Abstract
DNA gyrase brings negative supercoils into DNA and loosens up certain positive supercoils that collect during replication and transcription and is a notable antibacterial target. To fight against the menace of antibiotic-resistant bacterial infections, we have employed various computational tools like high throughput virtual screening (HTVS), standard precision (SP) docking, extra precision (XP) docking, and molecular dynamics (MD) simulation studies to identify some potential DNA gyrase inhibitors. A focused library of 5968 anti-bacterial compounds was screened using the HTVS docking protocol of the glide module of Maestro. The top 200 docked compounds were further filtered using SP and XP docking protocols, and their free binding energies were calculated using MM-GBSA studies. The binding and stability of the top two compounds which showed better docking scores than the co-crystallized ligand (Clorobiocin) of DNA gyrase (PDB ID: 1KZN) were further probed by MD simulation of 100 ns using GROMACS. MD simulation study suggested that the compounds AM1 and AM5 form a stable complex with DNA gyrase with a good number of hydrogen bonds. XP docking study showed that interaction with the crucial amino acids for compounds AM1 and AM5 was like the co-crystallized ligand. These compounds were also predicted to be drug-like molecules with good water solubility and excellent absorption profiles. Based on the above studies, herein we report compounds AM1 (1R,3S)-1-(2-((3-(ammoniomethyl)phenyl)amino)-2-oxoethyl)-3-carbamoylpiperidin-1-ium and AM5 (1'S,2 s,4R)-4-ammonio-6-ethyl-1'-methylspiro[chromane-2,4'-piperidin]-1'-ium as potential DNA gyrase inhibitors which can be further developed as a potential lead against the menace of antibiotic resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DNA gyrase is a topoisomerase II, an ATP-dependent enzyme that is essential for the processes of chromosome segregation, transcription, and replication. It is one of the most extensively researched and verified targets for the creation of novel antibacterial therapies. This enzyme is a good target for the development of antibacterial therapeutics with selective toxicity due to its lack in the mammalian organism and its critical function in the bacterial DNA replication cycle (Khan et al. 2018). Gyrase A and Gyrase B are the two subunits that make up the catalytically active heterotetrameric enzyme (i.e., A2B2). While the B subunit (DNA gyrase B) has ATPase activity and supplies enough energy for the DNA supercoiling, the A subunit is responsible for breaking and rejoining the double DNA strand (Dutta and Inouye 2000; Champoux 2003).
Gyrase is a crucial enzyme needed for bacterial cell survival that has emerged as a prime target for several antibiotics, notably quinolones. Resistance to quinolone antibiotics in Gram-negative bacteria results from mutations in the genes gyrA, gyrB, parC, and parE. The later genes encode parts of the topoisomerase IV enzyme, a secondary target for quinolones in Gram-negative bacteria and a homolog of gyrase (Webber et al. 2013). Gyrase appears to be the fluoroquinolones' main target in gram-negative bacteria like E. coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae, while topoisomerase IV is their preferred target in Gram-positive bacteria like Staphylococcus aureus and Streptococcus pneumoniae (Boutin et al. 2023). Gram-negative bacilli Escherichia coli and Salmonella enterica (subtype typhi) are among the top priority for which new therapeutics are warranted (Carpio Arévalo and Amorim 2021). Hence, targeting DNA gyrase might be a useful strategy, especially for the anti-microbial resistance caused by Gram-negative bacteria.
Given that DNA gyrase is a highly desirable pharmacological target, many inhibitors have been created and characterized, with quinolones and aminocoumarins being the most extensively researched substances (Maxwell 1997). Presently, the only DNA gyrase inhibitors utilized in clinical practice belong to the class of substances known as 6-fluoroquinolones. In-depth research in this area has been sparked by the variety of adverse effects and developing bacterial resistance in the absence of new antibacterial agents. By screening chemical libraries, new chemical entities with various scaffolds and DNA gyrase inhibitory capabilities have been identified. These compounds could make promising leads for the development of antibacterial agents (Khan et al. 2018).
