Abstract
The study explored the catalytic activities of chitosan-supported tetra (p-methoxyphenyl) porphyrin complexes 1–3 in the heterogeneous activation of the aerobic oxidation dimerization of 2-aminophenol (OAP) to 2-aminophenoxazine-3-one (APX) in the presence of bicarbonate, simulating the function of phenoxazinone enzyme synthase. The oxidation reaction was followed by recording the UV–vis spectra of the reaction mixture with time at λmax 433 nm. All chitosan-supported metalloporphyrin complexes 1–3 exhibited effective catalytic activities for OAP oxidation. Under optimal conditions, the chitosan-supported Cu(II) (Tp-OCH3PP) complex displayed the highest catalytic efficiency. Various parameters influencing the catalytic activity of Cu(II) (Tp-OCH3PP)/CTS 1 were studied. The observed rate constant of OAP oxidation exhibited a direct correlation with the concentration of supported catalyst 1 and followed Michaelis–Menten kinetics, indicating saturation of catalyst sites with increasing OAP concentration. The study investigated the impact of temperature, bicarbonate concentration, dissolved oxygen, and the reaction mechanism. Oxidation reaction of OAP catalyzed by 1 in the presence of nitro blue tetrazolium (NTB) revealed no superoxide anion O2−• was formed as a reactive species during the reaction. The chitosan-supported Cu(II) (Tp-OCH3PP) complex shows high catalytic stability and no significant changes up to the fifth run.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal complexes have great attention for catalytic oxidations of organic substrates with molecular dioxygen (Gandeepan et al. 2018; Kholdeeva and Zalomaeva 2016; Liang and Jiao 2017; Punniyamurthy et al. 2005; Trammell et al. 2019). Metalloporphyrins anchored onto solid supports have received a significant challenge in oxidizing organic substrates with molecular dioxygen (Ding et al. 2017; Ema et al. 2013; Ghafuri et al. 2016). Heterogeneous metalloporphyrin complexes show more enormous resistance to degradation and higher catalytic activity than their homogeneous analogous, separated quickly from the reaction mixtures and frequently reused (Moghadam et al. 2005; Rani et al. 2005).
Natural supports have attracted much attention, especially chitosan (CTS), an excellent candidate due to its biodegradability, non-toxicity antibacterial ability, and environmental collaboration (Guibal 2005). Owing to plenty of amino and hydroxyl functional groups in CTS and its derivatives, they are used extensively in the purification of inorganic heavy metal ions and the purification of waste containing organic dyes by adsorbent method and as catalysts support (Guibal 2005; Saka 2018; Šuláková et al. 2007).
The most abundant anion in natural water is bicarbonate (HCO3−), considered non-toxic and obtainable at low cost. In the presence of bicarbonate, transition metal salts and complexes have been investigated as efficient catalysts for H2O2 and O2 by producing several reactive oxygen species, such as superoxide ions, singlet oxygen, and hydroxyl radicals, in different oxidation reactions (El-khalafy et al. 2022). Bicarbonate has been reported as an effective co-catalyst for accelerating different oxidation reactions (Cheng et al. 2014; Dai et al. 2017; Kim et al. 2019; Lane et al. 2002; Lei et al. 2015; Li et al. 2014, 2012; Long et al. 2012; Monfared et al. 2010; Xu et al. 2011; Zhou et al. 2013).
Phenoxazinone synthase is considered a multicopper enzyme that activates the reaction coupling of two molecules of 3-hydroxy-4-methylanthranilic acid pentapeptide lactone to phenoxazinone chromophore with dioxygen, which was used as the final step for the biosynthesis of antineoplastic agent actinomycin D, which is clinically applied for the ministration of definite types of cancer (Barry et al. 1988; Barry et al. 1989; Dey & Mukherjee 2016; Freeman et al. 1993; Smith et al. 2006). The oxidative coupling of OAP to APX is investigated as questiomycin A that shows as a model reaction for phenoxazinone synthase, describes a six-electron oxidative coupling and occurs in three consecutive two-electron transfer processes (Barry et al., 1989).
Primarily, the oxidation of OAP to APX has been investigated in homogenous systems (Hassanein et al. 2008; Horváth et al. 2004; Jana et al. 2019; Podder and Mandal 2020; Simándi et al. 2004; Székely et al. 2015; Szigyártó et al. 2006; Zhang & Jiao 2010); however, only a few reports investigated the oxidation of OAP to APX in the heterogeneous system by applying complexes of transition metal supported on mesoporous silica and polymers, as catalysts (Chin et al. 2013; Hassanein et al. 2013; Korzec et al. 2019; Maurya et al. 2005).
