyrimidin-4(3H)-ones and isatins derivatives to obtain 77 in 65–73% yields. The reaction of 5-nitro-isatin or 5-bromo-isatin with 6-amino-3-methylthiouracil failed to undergo the nucleophilic attack by second 6-amino-3-methylthiouracil and stopped at the uncyclized stage (Dabiri et al. 2008) (Scheme 31).
In 2011, the reaction between isatins, 6-diaminopyrimidin-4(3H)-one and dimedone or cyclohexane-1,3-dione was reported in water to obtain the 5-spiropyrimido[4,5-b]quinoline derivatives 77 (Jadidi et al. 2011) (Scheme 32).
5-spiropyrimido[4,5-b]quinolines derivatives 78 were synthesized via a one-pot method of isatin derivatives, barbituric acid and different aromatic amines under solvent-free conditions (Vinoth and Lalitha 2022) (Scheme 33).
Other synthetic approaches
In 2006, pyrimido[4,5-b]quinolinones derivatives 79 were synthesized using a one-pot cyclization of 6-aminopyrimidin-4-one derivatives, dimedone and paraformaldehyde in the presence of basic catalyst such as triethylamine under microwave conditions. (Quiroga et al. 2006) (Scheme 34).
El-Gazzar et al. used α-β-unsaturated ketones and 6-aminothiouracil to synthesize a mixture of the oxidized form of 9-arylidene-5-aryl-2-thioxo-2,3,6,7,8,9-hexahydro-1H-pyrimido[4,5-b]quinoline 4-one 66 as a major product with yield of 60–70% and non-oxidized forms 65 (30–40% yield). The two products separated by crystallization with ethanol (El-Gazzar et al. 2007). The reaction is reported also for α-β-unsaturated ketones with diaryl moieties and 6-aminothiouracil (El-Gazzar et al. 2008) (Scheme 35).
In 2015, diversely functionalized pyrimido[4,5-b]quinoline-4(3H)-ones were achieved using one-pot multi-component reaction of substituted 2-bromobenzaldehyde, cyanoacetamide derivatives, Cu(OAc)2, K2CO3, aqueous ammonia and different aldehydes in DMSO. The reaction starts by a copper catalyzed amination of the bromobenzaldehydes followed by condensation with the aminoacetamide and cyclization to afford the corresponding 2-aminoquinoline-carboxamide in situ, which is then condensed with the added aldehyde to afford the target pyrimido[4,5-b]quinoline-4(3H)-ones 82 (Zhang et al. 2015) (Scheme 36).
In 2017, Panday et al. developed two methods for synthesizing pyrimido[4,5-b]quinolines using a one-pot C–C and C-N bond-forming strategy. They achieved this by the reaction of 6-aminouracils with either 2-bromobenzaldehydes or 2-bromobenzyl bromide derivatives in the presence of K2CO3 as a base and a catalytic amount of CuCl2 in DMF under microwave conditions for 30 min or in the presence of molecular oxygen, CuCl2 (10 mol%), and K2CO3 as a base in DMF under reflux conditions, respectively (Panday et al. 2018) (Scheme 37).
In 2022, Aknin et al. synthesized compound 85 among a long series of pyrimido[4,5-b]quinolines using a different method to be able to add the 5-N,N-dimethyl derivative (Scheme 38).
The biological activity of pyrimido[4,5-b]quinolines
Pyrimido[4,5-b]quinoline nucleus is present in nature in the co-enzyme F420 which is a co-factor in redox reaction in methanogen organisms (Fig. 1) (Hossain et al. 2015; Bender et al. 2016; Bashiri 2022). Development of co-enzyme F420 derivatives led to synthesis of many pyrimido[4,5-b]quinolines with antioxidant activity (Jayashree et al. 2010; Madhavi and Sudeepthi 2013). Many other activities of pyrimido[4,5-b]quinolines have been discovered such as antimicrobial, antiallergic, analgesic and anti-inflammatory (Althuis et al. 1980; El-Gazzar et al. 2008, 2009; Rajanarendar et al. 2012; Gouhar et al. 2017; Fathy et al. 2020). The anticancer activity of pyrimido[4,5-b]quinoline derivatives was established over the past years (Kimachi et al. 1997; Ikeuchi et al. 2000). In this work, we are presenting the recent research on the anticancer activity of pyrimido[4,5-b]quinolines.
The Anticancer activity of pyrimido[4,5-b]quinolines
In 2003, N-arylpyrimido[4,5-b]quinolin-4-amines were investigated as tyrosine kinase inhibitors anticancer agents. The compounds were assayed on Scr Enzyme. N-Aryl-5,10-dihydropyrimido[4,5-b]quinolin-4-amines 5a-d were more potent than the oxidized 3a and 3b form by 3000-fold. Within the active series, the presence of a methoxy group on the N-aryl moiety and the position of the solubilizing on the pyrimido[4,5-b]quinoline nucleus showed an impact on Src enzymatic and cell activity. Compound 5b, where the solubilizing CH3OCH2CH2O group was at C8, showed fourfold increasing in the activity than the 5c (CH3OCH2CH2O group at C7) and sevenfold more activity than 5d, its analog with no methoxy group on the N-aryl (Boschelli et al. 2003) (Fig. 2).