Recently, Pakamwong et al. have identified 2,2'-((2-hydroxy-3-(5-methyl-2,3-diphenyl-1H-indol-1-yl)propyl)azanediyl)bis(ethan-1-ol) as potential DNA gyrase inhibitor having an antimycobacterial activity (Pakamwong et al. 2022). Govender et al. have reported some potent spiropyrimidinetrione derivatives as DNA gyrase inhibitors with potent activity against mycobacterium tuberculosis (Govender et al. 2022). Alfonso et al. have discovered a few novel and structurally diverse DNA gyrase inhibitors through a fluorescence-based high-throughput screening assay (Alfonso et al. 2022). Saleh et al. have employed experimental and computational (molecular docking) studies to discover some cyclic diphenyl phosphonates as DNA gyrase inhibitors for fluoroquinolone-resistant pathogens (Saleh et al. 2022).
Recently, several authors have employed computational tools to identify novel inhibitors of various targets (Kumar et al. 2020; Rathi et al. 2020; Meena et al. 2021; Mukherjee et al. 2022). Jakhar et al. have employed quantitative structure–activity relationship (QSAR) modeling to identify a few novel DNA gyrase inhibitors (Jakhar et al. 2022). Hasan et al. have carried out in silico analysis of ciprofloxacin derivatives as potential DNA gyrase inhibitors (Hasan et al. 2021). Mohammed et al. have synthesized and performed docking studies of a few novel N4-piperazinyl ciprofloxacin analogues as potential DNA gyrase inhibitors (Mohammed et al. 2022). We wanted to identify some novel chemotypes which already might have antimicrobial activity and be developed as DNA gyrase inhibitors. Therefore, in the present study, we have employed various computational tools like high throughput virtual screening (HTVS), molecular docking, and molecular dynamics (MD) simulation studies to identify some potential DNA gyrase inhibitors. The findings of the current study might serve as a hit that can be optimized for DNA gyrase inhibition and in turn, develop it as a potent antimicrobial agent against antibiotic-resistant strains.
Materials and methods
All molecular modeling experiments and DFT studies were carried out using Maestro (v11.4) on an HP desktop with Linux Ubuntu 18.04.1 LTS as the operating system. Maestro is a small-molecule drug discovery suite from Schrodinger that has several integrated molecular modeling tools like Glide, Phase, Desmond, QikProp, LigPrep, etc. MD simulation was carried out using GROMACS 2018.3 on the LINUX system with NVIDIA GPU support.
Protein preparation
The protein structure (PDB ID 1KZN) was imported from the protein databank using the import option in the protein preparation wizard tool of the Maestro interface (Lafitte et al. 2002; Berman et al. 2003). The protein preparation wizard tool has a three-step integrated process where the imported protein structures are first pre-processed to fill hydrogens or any missing residues using an integrated Prime module. In the preprocessing step, bond orders are also corrected and then, water molecules beyond 5 Å were removed, added hydrogens were optimized and finally, protein structure was minimized using the OPLS3e (optimized potentials for liquid simulations) force field (Sastry et al. 2013).
Database preparation
For the present study, a focused library of 5968 anti-bacterial compounds was downloaded from the Asinex database. The Asinex database provides a variety of libraries of compounds for screening purposes. All the compounds were downloaded in mol2 format and imported using the LigPrep tool of Maestro (Sastry et al. 2013). Lowest energy 3D structures were generated for all the imported structures with corrected chiralities using the LigPrep tool. This exercise was carried out using an OPLS3e forcefield (Roos et al. 2019).
High-throughput virtual screening
HTVS mode in the Glide module of Maestro was employed to carry out high-throughput virtual screening. HTVS mode is the fastest docking mode in the Glide panel. It uses very less computational power as its scoring function does not use the explicit-water technology and descriptors used in XP mode. Hence, HTVS is preferred for the screening of a large library of compounds. But its algorithm is not able to weed out false positives. HTVS docking is a grid-based docking system, and hence, a receptor grid was first generated for the minimized protein structure (1KZN). The grid was generated using the co-crystallized ligand with the protein as a reference ligand (Friesner et al. 2004).