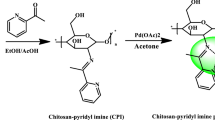
Our work aims to study the catalytic activity of porphyrin complexes anchored on chitosan 1–3 (Scheme 1) in combination with the bicarbonate as green-supported catalysts for the aerobic oxidative coupling of OAP to APX.
Experimental part
Materials and reagents
The solvents and reagents used were of analytical grade and commercially obtained. Tetra p-methoxy phenyl porphyrin (Tp-OCH3PP) was synthesized according to published procedures (Fadda et al. 2013; Korzec et al. 2019), Cu(II)Tp-OCH3PP, Co(II)Tp-OCH3PP, and Mn(III)Tp-OCH3PP complexes were synthesized following the procedure described previously (Bates et al. 2022).
Measurements
PerkinElmer L-17 spectrometer was used to record UV–vis spectra. PerkinElmer model 783 FT-IR spectrophotometer was used to record FT-IR spectra. ICP-OES was registered with PerkinElmer/Optima 7000 DV at the Scientific Research Center, Tanta University, Egypt.
Preparation and analysis of chitosan-supported porphyrin complexes M (Tp -OCH3PP) 1–3
In a three-neck flask, 2 g of chitosan with 2% acetic acid dissolved in 100 ml distilled water at 25 °C was stirred electromagnetically for 15 min. The colloidal solution was obtained by adding 100 ml of distilled water, then slowly adding 2.5% NaOH solution to reach the pH of the medium to 8.5. In 100 ml chloroform, dissolve 0.1 g of M(Tp-OCH3PP) and slowly add to the reaction flask with stirring. After 2 h, the mixture was filtered, and the filter cake was washed many times with distilled water and then, with alcohol, until no residue of M(Tp-OCH3PP) could be detected. The filter cake was dried, and the number of metals in porphyrin complexes supported on chitosan (1–3) was estimated using an ICP-OES Spectro flame Fig. S1. The amount of Cu(II), Co(II), and Mn(III) per gram of solid catalysts were 0.962, 0.991, and 0.445 mg/L, respectively, which was analogous to that obtained using UV–Vis spectroscopy (Baran et al. 2021).
Aerobic oxidation of OAP catalyzed by chitosan-supported M(Tp- OCH3PP) 1–3
In a typical reaction, a measured amount of chitosan-supported M(Tp-OCH3PP) and OAP in methanol was placed in a 10 ml volumetric flask, stirred, and heated in a thermostatic bath. The total reaction volume was adjusted to 7 ml by adding a solution of NaHCO3 in water to the reaction mixture. Catalytic reactions were followed by using UV–Vis spectroscopy at 433 nm.
Aerobic oxidation of OAP catalyzed by chitosan-supported Cu(II) (Tp-OCH3PP)
Aerobic oxidation of OAP was carried out by stirring a mixture of Cu(II) (Tp-OCH3PP)/CTS (1.2 X 10–4 M), OAP (7.5 X 10−2M) with NaHCO3 (0.05 M) in water (30 ml) at 28 °C. The mixture was extracted with 50 ml of diethyl ether. The organic layer was dried with anhydrous Na2SO4; then, the ether was evaporated and tested by TLC (Merck) using CHCl3–CH3OH mixture (20:1 by volume) as eluent to give the product, which showed the presence of 87% red crystals of APX with m.p. 254–256 °C along with unreacted OAP within 90 min.
Recovery and reuse of chitosan-supported Cu(II) (Tp- OCH3PP) 1
To study subsequent reuse of the catalyst supported on chitosan, at the end of each cycle, the catalyst 1 was isolated from the reaction medium by filtration, cleansed extensively with deionized water, dried, and then used in the following reaction cycle.
Results and discussion
Characterization of the supported catalyst
A light-green solid was obtained when M(Tp-OCH3PP) was immobilized on chitosan, which indicates the presence of M(Tp-OCH3PP) on the support.