In 2005, a series of 7-nitro-10-arylpyrimido[4,5-b]quinoline-2,4(3H,10H)-diones (5-deazaflavin derivatives) were studied out of a high-throughput screening to stabilize and promote p53 tumor suppressor protein and subsequently induce apoptosis. The compounds demonstrated good in vitro inhibition of HDM2 ubiquitin ligase (E3) activity that is responsible for the degradation of the p53 protein (Yang et al. 2005). In 2007, a study continued on the anticancer activity of the previous 5-deazaflavine. Compounds 72 induced apoptosis and promoted cell cycle arrest at G2 phase in the case of compounds 72a and 72c and G1 phase in case of 72b and 72d. The study proved that the nitro group is not essential for activity (Wilson et al. 2007). In 2013, depending on previous studies, another series of pyrimido[4,5-b]quinolines 72 was developed to evaluate the structure activity relationship (SAR) of 5-deazaflavins as HMD2 inhibitors. The most active compounds contained a trifluoromethyl or chloro-substituent at the C-9 position of pyrimido[4,5-b]quinoline nucleus, and this activity depended primarily on the presence of at least one additional halogen or methyl substituent of the phenyl group at N-10 (Dickens et al. 2013) (Fig. 3).
5-deazaflavins SAR as an antitumor nucleus attracted research (Ali et al. 2008). 5-deazaflavins were reported as selective inhibitor of tyrosyl-DNA Phosphodiesterase II (TDP2) recently after the discovery of its topoisomerase-mediated role in repairing of DNA damage (Raoof et al. 2013). The presence of a phenyl ring attached to position 10 is essential for activity, and the phenyl should have a substituent that can form a hydrogen bond with the receptor, and 3-hydroxyphenyl moiety showed one of the highest activities among the series. While the presence of a halo substituent on the C-8 was essential for activity, replacing it with its isosteres CN or CF3 groups retained the activity (Fig. 2). The other factor that was studied is the type of substituents that ensure solubility and cell permeability of the final product. 5-deazaflavins 72 served as essential nucleus for competitive inhibitors of TDP2 enzyme and subsequently anticancer agents (Raoof et al. 2013; Marchand et al. 2016) (Fig. 4).
In 2008, Naik et al. studied the DNA interaction properties of two pyrimido[4,5-b]quinoline-2-thiol/ol using absorption spectra, viscosity, and thermal denaturation experiments. Binding between 52, 53 and DNA manifested by their UV spectra by strong decrease in the absorption intensity at both peaks accompanied by a bathochromic shift. The results showed that the sulfur-containing 52 has more interaction with CT-DNA as compared to 52. The team also conducted the DNA cleavage by oxidative method. The cleavage study results showed that the sulfur-containing 53 had more nuclease activity than 52. The different binding affinity of 52, 53 to DNA is thought to be the reason for different DNA cleavage efficiency of 52 and 53 and subsequently their cytotoxic activity (Prakash Naik et al. 2008) (Fig. 5).
In 2009, a series of substituted or unsubstituted sulfonamide derivatives of pyrimido[4,5-b]quinolines 23 and 25 were designed as carbonic anhydrase inhibitors. These compounds have showed good activity against breast cancer against MCF-7 cells (Ghorab et al. 2009) (Fig. 6).
In 2011, 2,3,7,8,9,10-hexahydropyrimido[4,5-b]quinoline-4,6(1H,5H)-diones 12 were investigated for their anticancer activity against breast cancer. The compounds were evaluated on MCF-7 cell line and resulted in IC50 ranging from 38 to 52 µM (Ghorab et al. 2011) (Fig. 7).
In 2012, Abu-Hashem and Aly studied some fused pyrimido[4,5-b]quinolines as anticancer agents on different cell lines. Compounds 86, 87, 88 and 89 demonstrated potent anticancer activity on the KB cell line with IC50 12.9, 14.1, 15.3 and 17.1 µM, respectively. Compound 86 was the most potent and showed potent cytotoxicity against KB and CNE2 cancer cells lines (IC50 12.9 and 13.8 µM, respectively). The same compounds showed moderate cytotoxicity against MGC-803 and MCF-7 cancer cell lines (Abu-Hashem and Aly 2012) (Fig. 8).
In 2013, a series of tetrahydropyrimido[4,5-b]quinolines was subjected to preliminary screening on 60-cell lines be evaluated as anticancer agents. Compounds 16a, 17a, 90 and 91 showed the highest activities among the series. Compound 91 was the most active and showed broad spectrum anticancer activity with IC50 values of 46.9, 85.3 and 97.4 µM against GI, TGI and LC cell lines, respectively. The mechanism of action depends on DNA binding. Compound 91 showed high affinity to DNA compared to ethidium bromide in DNA binding test (Faidallah and Rostom 2013) (Fig. 9).
In 2016, a series of 5-deazaflavins was studied to understand the mechanism of their TDP2 inhibition. The compounds were selective competitive inhibitors of TDP2. The study suggested that 5-deazaflavins when used in combination with other topoisomerase II inhibitors anticancer drugs, the resistance to the anticancer drugs is reduced (Marchand et al. 2016) (Fig. 10).
In 2017, a new series of N-aryl-5,10-dihydropyrimido[4,5-b]quinolin-4-amines 5 was evaluated as dual EGFR and Src inhibitors. The series showed good antiproliferative activity against K562 and A549 cells. Compound 5e was the most active and showed IC50 values of 0.22 and 0.25 µM against K562 and A549 cell lines, respectively. It inhibited the tumor cells invasion of and induced apoptosis. The series can be considered for multi-target kinase inhibitors anticancer agents (Cui et al. 2017) (Fig. 11).
