Abstract
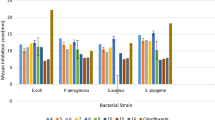
As an important class of compounds, 2-quinolones are isomeric to 4-quinolones and isosteric to coumarins. The compounds that have 2-quinolone moiety are associated with interesting biologic activities such as antibacterial, anticancer, antiviral, cardiotonic, and N-methyl-D-aspartate receptor inhibitor functions, among others. In the current study, based on the rational approach, lead molecules of the 2-quinolone skeleton were designed for binding to the bacterial DNA gyrase subunit A. Docking simulations and quantitative structure activity relationship (QSAR) analysis were performed using the Molegro Virtual Docker and Sarchitech softwares. Based on these studies, the 7-amino-4-methylquinolin-2(1H)-one parent compound and its carboxamides (JST 1–15) were synthesized using Conrad Limpach synthesis. The synthesized test compounds then were characterized by thin-layer chromatography and melting point determination, as well as by ultraviolet, infrared (IR), 1H-NMR, and MS studies. All synthesized and purified compounds were tested for antioxidant and antibacterial activity.







Similar content being viewed by others
References
Beckett AH, Stenlake JB (2005) Practical pharmaceutical chemistry, Part II, 4th edn. CBS Publishers and Distributors, New Delhi, India, pp 275–278
Boehm HJ, Boehringer M, Bur D, Gmuender H, Huber W, Klaus W, Kostrewa D, Kuehne H, Luebbers T, Meunier-Keller N, Mueller F (2000) Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization: promising alternative to random screening. J Med Chem 43:2664–2674. doi:10.1021/jm000017s
Chatterji M, Unniraman S, Mahadevan S, Nagaraja V (2001) Effect of different classes of inhibitors on DNA gyrase from Mycobacterium smegmatis. J Antimicrob Chemother 48:479–485. doi:10.1093/jac/48.4.479
Cooper CS, Klock PL, Chu DT, Hardy DJ, Swanson RN, Plattner JJ (1992) Preparation and in vitro and in vivo evaluation of quinolines with selective activity against gram positive organisms. J Med Chem 35:1392–1398. doi:10.1021/jm00086a007
Cruikshank R, Duguid JP, Marmion BP, Swain RH (1975) Medical microbiology, 2nd edn. Churchill Livingstone, NY, USA, p 190
Danysz W, Parsons CG (1998) Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50:597–664
Dodia N, Shah A (2001) Synthesis and anti-HIV studies of some substituted pyrimidinediones, ethoxypyranon [3,2-C] quinolines and hydrazinopyrano [3,2-C] quinolines. Indian J Pharm Sci 63:211–215
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (2004) Vogel’s textbook of practical organic chemistry, 5th edn. Pearson Education, Delhi, India, p 1074
Heddle J, Maxwell A (2002) Quinolone-binding pocket of DNA gyrase: role of GyrB. Antimicrob Agents Chemother 46:1805–1815. doi:10.1128/AAC.46.6.1805-1815.2002
Jain SC, Pandey MK, Upadhyay RK, Kumar R, Hundal G, Hundal MS (2006) Alkaloids from Toddalia aculeata. Phytochemistry 67:1005–1010. doi:10.1016/j.phytochem.2006.03.012
Joseph B, Darro F, Behard A, Frydman A, Lesur B, Collignon F (2002) 3-Aryl-2-quinolone derivatives: synthesis and characterization of in vitro and in vivo antitumor effects with emphasis on a new therapeutical target connected with cell migration. J Med Chem 45:2543–2555. doi:10.1021/jm010978m
Leclerc G, Marciniak G, Decker N, Schwartz J (1986) Cardiotonic agents. 1. Synthesis and structure-activity relationships in a new class of 3-, 4-, and 5-pyridyl-2(1H)-quinolone derivatives. J Med Chem 29:2427–2432. doi:10.1021/jm00162a002
Lee HZ (1997) Inhibitory effect of 2-phenyl-4-quinolone on serotonin-mediated changes in the morphology and permeability of endothelial monolayers. Eur J Pharmacol 335:245–254. doi:10.1016/S0014-2999(97)01203-X
Milecki J, Baker SP, Standifer KM, Ishizu T, Chida Y, Kusiak JW (1987) Carbostyril derivatives having potent β-adrenergic agonist properties. J Med Chem 30:1563–1566. doi:10.1021/jm00392a006
Pan XS, Fisher LM (1998) DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in streptococcus pneumonia. Antimicrob Agents Chemother 42:2810–2816
Raitio KH, Savinainen JR, Vepsalainen J, Laitinen JT, Poso A, Jarvinen T, Nevalainen T (2006) Synthesis and SAR studies of 2-oxoquinoline derivatives as CB2 receptor inverse agonists. J Med Chem 49:2022–2027. doi:10.1021/jm050879z
Rajakumar DV, Rao MN (1995) Antioxidant properties of phenyl styryl ketones. Free Radic Res 22:309–317. doi:10.3109/10715769509145643
Schechner M, Scirockin F, Stote RH, Dejaegere AP (2004) Functionality maps of the ATP-binding site of DNA gyrase-B: generation of a consensus model of ligand binding. J Med Chem 47:4373–4390. doi:10.1021/jm0311184
Selassie CD (2003) History of quantitative structure-activity relationship: Burger’s medicinal chemistry and drug discovery, vol I, 6th edn. Wiley InterScience, Hoboken, NJ, pp 15–23
Taylor PJ (2005) Hydrophobic properties of drug in comprehensive medicinal chemistry, vol IV, 1st edn. Elsevier, Pergamon Press, Oxford, UK, pp 270–273
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321. doi:10.1021/jm051197e
Tikhonova IG, Baskin II, Palyulin VA, Zefirov NS (2003) CoMFA and homology-based models of the glycine binding site of N-methyl-D-aspartate receptor. J Med Chem 46:1609–1616. doi:10.1021/jm0210156
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayashree, B.S., Thomas, S. & Nayak, Y. Design and synthesis of 2-quinolones as antioxidants and antimicrobials: a rational approach. Med Chem Res 19, 193–209 (2010). https://doi.org/10.1007/s00044-009-9184-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9184-x