Immobilization of Cu(II) (Tp-OCH3PP) to CTS was further verified by UV–Vis spectra analyses (Fig. 1A). For the Cu(II) (Tp-OCH3PP) UV–Vis spectra, its soret peak at 417 nm was red-shifted to the Soret peak of Cu(II) (Tp-OCH3PP)/CTS at 425 nm. The soret peak red shift confirmed the coordination between Cu(II)T(p-OCH3)PP and CTS (Kobayashi and Yanagawa 1972; Yoshimura et al. 1992). In addition, the same peaks were obtained from the extracted chloroform of the powder of the solid compound, which again shows that the Cu(II)T(p-OCH3)PP has been anchored on the support.
The FT-IR spectra of the immobilizing catalyst on chitosan show that when Cu(II)T(p-OCH3)PP anchored on chitosan to form a supported complex. The carbonyl band is observed to shift from 1645 to 1636 cm−1, and especially, the amide II also shifted from 1583 to 1562 cm−1. The presence of a peak at 635 cm−1 for Cu(II) (Tp-OCH3PP)/CTS indicates the formation of a Cu–N(axial) coordination bond between Cu(II) (Tp-OCH3PP) and CTS (Boucher and Katz 1967) (Fig. 1B). Because the characteristic absorption of Cu–N(equatorial) bond in Cu(II)T(p-OCH3)PP appeared at 995 cm−1 (Wang et al. 2009), suggesting that a strong coordination links of copper ion to N electron-donor atom in the Cu(II) (Tp-OCH3PP)/CTS complex were formed.
Catalytic oxidation of 2-aminophenol
The activities of porphyrin complexes supported on CTS 1–3 were investigated in the aerobic oxidative dimerization of (OAP) to (APX) in the presence of bicarbonate in methanol–water (50/50 v/v) as solvent (Scheme 2).
Data illustrated in Fig. 2 demonstrate that all porphyrin complexes anchored in chitosan 1–3 with HCO3− show high catalytic activity for the aerobic oxidation of OAP to APX compared to chitosan. The catalytic system Cu(II)T(p-OCH3)PP/CTS 1 showed higher reactivity toward oxidation of OAP compared to Mn(III) T(p-OCH3)PP/CTS 2 and Co(II) T(p-OCH3)PP/CTS 3; it was therefore, chosen for more detailed study.
Production of APX was recorded by UV–vis spectrophotometer as a function with time at 433 nm. Figure 3A, B shows that the characteristic band at 433 nm raised with time in the presence of the catalytic amount of Cu(II)T(p-OCH3)PP/CTS/HCO3−, indicating the oxidation of OAP to APX (Kaizer et al. 2008). The observed rate constant, kobs, was calculated using the first-order plot slope according to the following equation:
The plot of ln{(At − Aα)/(A0 − Aα)} at 433 nm with reaction time in Fig. 3C indicates a pseudo-first-order rate concerning OAP concentration (kobs = 0.1743 min−1).
Rate constants kobs obtained from the plots of ln(A0/At) against time at absorbance 433 nm show that the reaction catalyzed by the catalytic system Cu(II) Tp-OCH3PP/CTS/HCO3− is twelve times faster than in the absence of the catalyst and two times more active than soluble Cu(II) Tp-OCH3PP/HCO3− as shown in Fig. 4.
Data demonstrated in Fig. 5 revealed that the oxidation reaction rate in the presence of bicarbonate is six times faster than in the absence of bicarbonate. The addition of HCO3− accelerated the rate of reaction as described previously (Dai et al. 2017; Lei et al. 2015; Li et al. 2014), so Cu(II)Tp-OCH3PP/CTS/HCO3− complex is more reactive than Cu(II) Tp-OCH3PP/CTS.
The oxidation reaction of OAP performed in the presence of chitosan and bicarbonate without Cu(II) Tp-OCH3PP complex was prolonged, as shown in Fig. 2. This result suggests that the conversion of OAP to APX was affected by catalytic oxidation and not only due to the adsorption process of the OAP from the reaction medium to chitosan.