In 2020, the development of new 5-deazaflavins inspired from the potent reported compound SV-5–153 (Raoof et al. 2013). The study focused on two derivatives ZW-1231 and ZW-1288 to improve cellular permeability and medicinal characteristics of SV-5–153 and its reported derivatives. The two deazaflavins are selective to TDP2 and inactive against TDP1. Despite adding an aromatic ring to N-3 in ZW-1288 and the presence of a bulky hydrophobic benzyl moiety to N-10 phenyl ring in ZW-1231 which was a significant structural deviation from the parent SV-5–153. The key amino acid residue L313 was at the same proximity on binding site of hTDP2 as SV-5–153 (Kiselev et al. 2020) (Fig. 12).
In 2022, Aknin et al. evaluated a new series of pyrimido[4,5-b]quinoline as potential inhibitors of thymidine phosphorylase. Thymidine phosphorylase is essential in the process of angiogenesis and metastasis of tumors. The most active compounds showed good to moderate IC50 (Aknin et al. 2022) (Fig. 13).
Finally in 2023, Ibrahim et al. synthesized a series of pyrimido[4,5-b]quinoline and 3,4-dihydropyrimido[4,5-b]quinoline as potential anticancer against the MCF-7 cell line with IC50 ranging from 1.67 to 37 µM. Compound 47b was the most active. It induced cell cycle arrest and promoted apoptosis. Compound 47b induced the pro-apoptotic proteins p53, Bax, caspase-7 and inhibited the antiapoptotic protein Bcl-2 and showed good HER2 inhibition with IC50 of 0.073 µM (Ibrahim et al. 2023a) (Fig. 14).
Conclusion
Synthesis of pyrimido[4,5-b]quinolines has been a challenging topic; it was first reported in the 1960s. In the last years, major advances in pyrimido[4,5-b]quinoline synthesis have come about. In this review, we have covered the synthesis of different pyrimido[4,5-b]quinoline derivatives through different pathways from 2003 to 2023. Quinoline derivatives were one of the starting materials and cyclized with different cyclization agents. Multi-component one-pot synthesis played important role to achieve the target pyrimido[4,5-b]quinolines. One example is the use of aldehydes, amines and barbituric or thiobarbituric acid and the one-pot reaction of 6-aminouracil derivatives, aldehydes, and cyclohexanone derivatives. Another approach is using 5-chlorouracil derivatives and amines. Isatin derivatives were used to obtain spiropyrimido[4,5-b]quinoline derivatives at C-5. Many green chemistry techniques were employed to achieve functionalized pyrimido[4,5-b]quinolines. Pyrimido[4,5-b]quinoline scaffolds showed diverse biological activities and the anticancer activity was prominent over the last twenty years. Pyrimido[4,5-b]quinoline derivatives showed anticancer activity on cancer cell lines such as MCF-7, A549, K562 and others. Literature survey revealed that pyrimido[4,5-b]quinoline derivatives have targeted cancer pathways such as tyrosine kinases, tyrosyl-DNA Phosphodiesterase II, and HDM2 ubiquitin ligase (E3) which makes it promising nucleus for anticancer drugs. By highlighting the milestones achieved in this topic, this comprehensive review aims to inspire further advancements in the design and development of novel pyrimido[4,5-b]quinoline derivatives with enhanced anticancer potential.
References
Abdel-Gawad SM, El-Gaby MSA, Heiba HI, Aly HM, Ghorab MM (2005) Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido[4,5-b]quinoline derivatives. J Chin Chem Soc 52(6):1227–1236. https://doi.org/10.1002/jccs.200500177
Abu-Hashem AA, Aly AS (2012) Synthesis of new pyrazole, triazole, and thiazolidine-pyrimido [4, 5-b] quinoline derivatives with potential antitumor activity. Arch Pharm Res 35(3):437–445. https://doi.org/10.1007/s12272-012-0306-5
Aknin K, Desbène-Finck S, Helissey P, Giorgi-Renault S (2010) A new synthetic approach to functionalize pyrimido[4,5-b]quinoline-2,4(1H,3H)-diones via a three-component one-pot reaction. Mol Divers 14(1):123–130. https://doi.org/10.1007/s11030-009-9154-8
Aknin K, Bontemps A, Farce A, Merlet E, Belmont P, Helissey P, Chavatte P, Sari M-A, Giorgi-Renault S, Desbène-Finck S (2022) Polycyclic nitrogen heterocycles as potential thymidine phosphorylase inhibitors: synthesis, biological evaluation, and molecular docking study. J Enzyme Inhib Med Chem 37(1):252–268. https://doi.org/10.1080/14756366.2021.2001806