Molecular docking
Based on the docking score, the compounds were ranked in the HTVS experiment, and the top 200 compounds were taken up for docking in the standard precision (SP) mode of Glide. The SP mode uses a sampling method based on anchor and refined growth. The top 50 compounds based on the docking score in SP docking were selected for XP (extra precision) docking. XP mode is less forgiving than SP mode, and penalties are levied for violations like inadequate solvation as predicted by statistical results (Friesner et al. 2006).
Free binding energy calculations
The top five compounds from XP docking studies were put for free binding energy calculations using the MM-GBSA (Molecular Mechanics, the Generalized Born model and Solvent Accessibility) tool of the Prime module of Maestro. Prime utilizes the VSGB 2.0 solvation model and OPLS3e force field to simulate these interactions. Ligands were ranked based on MM-GBSA ∆G bind scores (Genheden and Ryde 2015).
MD simulation study
The top two compounds based on the above studies were taken up for MD simulation for more insight into the interaction and stability of the protein–ligand complex. GROMACS (version 2018.3) was used with AMBER ff99SB-ILDN forcefield and tip3p solvent model (Berendsen et al. 1995). The Antechamber tool of AMBERTOOLS was employed for generating the ligand topology. The system was neutralized by adding counter ions (Na+/Cl). The solvated complex was subjected to energy minimization by employing conjugate gradient methods and the steepest descent (SD) algorithm. Post minimization, two-phase equilibration was carried out, and the equilibrated complex was put for MD simulation at 300 K and 1 bar for 100 ns with a time step of 2 fs.
ADME analysis
ADME (absorption, distribution, metabolism, and excretion) analysis was done using SwissADME which is a web-based tool. SwissADME helps us to forecast ADME parameters, pharmacokinetic qualities, drug-like nature and medicinal chemistry friendliness by harnessing its ability to compute physicochemical descriptors (Daina et al. 2017).
Results and discussion
Molecular docking and MM-GBSA studies
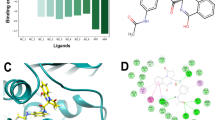
From a library of 5968 compounds, initial screening was done using HTVS docking and based on the docking score, the top 200 compounds were selected for SP docking studies. As SP docking algorithms are more refined than HTVS docking, therefore the second level of screening was done using SP mode. The top 50 compounds based on the docking score in the SP mode were selected for docking in the XP mode. The docking results were compared with the standard ligand (Clorobiocin) co-crystallized with the target protein. The docking score of Clorobiocin was found to be −6.078 kcal/mol, and it showed H–bond interactions with GLY77 and THR165 amino acid residues (Fig. 1f). The docking score of clorobiocin was found to be similar to the one reported by Elseginy et al. which was −6.3 kcal/mol (Elseginy and Anwar 2022). Hydrophobic interactions were observed with VAL43, ALA47, VAL71, ILE78, PRO79, ILE90, ALA96, VAL118, GLY119, VAL120, MET166, AND VAL167 residues. Polar interactions were observed with ASN46, GLN72, HIS95, and THR165 residues. Charged negative interactions were observed with ASP49, GLU50, and ASP73 amino acid residues. Hydrophobic interactions were also similar to one reported by Elseginy et al. which were with VAL43, ALA47, VAL67, VAL71, ILE78, PRO79, MET95, and VAL120 residues (Elseginy and Anwar 2022).
In comparison with Clorobiocin, all five selected compounds showed a better docking score which was > −8.0 kcal/mol (Table 1). Compound AM5 showed the best docking score of −10.160 kcal/mol while compound AM4 showed the least docking score of −8.007 kcal/mol which was still better than Clorobiocin. Non-bonding interactions like H–bonding, hydrophobic, polar interactions, etc., were also similar to Clorobiocin as shown in Table 1 and Fig. 1. Salt-bridge type interactions were also observed with all these ligands except AM3 and AM4 with ASP73 residue which was not observed for Clorobiocin. Compounds AM3 and AM4 showed salt-bridge interaction with ASP49 residues. The ligand interaction diagram of all the top ligands is shown in Fig. 1.