Influence of operational variables on the oxidation of OAP
Effect of bicarbonate concentration on OAP's aerobic oxidation rate
To identify the impact of bicarbonate on the rate of aerobic oxidation of OAP, different concentration in the range of 0.0: 0.212 M was used. As indicated in Fig. 6, the rate constant kobs increased with raising the concentration of HCO3−, recording the maximum at 0.127 M HCO3−, and then de-escalating. This behavior is analogous to the decolorization of organic dye by the Co2+/HCO3−/H2O2 system (Xu et al. 2011; Li et al. 2012), indicating that the complex formed between Cu(II) complex and HCO3− is of great interest in OAP oxidation due to the bicarbonate ions act as proton acceptors which property enhances the reaction up to a certain concentration. The decrease in the rate of aerobic oxidation at higher concentrations of HCO3− may be due to the formation of species [Cu(II)(HCO3−)2] and [Cu(II)(CO3−2)2]2− are predominant (Zhang et al. 2016), and the equatorial positions attainable not sufficient to bind OAP and O2. Then, the efficiency decreased (Xu et al. 2011).
Influence of Cu(II) TP-OCH3PP/CTS concentration on the oxidation of OAP
The results shown in Fig. 7 indicated that the oxidative dimerization of OAP to APX, under the experimental conditions, is highly dependent on the Cu(II) TP-OCH3PP/CTS concentration.
Effect of substrate concentration on the rate of oxidation of OAP
To demonstrate the influence of substrate concentration on the rate of aerobic dimerization of OAP, different concentrations of OAP from 1.425 × 10–3 to 14.25 × 10–3 M were used. Figure 8 shows that the reaction rate increased gradually from 1.425 × 10–3 to 8.55 × 10–3 and then leveled off. A double reciprocal Lineweaver plot (Fig. 9) showed that the rate obeys the Michaelis–Menten kinetic application (Lineweaver and Burk 1934; Michaelis and Menten 1913). The data calculated from the Lineweaver–Burk plot found that the highest reaction rate Vmax = 1.4459 min−1 and Michaelis constant KM = 0.0750 M.
Effect of OAP concentration on the observed rate constant. For reaction conditions, see Fig. 3
Lineweaver–Burk plot of the data in Fig. 8
Effect of the reaction temperature on the rate of aerobic oxidation of OAP
Figure 10 shows an apparent increase in the kobs values as the reaction temperature increased from 302 to 333 K. The activation parameters for the aerobic oxidation of the OAP reaction were determined from the temperature dependence of the kinetic constant kobs. As shown in Fig. 11, the Arrhenius plot of (ln kobs versus 1/T) using kobs values at 302, 313,323, and 333K is linear with a correlation coefficient of 98.3%. From the slope of this line, the apparent activation energy (Ea) was calculated to be 14.10 k J mol−1. The other activation parameters ΔH # and ΔS# were obtained by Eyring’s equation in which ΔH# = 11.46 k J mol−1 and ΔS# = − 26.60 J mol−1 K−1. The negative entropy of activation in this reaction indicates that substrate-complex association occurs at the transition state (Podder and Mandal 2020).
Effect of dissolved oxygen on the rate of oxidation of OAP
Increasing the amount of dissolved oxygen in the reaction medium could positively increase the aerobic oxidative dimerization reaction rate of OAP to APX. Therefore, experiments were conducted under identical reaction conditions, except that the reaction mixture was purged with N2, air, and oxygen. Data obtained indicated that 90% of OAP were oxidized under purging conditions with oxygen, while only 52% and 9% of OAP was oxidized under air and N2, respectively, illustrating that raising the concentration of O2 in the reaction solution led to increasing aerobic oxidation reaction of OAP efficiency. Similar behavior was observed elsewhere (Fang et al. 2013; Shimizu et al. 2012).
ROS detection during oxidation of 2-aminophenol
During the oxidation of OAP with oxygen catalyzed by Cu(II)T(p-OCH3)PP/CTS/HCO3−, several (ROS) intermediates such as superoxide anion formation O2−•and H2O2 could be generated. Experiments were conducted in the presence of nitro blue tetrazolium (NBT), specifically applied to detect O2−• species to clarify the generation and transformation of ROS species in the present study (Zhang et al. 2016). No characteristic absorption band at 560 nm of diformazan is produced from the reaction of O2−• and NBT, as shown in Fig. S2. These data demonstrated that the aerobic oxidation of OAP catalyzed by Cu(II)T(p-OCH3)PP/CTS under present experimental conditions was not due to O2−•. To indicate whether H2O2 or H2O formed in the aerobic oxidation of OAP catalyzed by Cu(II)T(p-OCH3)PP/CTS in the absence or presence of an alkaline bicarbonate medium. H2O2 examined by potassium iodide KI determination during the reaction and the formation of I3− ions formed from the reaction of KI with H2O2 if presence followed by using the absorption spectrum (where λmax of I3− shows two bands at 285 and 350 nm) (Podder & Mandal 2020). No characteristic absorption band at 285 and 350 nm was formed during the reaction period related to the formation of I3− and consequently, there is no indication for H2O2 formation in the absence or presence of bicarbonate as shown in Fig. S3, the only difference observed that the reaction rate was faster in using bicarbonate which due to the ability of bicarbonate to accept the proton from OAP which enhances the reaction.