Ali HI, Tomita K, Akaho E, Kunishima M, Kawashima Y, Yamagishi T, Ikeya H, Nagamatsu T (2008) Antitumor studies – Part 2: Structure–activity relationship study for flavin analogs including investigations on their in vitro antitumor assay and docking simulation into protein tyrosine kinase. Eur J Med Chem 43(7):1376–1389. https://doi.org/10.1016/j.ejmech.2007.10.011
Althuis TH, Moore PF, Hess HJ (1979) Development of ethyl 3,4-dihydro-4-oxopyrimido[4,5-b]quinoline-2-carboxylate, a new prototype with oral antiallergy activity. J Med Chem 22(1):44–48. https://doi.org/10.1021/jm00187a011
Althuis TH, Kadin SB, Czuba LJ, Moore PF, Hess HJ (1980) Structure-activity relationships in a series of novel 3,4-dihydro-4-oxopyrimido[4,5-b]quinoline-2-carboxylic acid antiallergy agents. J Med Chem 23(3):262–269. https://doi.org/10.1021/jm00177a010
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23. https://doi.org/10.1016/j.breast.2022.08.010
Bashiri G (2022) Cofactor F420, an emerging redox power in biosynthesis of secondary metabolites. Biochem Soc Trans 50(1):253–267. https://doi.org/10.1042/BST20211286
Bender M, Mouritsen H, Christoffers J (2016) A robust synthesis of 7,8-didemethyl-8-hydroxy-5-deazariboflavin. Beilstein J Org Chem 12:912–917. https://doi.org/10.3762/bjoc.12.89
Bharti R, Kumari P, Parvin T, Choudhury LH (2017) Molecular diversity from the three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes and 6-aminouracils: a reaction condition dependent MCR. RSC Adv 7(7):3928–3933. https://doi.org/10.1039/C6RA18828A
Boschelli DH, Powell D, Golas JM, Boschelli F (2003) Inhibition of Src kinase activity by 4-anilino-5,10-dihydro-pyrimido[4,5-b]quinolines. Bioorg Med Chem Lett 13(18):2977–2980. https://doi.org/10.1016/S0960-894X(03)00628-0
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Carlson EE, Kiessling LL (2004) Improved chemical syntheses of 1- and 5-deazariboflavin. J Org Chem 69(7):2614–2617. https://doi.org/10.1021/jo049859f
Chandra A, Upadhyay S, Singh B, Sharma N, Singh RM (2011) Base-catalyzed cyclization reaction of 2-chloroquinoline-3-carbonitriles and guanidine hydrochloride: a rapid synthesis of 2-amino-3H-pyrimido[4,5-b]quinolin-4-ones. Tetrahedron 67(47):9219–9224. https://doi.org/10.1016/j.tet.2011.09.032
Cui Z, Chen S, Wang Y, Gao C, Chen Y, Tan C, Jiang Y (2017) Design, synthesis and evaluation of azaacridine derivatives as dual-target EGFR and Src kinase inhibitors for antitumor treatment. Eur J Med Chem 136:372–381. https://doi.org/10.1016/j.ejmech.2017.05.006
Dabiri M, Azimi SC, Khavasi HR, Bazgir A (2008) A novel reaction of 6-amino-uracils and isatins. Tetrahedron 64(30–31):7307–7311. https://doi.org/10.1016/j.tet.2008.05.063
Dickens MP, Roxburgh P, Hock A, Mezna M, Kellam B, Vousden KH, Fischer PM (2013) 5-Deazaflavin derivatives as inhibitors of p53 ubiquitination by HDM2. Bioorg Med Chem 21(22):6868–6877. https://doi.org/10.1016/j.bmc.2013.09.038
Du B-X, Zhao B, Cai G, Li Y-L, Wang X-S (2012) Mild and efficient one-pot three-component synthesis of benzopyrimidoquinoline-tetraone derivatives in ionic liquids. J Chem Res 36(8):453–456. https://doi.org/10.3184/174751912X13384724679874
El-Gazzar ABA, Gaafar AM, Youssef MM, Abu-Hashem AA, Badria FA (2007) Synthesis and anti-oxidant activity of novel pyrimido[4,5- b ]quinolin-4-one derivatives with a new ring system. Phosphorus Sulfur Silicon Relat Elem 182(9):2009–2037. https://doi.org/10.1080/10426500701369864
El-Gazzar A-RBA, El-Enany MM, Mahmoud MN (2008) Synthesis, analgesic, anti-inflammatory, and antimicrobial activity of some novel pyrimido[4,5-b]quinolin-4-ones. Bioorg Med Chem 16(6):3261–3273. https://doi.org/10.1016/j.bmc.2007.12.012
El-Gazzar ABA, Hafez HN, Nawwar GAM (2009) New acyclic nucleosides analogues as potential analgesic, anti-inflammatory, anti-oxidant and anti-microbial derived from pyrimido[4,5-b]quinolines. Eur J Med Chem 44(4):1427–1436. https://doi.org/10.1016/j.ejmech.2008.09.030