Many hydrophobic residues, including VAL43, ALA47, ILE78, PRO79, ILE90, MET91, VAL120, VAL67, ARG136, and ASN46, line the binding pocket. These residues are reported to be conserved in type II topoisomerases, DNA gyrase A, and DNA gyrase B across the species. For ATP hydrolysis to occur, ASN46 must coordinate with the Mg + 2 ion in the ATP binding pocket (Lewis et al. 1996). Therefore, our docked compounds needed to bind with ASN46 residues. It was found that all the top five ranked compounds except AM3 formed H–bond interactions with ASN46 residues. All the compounds showed hydrophobic interactions with these conserved amino acid residues which might be crucial for their potential inhibition of Gyr B. The docking results are also similar to the hydrophobic interactions reported by some other recently published studies (Carpio Arévalo and Amorim 2021; Amorim et al. 2022).
Free binding energy calculations were also carried out for the top five ligands in the complex with the protein. The compounds were ranked based on dG bind energy, and all the selected compounds showed favorable binding free energy profiles (Table 1). Compound AM1 showed the best dG bind energy of −59.32 kcal/mol, while compound AM4 showed the lowest value of −40.20 kcal/mol. Clorobiocin showed a dG bind score of −64.36 kcal/mol, and hence, compounds AM1, AM2 and AM5 were found to show a comparable free binding energy profile with the standard Clorobiocin.
MD simulation study
The stability of the DNA gyrase and its identified inhibitors (AM1 and AM5) based on the docking studies was further validated through an MD simulation study for 100 ns. Docking studies are devoid of any water molecule, and there is no control over the temperature and pressure parameters also. Therefore, the MD simulation study gives a better understanding of the complex stability in the aqueous environment and at room temperature and pressure. Various information like the conformational changes in the ligands, protein residues and changes in their interaction during the simulation period, stability, etc., are collected during the simulation and are represented as various plots like RMSD (root mean square deviation), SASA (solvent accessible surface area), H–bond plot, RMSF (root mean square fluctuation), the radius of gyration (Rg) and root mean square fluctuation (RMSF).
RMSD analysis
RMSD plots are very crucial in MD simulation studies. It gives an insight into the overall stability of the protein–ligand complex which can be analyzed over two phases, i.e., the equilibrium phase and the production phase. The first frame is taken as the reference frame and all subsequent frames are superimposed over it to obtain the RMSD plot to time. As shown in Fig. 2, the RMSD of Cα-atoms was computed at the beginning and for the AM1-DNA gyrase complex (black color in Fig. 2), the system reached the equilibrium phase at around 10 ns. For the AM5-DNA gyrase complex (red color in Fig. 2), equilibrium was attained at around 20 ns. During the post-equilibrium phase, both the complexes remained stable throughout the simulation period as shown by the average RMSD deviation between 0.1 to 0.4 nm. The RMSD plot suggested that the compounds AM1 and AM5 might form a stable complex with DNA gyrase.
SASA and H–bond analysis
Intermolecular hydrogen bonding is one of the most important interactions between protein and ligand and plays a key role in the stability of the complex. The distribution and number of H–bonding between the ligand and protein during MD simulation are depicted in Fig. 3a. For the AM1-DNA gyrase complex (black color), there were a few poses which showed 7 H–bonds, while many of the poses showed 5–6 H–bonds. The average density of the H–bond was strongly distributed around 3 and 4 which suggests that AM1-DNA gyrase might form a stable complex. For the AM5-DNA gyrase complex, a few poses showed 3 to 4 H–bonds, while the average H–bonding interaction was 2, implying that the AM5-DNA gyrase complex might also be stable through the simulation period.
SASA plot represents the change inaccessibility of protein to the solvent. It gives an insight into the analysis of the folding pattern of the inner hydrophobic core to the outer hydrophilic surface of the protein structure. As depicted in Fig. 3b, the SASA for AM1-DNA gyrase complex (black) and AM1-DNA gyrase complex (red) was in the range of 100–120 nm2 which suggests that there were no major changes in the folding pattern of the protein due to binding with the ligands and hence, might have remained stable throughout the simulation period.
Rg and RMSF analysis
The radius of gyration serves as a measure of the compactness of protein structures. It is focused on how regular secondary structures can be tightly packed into the three-dimensional (3D) structure of proteins. A decrease in protein structural compactness is implied by an increase in Rg values, indicating higher flexibility and less stability. As shown in Fig. 4a, the Rg values for both complexes were in the range of 1.63–1.73 nm with major clustering in the range of 1.65–1.70 nm. This indicates that the protein maintained its compactness even after binding with the ligands and hence, furthers the RMSD, finding that the protein–ligand complex was stable.