Proposed mechanism of the catalytic oxidation 2-aminophenol
Based on the kinetic results obtained and results obtained from the effect of bicarbonate on the rate of oxidation coupling on OAP, Fig. 6. HCO3− is necessary in the Cu(II) T(p-OCH3)PP/CTS –HCO3− system. One reason may be that HCO3− acts as a proton acceptor for H+ion released during OAP oxidation, thus enhancing the rate of APX production. Also, HCO3− can form a complex with Cu(II) T(p-OCH3)PP/CTS, making the catalyst much more reactive. A plausible mechanism for the aerobic oxidative coupling of OAP to APX catalyzed by Cu(II) T(p-OCH3)PP/CTS (Cu(II)L) system is proposed (Scheme 3). The first step involves an interaction between (Cu(II)L) and HCO3¯ to produce Cu(II)L(HCO3−) complex, which binds with 2-aminophenol in a pre-equilibrium step to form complex-substrate adduct (LCu(II)) (HCO3−) ( OAP) (Eq. 1). The formed adduct reacts with one mole of O2 (Eq. 2) to form (OH) species by exchanging with OAP proton which forms free radical intermediate OAP. Fast disproportionation occurred for unstable OAP. in a reduction step (Eq. 3), forming Cu(I) L(HCO3−) and 2-benzoquinone monoimine (BQMI) (Maurya et al. 2005). BQMI is considered the key intermediate, converted into the product APX as multiple oxidative dehydrogenation steps, including BQMI, OAP, and dioxygen as reactants required on the way to form APX described in Scheme 3 (Hassanein et al. 2013).
Catalyst reuse
The Cu(II)T(p-OCH3)PP complex supported on chitosan with NaHCO3 was readily recycled from the reaction media by simple filtration, washed several times with water, and reused for subsequent studies. Cu(II)T(p-OCH3)PP/CTS shows high catalytic stability and no significant changes till the fifth run.
Comparison with other methods
Table 1 compares the results obtained in terms of catalytic oxidation efficiency, stability, reaction time, solvent, temperature, and reaction yield in the presence of the Cu(II) Tp-OCH3PP/CTS presented in this work to other previously published results. It can be seen from Table 1. The heterogeneous catalyst used Cu(II) Tp-OCH3PP/CTS/HCO3− gave a higher oxidation yield of APX with a shorter reaction time required and low temperature. It also shows high catalytic stability and no significant changes until the fifth run. Compared to the other heterogeneous catalysts used in the comparison.
Conclusion
Three metalloporphyrins Cu(II), Co(II), and Mn(III) (TP-OCH3 PP) anchored on chitosan have been synthases and investigated for aerobic oxidation of OAP to APX in bicarbonate solution. The three complexes 1–3 showed high catalytic activity, and Cu(II)T(p-OCH3)PP/CTS 1 showed the higher reactive catalyst toward oxidation of OAP. The influence of reaction conditions on the catalytic efficiency of Cu(II)T(p-OCH3)PP/CTS/HCO3− has been investigated. The rate of oxidation coupling of OAP to APX showed a linear dependence on catalyst concentration and obeyed a Michaelis–Menten kinetic system for saturation of catalyst sites by raising the concentration of OAP. The effect of temperature, bicarbonate concentration, dissolved oxygen, and the proposed mechanism has been investigated. Oxidation reaction of OAP catalyzed by 1 in the presence of nitro blue tetrazolium (NTB) revealed no superoxide anion O2−• was formed as a reactive species during the reaction. Cu(II)T((p-OCH3)PP/CTS/HCO3− shows high catalytic stability and no significant changes till the fifth run.
Data availability
Data available on request from the authors.