El-Gohary NS (2013) Synthesis and in vitro antitumor activity of new quinoline, pyrimido[4,5-b]quinoline, [1,2,3]triazino[4,5-b]quinoline, and [1,2,4]triazolo[2′,3′:3,4]pyrimido[6,5-b]quinoline analogs. Med Chem Res 22(11):5236–5247. https://doi.org/10.1007/s00044-013-0519-2
Faidallah HM, Rostom SAF (2013) Synthesis, in vitro antitumor evaluation and DNA-binding study of novel tetrahydroquinolines and some derived tricyclic and tetracyclic ring systems. Eur J Med Chem 63:133–143. https://doi.org/10.1016/j.ejmech.2013.02.006
Faidallah HM, Saqer AA, Alamry KA, Khan KA, Asiri AM (2014) Synthesis and biological evaluation of some novel tetrahydroquinolines as anticancer and antimicrobial agents. J Enzyme Inhib Med Chem 29(3):367–378. https://doi.org/10.3109/14756366.2013.787421
Fathy U, Gouhar RS, Younis A, El-Ghonemy DH (2020) Synthesis of novel pyrimido[4,5-b]quinoline-4-one derivatives and assessment as antimicrobial and antioxidant agents. Pharmacogn J 13(2):550–562. https://doi.org/10.5530/pj.2021.13.69
Foroughi Kaldareh M, Mokhtary M, Nikpassand M (2020) Nicotinic acid-supported cobalt ferrite-catalyzed one-pot synthesis of substituted chromeno[3,4-b]quinolines. Appl Organomet Chem 34(4):e5469. https://doi.org/10.1002/aoc.5469
Gholami A, Mokhtary M, Nikpassand M (2020) Choline chloride/Oxalic acid (ChCl/Oxa) catalyzed one-pot synthesis of novel azo and sulfonated pyrimido[4,5-b]quinoline derivatives. Dyes Pigm 180:108453. https://doi.org/10.1016/j.dyepig.2020.108453
Ghorab MM, Ragab FA, Hamed MM (2009) Design, synthesis and anticancer evaluation of novel tetrahydroquinoline derivatives containing sulfonamide moiety. Eur J Med Chem 44(10):4211–4217. https://doi.org/10.1016/j.ejmech.2009.05.017
Ghorab MM, Ragab FA, Heiba HI, Ghorab WM (2011) Design and synthesis of some novel quinoline derivatives as anticancer and radiosensitizing agents targeting VEGFR tyrosine kinase. J Heterocycl Chem 48(6):1269–1279. https://doi.org/10.1002/jhet.749
Gouhar RS, Abou-Elmagd WSI, El-Zahar MI, Kamel MM, El-Ghonamy DH (2017) Synthesis of novel 5,6,7,8,9,10-hexahydropyrimido[4,5-b]quinoline derivatives for antimicrobial and anti-oxidant evaluation. Res Chem Intermed 43(3):1301–1327. https://doi.org/10.1007/s11164-016-2699-0
Guo HY, Yu Y (2010) One-pot synthesis of 7-aryl-11,12-dihydrobenzo[h]pyrimido-[4,5-b]quinoline-8,10(7H,9H)-diones via three-component reaction in ionic liquid. Chin Chem Lett 21(12):1435–1438. https://doi.org/10.1016/j.cclet.2010.07.017
Hassan NA, Hegab MI, Abdel-Motti FM, Hebah SHA, Abdel-Megeid FME, Hashem AI (2007) Three-component, one-pot synthesis of pyrimido[4,5- b]-quinoline and pyrido[2,3-d]pyrimidine derivatives. J Heterocycl Chem 44(4):775–782. https://doi.org/10.1002/jhet.5570440404
Hossain MS, Le CQ, Joseph E, Nguyen TQ, Johnson-Winters K, Foss FW (2015) Convenient synthesis of deazaflavin cofactor FO and its activity in F 420 -dependent NADP reductase. Org Biomol Chem 13(18):5082–5085. https://doi.org/10.1039/C5OB00365B
Ibrahim NSM, Kadry HH, Zaher AF, Mohamed KO (2023a) Synthesis of novel pyrimido[4,5-b ]quinolines as potential anticancer agents and HER2 inhibitors. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.14307
Ibrahim NSM, Kadry HH, Zaher AF, Mohamed KO (2023b) Synthesis of novel pyrimido[4,5- b]quinoline derivatives as dual EGFR/HER2 inhibitors as anticancer agents. Arch Pharm. https://doi.org/10.1002/ardp.202300513
Ikeuchi Y, Sumiya M, Kawamoto T, Akimoto N, Mikata Y, Kishigami M, Yano S, Sasaki T, Yoneda F (2000) Synthesis and antitumor activities of novel 5-deazaflavin-sialic acid conjugate molecules. Bioorg Med Chem 8(8):2027–2035. https://doi.org/10.1016/S0968-0896(00)00124-3
Jadidi K, Ghahremanzadeh R, Mirzaei P, Bazgir A (2011) Three-component synthesis of spiro[indoline-3,5′-pyrimido[4,5-b]quinoline]-triones in water. J Heterocycl Chem 48(5):1014–1018. https://doi.org/10.1002/jhet.655
Jalili F, Zarei M, Zolfigol MA, Khazaei A (2022) Application of novel metal–organic framework [Zr-UiO-66-PDC-SO 3 H]FeCl 4 in the synthesis of dihydrobenzo[g ]pyrimido[4,5-b]quinoline derivatives. RSC Adv 12(15):9058–9068. https://doi.org/10.1039/D1RA08710J
Jayashree BS, Thomas S, Nayak Y (2010) Design and synthesis of 2-quinolones as antioxidants and antimicrobials: a rational approach. Med Chem Res 19(2):193–209. https://doi.org/10.1007/s00044-009-9184-x
Ji S-J, Ni S-N, Yang F, Shi J-W, Dou G-L, Li X-Y, Wang X-S, Shi D-Q (2008) An Efficient synthesis of pyrimido[4,5- b ]quinoline and indeno[2′,1′:5,6]pyrido[2,3- d ]pyrimidine derivatives via multicomponent reactions in ionic liquid. J Heterocycl Chem 45(3):693–702. https://doi.org/10.1002/jhet.5570450310
Jourshari S, Mamaghani M, Tabatabaeian M, Shirini K, Rassa F, Langhari M (2012) An efficient ultrasound promoted one-pot three-component synthesis and antibacterial activities of novel pyrimido[4,5-b]quinoline- 4,6(3H,5H,7H,10H)-dione derivatives. Lett Org Chem 9(9):664–670. https://doi.org/10.2174/157017812803521207
Kankanala J, Ribeiro CJA, Kiselev E, Ravji A, Williams J, Xie J, Aihara H, Pommier Y, Wang Z (2019) Novel deazaflavin analogues potently inhibited tyrosyl DNA phosphodiesterase 2 (TDP2) and strongly sensitized cancer cells toward treatment with topoisomerase II (TOP2) poison etoposide. J Med Chem 62(9):4669–4682. https://doi.org/10.1021/acs.jmedchem.9b00274
Khalafi-Nezhad A, Sarikhani S, Shahidzadeh ES, Panahi F (2012) l-Proline-promoted three-component reaction of anilines, aldehydes and barbituric acids/malononitrile: regioselective synthesis of 5-arylpyrimido[4,5-b]quinoline-diones and 2-amino-4-arylquinoline-3-carbonitriles in water. Green Chem 14(10):2876. https://doi.org/10.1039/c2gc35765h
Khurana JM, Chaudhary A, Nand B, Lumb A (2012) Aqua mediated indium(III) chloride catalyzed synthesis of fused pyrimidines and pyrazoles. Tetrahedron Lett 53(24):3018–3022. https://doi.org/10.1016/j.tetlet.2012.04.001
Kimachi T, Sugita K, Tamura Y, Kagawa M, Yamasaki K, Yoneda F, Sasaki T (1997) Synthesis and cytotoxicity of 5-deazaflavins containing o- and p-quinone moieties. Bioorg Med Chem Lett 7(6):753–756. https://doi.org/10.1016/S0960-894X(97)00100-5
Kiselev E, Ravji A, Kankanala J, Xie J, Wang Z, Pommier Y (2020) Novel deazaflavin tyrosyl-DNA phosphodiesterase 2 (TDP2) inhibitors. DNA Repair (amst) 85:102747. https://doi.org/10.1016/j.dnarep.2019.102747
Kozlov NG, Basalaeva LI (2007) Reaction of hexahydropyirimidine-2,4,6-trione with naphthalen-2-amine and benzaldehydes. Russ J Org Chem 43(3):432–438. https://doi.org/10.1134/S1070428007030190
Kozlov NG, Bondarev SL, Odnoburtsev BA, Basalaeva LI (2007) Synthesis of arylmethylpyrimidinetriones and pyrimidoquinolinediones with fluorescent and nonlinear-optical properties. Russ J Appl Chem 80(7):1101–1104. https://doi.org/10.1134/S1070427207070178
Kumar RN, Suresh T, Mohan PS (2003) Synthesis and antibacterial activity of pyrimido[4,5-b]quinolines. Indian J Chem Sect B 42(03):688–689
Kumbhar D, Chandam D, Patil R, Jadhav S, Patil D, Patravale A, Deshmukh M (2018) Synthesis and antimicrobial activity of novel derivatives of 7-aryl-10-thioxo-7, 10, 11, 12 – tertahydro-9 H -benzo[H] pyrimido [4,5-b] quinoline-8-one. J Heterocycl Chem 55(3):692–698. https://doi.org/10.1002/jhet.3089
Madhavi K, Sudeepthi P (2013) Synthesis of cyanoacetylated derivatives of some heteroaryl amines as analgesic and antioxidant agents. IJPSN 5(4):1879–1884
Maleki B, Mofrad AV (2017) Efficient synthesis of quinazoline derivatives catalyzed by flourinated alcohol. Res Chem Intermed 43(5):3111–3120. https://doi.org/10.1007/s11164-016-2813-3
Maleki B, Seresht ER, Ebrahimi Z (2015) Friedlander synthesis of quinolines promoted by polymer-bound sulfonic Acid. Org Prep Proced Int 47(2):149–160. https://doi.org/10.1080/00304948.2015.1005986
Mamaghani M, Jamali Moghadam M, Hossein Nia R (2017) A facile ZrO2 nanoparticles catalyzed synthesis of 2-amino-5-arylpyrimido[4,5-b]quinolinediones. J Iran Chem Soc 14(2):395–401. https://doi.org/10.1007/s13738-016-0988-6
Marchand C, Abdelmalak M, Kankanala J, Huang S-Y, Kiselev E, Fesen K, Kurahashi K, Sasanuma H, Takeda S, Aihara H, Wang Z, Pommier Y (2016) Deazaflavin inhibitors of tyrosyl-DNA phosphodiesterase 2 (TDP2) specific for the human enzyme and active against cellular TDP2. ACS Chem Biol 11(7):1925–1933. https://doi.org/10.1021/acschembio.5b01047
Marjani AP, Khalafy J, Ebrahimlo ARM, RolfH P (2011) Synthesis of some pyrimido[4,5-b]quinoline derivatives. Bull Korean Chem Soc 32(7):2183–2186. https://doi.org/10.5012/bkcs.2011.32.7.2183