References
Baran T, Visibile A, Busch M, He X, Wojtyla S, Rondinini S, Vertova A (2021) Copper oxide-based photocatalysts and photocathodes: fundamentals and recent advances. Molecules 26(23):7271
Barry CE, Nayar PG, Begley TP (1988) Phenoxazinone synthase: enzymatic catalysis of an aminophenol oxidative cascade. J Am Chem Soc 110(10):3333–3334
Barry CE III, Nayar PG, Begley TP (1989) Phenoxazinone synthase: a mechanism for the formation of the phenoxazinone chromophore of actinomycin. Biochemistry 28(15):6323–6333
Bates JS, Khamespanah F, Cullen DA, Al-Omari AA, Hopkins MN, Martinez JJ, Stahl SS (2022) Molecular catalyst synthesis strategies to prepare atomically dispersed Fe-NC heterogeneous catalysts. J Am Chem Soc 144(41):18797–18802
Boucher LJ, Katz JJ (1967) The infrared spectra of metalloporphyrins (4000–160 cm-1). J Am Chem Soc 89(6):1340–1345
Cheng L, Wei M, Huang L, Pan F, Xia D, Li X, Xu A (2014) Efficient H2O2 oxidation of organic dyes catalyzed by simple copper(II) ions in bicarbonate aqueous solution. Ind Eng Chem Res 53(9):3478–3485
Chin T-K, Endud S, Jamil S, Budagumpi S, Lintang HO (2013) Oxidative dimerization of o-aminophenol by heterogeneous mesoporous material modified with biomimetic salen-type copper(II) complex. Catal Lett 143:282–288
Dai D, Yang Z, Yao Y, Chen L, Jia G, Luo L (2017) Highly efficient removal of organic contaminants based on peroxymonosulfate activation by iron phthalocyanine: mechanism and the bicarbonate ion enhancement effect. Catal Sci Technol 7(4):934–942
Dey SK, Mukherjee A (2016) Catechol oxidase and phenoxazinone synthase: biomimetic functional models and mechanistic studies. Coord Chem Rev 310:80–115
Ding ZD, Zhu W, Li T, Shen R, Li Y, Li Z, Ren X, Gu ZG (2017) A metalloporphyrin-based porous organic polymer as an efficient catalyst for the catalytic Oxidation of olefins and arylalkanes. Dalton Trans 46(34):11372–11379
El-khalafy S, Etaiw SE-D, Hassanein M (2022) Catalytic activity of CuI/CuII cyanide based phenanthroline-bicarbonate system for enhancing aerobic Oxidation of 2,6-di-tert-butylphenol. J Saudi Chem Soc 26(3):101466
Ema T, Miyazaki Y, Taniguchi T, Takada J (2013) Robust porphyrin catalysts immobilized on biogenous iron oxide for the repetitive conversions of epoxides and CO2 into cyclic carbonates. Green Chem 15(9):2485–2492
Fadda AA, El-Mekawy RE, El-Shafei A, Freeman HS, Hinks D, El-Fedawy M (2013) Design, synthesis, and pharmacological screening of novel porphyrin derivatives. Hindawi Publ Corp J Chem 2013:1–12
Fang G-D, Zhou D-M, Dionysiou DD (2013) Superoxide mediated production of hydroxyl radicals by magnetite nanoparticles: demonstration in the degradation of 2-chlorobiphenyl. J Hazard Mater 250:68–75
Freeman JC, Nayar PG, Begley TP, Villafranca JJ (1993) Stoichiometry and spectroscopic identity of copper centers in phenoxazinone synthase: a new addition to the blue copper oxidase family. Biochemistry 32(18):4826–4830
Gandeepan P, Müller T, Zell D, Cera G, Warratz S, Ackermann L (2018) 3d transition metals for C–H activation. Chem Rev 119(4):2192–2452
Ghafuri H, Rahmani S, Rahimi R, Mohammadiyan E (2016) Synthesis and characterization of benzilic alcohol metalloporphyrin and its nanocomposite with graphene oxide (GO–CoTHMP) and investigation of their efficiency in the removal of environmental pollutants. RSC Adv 6(67):62916–62922
Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30(1):71–109
Hassanein M, Abdo M, Gerges S, El-Khalafy S (2008) Study of the oxidation of 2-aminophenol by molecular oxygen catalyzed by cobalt(II) phthalocyaninetetrasodiumsulfonate in water. J Mol Catal a: Chem 287(1–2):53–56