Mohire PP, Patil RB, Chandam DR, Jadhav SJ, Patravale AA, Kumbhar DR, Ghosh JS, Deshmukh MB (2017) Low transition temperature mixtures prompted one-pot synthesis of 5, 10 dihydropyrimido[4,5-b]quinoline-2,4(1H,3H)-dione derivatives. Res Chem Intermed 43(12):7013–7028. https://doi.org/10.1007/s11164-017-3033-1
Mokhtary M, Mirfarjood Langroudi SA (2014) Polyvinylpolypyrrolidone-supported boron trifluoride: a mild and efficient catalyst for the synthesis of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines. Monatsh Chem 145(9):1489–1494. https://doi.org/10.1007/s00706-014-1206-9
Moosavi-Zare AR, Goudarziafshar H, Bahrami Z (2023) Nano-[Cu-4C3NSP](Cl)2 as a new catalyst for the preparation of pyrimido[4,5-b]quinoline derivatives. Res Chem Intermed 49(2):507–523. https://doi.org/10.1007/s11164-022-04901-8
Mosslemin MH, Zarenezhad E, Shams N, Rad MNS, Anaraki-Ardakani H, Fayazipoor R (2014) Green synthesis of 5-aryl-(1 H,3 H,5 H,10 H )-pyrimido[4,5-b]quinoline-2,4-diones catalysed by 1,4-diazabicyclo[222]octane in water. J Chem Res 38(3):169–171. https://doi.org/10.3184/174751914X13917105358323
Nagamatsu T, Hashiguchi Y, Yoneda F (1984) A new, general, and convenient synthesis of 5-deazaflavins (5-deazaisoalloxazines) and bis-(5-deazaflavin-10-yl)alkanes. J Chem Soc Perkin 1:561. https://doi.org/10.1039/p19840000561
Nongthombam GS, Nongkhlaw R (2018) Experimental and theoretical studies on SPION@glutathione catalyzed synthesis of indolyl chromene, indolo xanthene, and pyrimido[4,5- b]quinoline. Synth Commun 48(5):541–552. https://doi.org/10.1080/00397911.2017.1410893
Nongthombam GS, Kharmawlong GK, Kumar JE, Nongkhlaw R (2018) UV365 light promoted catalyst-free synthesis of pyrimido[4,5-b] quinoline-2,4-diones in aqueous-glycerol medium. New J Chem 42(12):9436–9442. https://doi.org/10.1039/c8nj01459k
Panday AK, Mishra R, Jana A, Parvin T, Choudhury LH (2018) Synthesis of pyrimidine fused quinolines by ligand-free copper-catalyzed domino reactions. J Org Chem 83(7):3624–3632. https://doi.org/10.1021/acs.joc.7b03272
Prakash Naik HR, Bhojya Naik HS, Ravikumar Naik TR, Naik HR, Lamani DS, Aravinda T (2008) Pyrimido[4,5-b]quinoline-2-thiol/ol: microwave-induced one-pot synthesis, DNA binding and cleavage studies. J Sulphur Chem 29(6):583–592. https://doi.org/10.1080/17415990802382890
Quiroga J, Cruz S, Insuasty B, Abonía R, Nogueras M, Cobo J (2006) Three-component synthesis of hexahydropyridopyrimidine–spirocyclohexanetriones induced by microwave. Tetrahedron Lett 47(1):27–30. https://doi.org/10.1016/j.tetlet.2005.10.120
Rajanarendar E, Nagi Reddy M, Rama Krishna S, Rama Murthy K, Reddy YN, Rajam MV (2012) Design, synthesis, antimicrobial, anti-inflammatory and analgesic activity of novel isoxazolyl pyrimido[4,5-b]quinolines and isoxazolyl chromeno[2,3-d]pyrimidin-4-ones. Eur J Med Chem 55:273–283. https://doi.org/10.1016/j.ejmech.2012.07.029
Rane BS, Deshmukh SV, Ghagare MG, Rote RV, Jachak MN (2012) Synthesis of spiro-pyrimido [4,5-b]quinoline and study of their antimicrobial activities. J Chem Pharm Res 4(7):3562–3567
Raoof A, Depledge P, Hamilton NM, Hamilton NS, Hitchin JR, Hopkins GV, Jordan AM, Maguire LA, McGonagle AE, Mould DP, Rushbrooke M, Small HF, Smith KM, Thomson GJ, Turlais F, Waddell ID, Waszkowycz B, Watson AJ, Ogilvie DJ (2013) Toxoflavins and deazaflavins as the first reported selective small molecule inhibitors of tyrosyl-DNA phosphodiesterase II. J Med Chem 56(16):6352–6370. https://doi.org/10.1021/jm400568p
Reddy SS, Reddy MVK, Reddy PVG (2018) β-cyclodextrin in water: as an efficient green protocol for the synthesis of pyrimido[4, 5-b]quinoline-diones. ChemistrySelect 3(16):4283–4288. https://doi.org/10.1002/slct.201800208
Sachdeva H, Khaturia S, Saquib M, Khatik N, Khandelwal AR, Meena R, Sharma K (2022) Oxygen- and sulphur-containing heterocyclic compounds as potential anticancer agents. Appl Biochem Biotechnol. https://doi.org/10.1007/S12010-022-04099-W
Safari J, Tavakoli M, Ghasemzadeh MA (2020) A highly effective synthesis of pyrimido[4,5-b]quinoline-tetraones using H3PW12O40/chitosan/NiCo2O4 as a novel magnetic nanocomposite. Polyhedron 182:114459. https://doi.org/10.1016/j.poly.2020.114459