Hassanein M, El-Khalafy S, Shendy S (2013) 5,10,15,20-Tetrakis-(4-sulfonatophenyl) porphyrinatocobalt(II) supported on ion exchange resin as reusable and effective catalyst for the oxidative coupling of 2-aminophenol to 2-aminophenoxazine-3-one. Catal Commun 40:125–128
Horváth T, Kaizer J, Speier G (2004) Functional phenoxazinone synthase models: kinetic studies on the copper-catalyzed oxygenation of 2-aminophenol. J Mol Catal a: Chem 215(1–2):9–15
Jana NC, Patra M, Brandão P, Panja A (2019) Synthesis, structure and diverse coordination chemistry of cobalt(III) complexes derived from a Schiff base ligand and their biomimetic catalytic Oxidation of o-aminophenols. Polyhedron 164:23–34
Kaizer J, Baráth G, Csonka R, Speier G, Korecz L, Rockenbauer A, Párkányi L (2008) Catechol oxidase and phenoxazinone synthase activity of a manganese(II) isoindoline complex. J Inorg Biochem 102(4):773–780
Kholdeeva OA, Zalomaeva OV (2016) Recent advances in transition-metal-catalyzed selective oxidation of substituted phenols and methoxyarenes with environmentally benign oxidants. Coord Chem Rev 306:302–330
Kim J, Lee SH, Tieves F, Paul CE, Hollmann F, Park CB (2019) Nicotinamide adenine dinucleotide as a photocatalyst. Sci Adv 5(7):eaax0501
Kobayashi H, Yanagawa Y (1972) Electronic spectra and electronic structure of iron(II) tetraphenylporphins. Bull Chem Soc Jpn 45(2):450–456
Korzec M, Senkała S, Rzycka-Korzec R, Kotowicz S, Schab-Balcerzak E, Polański J (2019) A highly selective and sensitive sensor with imine and phenyl-ethynyl-phenyl units for the visual and fluorescent detection of copper in water. J Photochem Photobiol, A 382:111893
Lane BS, Vogt M, DeRose VJ, Burgess K (2002) Manganese-catalyzed epoxidations of alkenes in bicarbonate solutions. J Am Chem Soc 124(40):11946–11954
Lei Y, Chen C-S, Tu Y-J, Huang Y-H, Zhang H (2015) Heterogeneous degradation of organic pollutants by persulfate activated by CuO–Fe3O4: mechanism, stability, and effects of pH and bicarbonate ions. Environ Sci Technol 49(11):6838–6845
Li X, Xiong Z, Ruan X, Xia D, Zeng Q, Xu A (2012) Kinetics and mechanism of organic pollutants degradation with cobalt–bicarbonate–hydrogen peroxide system: investigation of the role of substrates. Appl Catal A 411:24–30
Li X, Shi W, Cheng Q, Huang L, Wei M, Cheng L, Xu A (2014) Catalytic activation of dioxygen to hydroxyl radical and efficient oxidation of o-aminophenol by cobalt(II) ions in bicarbonate aqueous solution. Appl Catal A 475:297–304
Liang Y-F, Jiao N (2017) Oxygenation via C–H/C–C bond activation with molecular oxygen. Acc Chem Res 50(7):1640–1653
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666
Long X, Yang Z, Wang H, Chen M, Peng K, Zeng Q, Xu A (2012) Selective degradation of orange II with the cobalt(II)–bicarbonate–hydrogen peroxide system. Ind Eng Chem Res 51(37):11998–12003
Maurya MR, Sikarwar S, Joseph T, Halligudi S (2005) Bis (2-[α-hydroxyethyl] benzimidazolato) copper(II) anchored onto chloromethylated polystyrene for the biomimetic oxidative coupling of 2-aminophenol to 2-aminophenoxazine-3-one. J Mol Catal a Chem 236(1–2):132–138
Michaelis L, Menten ML (1913) Die kinetik der invertinwirkung. Biochem 49(333–369):352
Moghadam M, Tangestaninejad S, Mirkhani V, Mohammadpoor-Baltork I, Kargar H (2005) Mild and efficient Oxidation of alcohols with sodium periodate catalyzed by polystyrene-bound Mn(III) porphyrin. Bioorg Med Chem 13(8):2901–2905
Monfared HH, Aghapoor V, Ghorbanloo M, Mayer P (2010) Highly selective olefin epoxidation with the bicarbonate activation of hydrogen peroxide in the presence of manganese(III) meso-tetraphenylporphyrin complex: optimization of effective parameters using the Taguchi method. Appl Catal A 372(2):209–216
Podder N, Mandal S (2020) Aerobic oxidation of 2-aminophenol catalysed by a series of mononuclear copper(ii) complexes: phenoxazinone synthase-like activity and mechanistic study. New J Chem 44(29):12793–12805
Punniyamurthy T, Velusamy S, Iqbal J (2005) Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem Rev 105(6):2329–2364
Rani VR, Kishan MR, Kulkarni S, Raghavan K (2005) Immobilization of metalloporphyrin complexes in molecular sieves and their catalytic activity. Catal Commun 6(8):531–538
Saka ET (2018) Preparation, characterization of new Co(II) and Cu(II) phthalocyanines and their catalytic performances in aerobic Oxidation of substituted phenols. J Incl Phenom Macrocycl Chem 91:61–69
Shimizu A, Tokumura M, Nakajima K, Kawase Y (2012) Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: roles of decomposition by the Fenton reaction and adsorption/precipitation. J Hazard Mater 201:60–67
Simándi TM, Simándi LI, Győr M, Rockenbauer A, Gömöry Á (2004) Kinetics and mechanism of the ferroxime(II)-catalysed biomimetic Oxidation of 2-aminophenol by dioxygen. A functional phenoxazinone synthase model. Dalton Trans 7:1056–1060
Smith AW, Camara-Artigas A, Wang M, Allen JP, Francisco WA (2006) Structure of phenoxazinone synthase from Streptomyces antibioticus reveals a new type 2 copper center. Biochemistry 45(14):4378–4387
Šuláková R, Hrdina R, Soares GM (2007) Oxidation of azo textile soluble dyes with hydrogen peroxide in the presence of Cu(II)–chitosan heterogeneous catalysts. Dyes Pigm 73(1):19–24
Székely G, Bagi N, Kaizer J, Speier G (2015) Oxidation of 3,5-di-tert-butylcatechol and 2-aminophenol by molecular oxygen catalyzed by an organocatalyst. New J Chem 39(8):5908–5911
Szigyártó IC, Simándi TM, Simándi LI, Korecz L, Nagy N (2006) A functional phenoxazinone synthase model based on dioximatomanganese(II): kinetics and mechanism of the catalytic Oxidation of 2-aminophenols by dioxygen. J Mol Catal a: Chem 251(1–2):270–276
Trammell R, Rajabimoghadam K, Garcia-Bosch I (2019) Copper-promoted functionalization of organic molecules: from biologically relevant Cu/O2 model systems to organometallic transformations. Chem Rev 119(4):2954–3031
Wang R, Jiao W, Gao B (2009) Efficient biomimetic aerobic Oxidation of phenylethane catalyzed by P (4VP-co-St)/SiO2-supported metalloporphyrins. Appl Surf Sci 255(17):7766–7772
Xu A, Li X, Ye S, Yin G, Zeng Q (2011) Catalyzed oxidative degradation of methylene blue by in situ generated cobalt(II)-bicarbonate complexes with hydrogen peroxide. Appl Catal B 102(1–2):37–43
Yoshimura T, Toi H, Inaba S, Ogoshi H (1992) Bis (1-methylimidazole) iron(II) complexes of porphyrins substituted with highly electron-withdrawing CF3 groups: electronic spectra with split Q-bands and MCD spectra with unusual features. Bull Chem Soc Jpn 65(7):1915–1919
Zhang C, Jiao N (2010) Dioxygen activation under ambient conditions: Cu-catalyzed oxidative amidation−diketonization of terminal alkynes leading to α-ketoamides. J Am Chem Soc 132(1):28–29
Zhang D, Shi W, Cheng Q, Li X, Xu A (2016) Dioxygen-mediated Oxidation of hydroquinone with cobalt ions in a bicarbonate aqueous solution for the production of active radicals. New J Chem 40(6):5562–5567
Zhou L, Song W, Chen Z, Yin G (2013) Degradation of organic pollutants in wastewater by bicarbonate-activated hydrogen peroxide with a supported cobalt catalyst. Environ Sci Technol 47(8):3833–3839
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Khalafy, S.H., Hassanein, M.T. & Mubarak, A.A. Efficient and green oxidation of 2-aminophenol catalyzed by tetra-(p-methoxyphenyl) porphyrin complexes anchored on chitosan in bicarbonate solution. Chem. Pap. 78, 1205–1215 (2024). https://doi.org/10.1007/s11696-023-03159-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03159-7