Sarhan AE-WAO, Hozien ZA, El-Sherief HAH (2001) Synthesis, characterization and reactions of 2-deoxo-5-deazaalloxazines. Bioorg Med Chem 9(11):2993–2998. https://doi.org/10.1016/S0968-0896(01)00194-8
Shelar DP, Rote RV, Patil SR, Jachak MN (2012) Effects of homogeneous media, binary mixtures and microheterogeneous media on the fluorescence and fluorescence probe properties of some benzo[b][1,8]naphthyridiens with HSA and BSA. Luminescence 27(5):398–413. https://doi.org/10.1002/bio.1364
Shi D-Q, Niu L-H, Yao H, Jiang H (2009) An efficient synthesis of pyrimido[4,5- b ]quinoline derivatives via three-component reaction in aqueous media. J Heterocycl Chem 46(2):237–242. https://doi.org/10.1002/jhet.57
Singh RM, Sharma N, Kumar R, Asthana M, Upadhyay S (2012) An alternative synthesis of pyrimido[4,5-b]quinoline-4-ones via metal-free amination in water and vilsmeier-haack cyclization. Tetrahedron 68(50):10318–10325. https://doi.org/10.1016/j.tet.2012.10.004
Singh JB, Mishra K, Gupta T, Singh RM (2018) Copper-catalyzed cascade reaction: synthesis of pyrimido[4,5- b]quinolinones from 2-chloroquinoline-3-carbonitriles with (aryl)methanamines. New J Chem 42(5):3310–3314. https://doi.org/10.1039/C7NJ04689H
Singh Dolly H, Singh Chimni S, Kumar S (1995) Acid catalysed enamine induced transformations of 1,3-dimethyl-5-formyluracil. A unique annulation reaction with enaminones. Tetrahedron 51(46):12775–12780. https://doi.org/10.1016/0040-4020(95)00831-R
Tabibi T, Esmaeili AA (2023) Efficient and green synthesis of novel hexahydro-5H-thiazolo[2’,3’:2,3]pyrimido[4,5-b]quinoline derivatives. Mol Divers 27(1):477–486. https://doi.org/10.1007/s11030-022-10439-z
Vinoth N, Lalitha A (2022) Catalyst-free three-component synthesis, antibacterial, antifungal, and docking studies of spiroindoline derivatives. Polycycl Aromat Compd 42(2):517–533. https://doi.org/10.1080/10406638.2020.1744025
Wang X-S, Li Q, Wu J-R, Zhang M-M (2009) Green method for the synthesis of benzo[f]pyrimido[4,5- b ]quinoline derivatives catalyzed by iodine in aqueous media. Synth Commun 39(17):3069–3080. https://doi.org/10.1080/00397910902730929
Wilson JM, Henderson G, Black F, Sutherland A, Ludwig RL, Vousden KH, Robins DJ (2007) Synthesis of 5-deazaflavin derivatives and their activation of p53 in cells. Bioorg Med Chem 15(1):77–86. https://doi.org/10.1016/j.bmc.2006.10.011
Xu J, Zhang LH, Liu XB, Ma W, Ma L, Ma Y, Yang JN, Wang DL (2018) Catalyst-free, one-pot, three-component synthesis of pyrimido[4,5-b] quinoline-4-amines under microwave irradiation. J Chem Res 42(10):525–528. https://doi.org/10.3184/174751918X15366615479040
Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV, Safiran YJ, Oberoi P, Kenten JH, Phillips AC, Weissman AM, Vousden KH (2005) Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7(6):547–559. https://doi.org/10.1016/j.ccr.2005.04.029
Yoneda F, Mori K, Sakuma Y, Yamaguchi H (1980a) A novel synthesis of pyrimido[4,5-b]quinoline-2(3H),4(10H)-diones (5-deazaflavins) and analogues by the oxidative cyclization of 5,5′-arylmethylenebis-(6-alkylamino-3-methyluracils). J Chem Soc, Perkin Trans 1:978–981. https://doi.org/10.1039/P19800000978
Yoneda F, Sakuma Y, Koshiro A (1980b) Disproportionation in hydrolysis of pyrimido[4,5-b]quinoline-2(3H),4(10H)-diones (5-deazaflavins). J Chem Soc Perkin 1:293. https://doi.org/10.1039/p19800000293
Zhang X, Guo X, Fan X (2015) Synthesis of 2-Aminoquinoline-3-carboamides and Pyrimido[4,5- b ]quinolin-4-ones through copper-catalyzed one-pot multicomponent reactions. Chem Asian J 10(1):106–111. https://doi.org/10.1002/asia.201402962
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome. We also confirm that the manuscript has been read and approved by all named authors and that there are no other people who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, N.S.M., Kadry, H.H., Zaher, A.F. et al. Review: synthesis and anticancer activity of pyrimido[4,5-b]quinolines in the last twenty years. Chem. Pap. 78, 2729–2755 (2024). https://doi.org/10.1007/s11696-024-03316-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03316-6