The average variation of a protein residue over time from a reference position is measured by the RMSF. As a result, RMSF gives information about the structural elements that deviate the most or the least from their mean structure. The RMSF plot of the complex is depicted in Fig. 4b. The loop of DNA gyrase B generated by residues 98 through 118 of the protein closes the ATP binding pocket and has been reported to be disordered (Holdgate et al. 1997), and there is a clear rise in fluctuation in the disordered region of the protein. Apart from the disordered region, the fluctuation was within 0 to 0.4 nm which is in the acceptable range of 3.4 Å (Li et al. 2011). This finding is also in accordance with the reported literature.
ADME analysis
Initially, we thought to do ADME profiling of the compounds to select only drug-like molecules from the library of 5968 compounds. But then we did not want to miss out on any potent inhibitor of DNA gyrase because of the unfavorable ADME profile. As this is an in silico study, the potent DNA gyrase inhibitors could be identified at this stage and then, their pharmacokinetic properties can be optimized. Therefore, we ran ADME profiling of only the top five compounds that were identified using XP docking studies. Several parameters were computed for all the compounds, and some of them like molecular weight, the number of H–bond donors and acceptors, polar surface area (PSA), solubility, gastrointestinal (GI) absorption, blood–brain-barrier (BBB) permeation, Lipinski’s rule of five violations, PAINS alert, lead-likeness, and synthetic feasibility are reported in Table 2. All the compounds were lead-like molecules with no violation of the rule of five. There were no PAINS alerts for any compounds except AM2. They also showed good GI absorption (especially AM3, AM4, and AM5). It was interesting to note that the molecules from AM3 to AM5 had three rings in their structure compared to Am1 and AM2 with two rings. Thus, they might show greater lipophilicity and hence, increased GI absorption. The prevailing consensus has been that an oral drug's bioavailability is mostly influenced by its physicochemical attributes. The molecular weight, pK, and lipophilicity, as measured by the octanol/water partition coefficient, are some of these properties (Log D). But several reports are suggesting that this is a too simplistic view. For many molecules, no correlation could be found between Log D and GI absorption (Barthe et al. 1999). The water solubility and intestinal membrane permeability of pharmaceuticals are their inherent qualities that affect oral absorption, according to the Biopharmaceutics Classification System (BCS) of the US Food and Drug Administration. Due to its function in drug dissolving within the gastric lumina, solubility is one of the important parameters that determine the oral bioavailability of drug molecules (Azman et al. 2022). Since these molecules are predicted to be very soluble hence, increasing lipophilicity might lead to an increase in GI absorption.
All the compounds were predicted to be water-soluble, and none of them could cross BBB. Charge, mass and lipophilicity of a molecule impact its ability to cross BBB (Kasinathan et al. 2015). These molecules are designed to target bacterial DNA gyrase of the bacterial species which mostly cause infection outside the brain, and hence, non-permeability of BBB can be advantageous in avoiding unwanted CNS toxicity. They were also predicted to be Pgp substrates except for AM2 and AM4, and none of them inhibited CYP1A2 and its other isoforms like CYP2C19, CYP2C9, CYP2D6, and CYP3A4. Synthetic accessibility scores also suggested that these compounds can be synthesized. Based on the ADME predictions, the selected compounds AM1 and AM5 seem to be drug-like inhibitors of the DNA gyrase enzyme.
Conclusion
In the present study, we have employed HTVS docking to screen a focused library of antibacterial compounds to identify potential DNA gyrase inhibitors. The top 200 compounds were further filtered with the help of more advanced docking algorithms like SP and XP docking. Finally, based on the docking score and MM-GBSA studies, the top two compounds were selected for MD simulation studies. The docking score of the co-crystallized ligand (Clorobiocin) was found to be −6.078 kcal/mol and in its comparison, the top five selected compounds showed a docking score of > −8.0 kcal/mol. Both the compounds showed potential H–bond interaction with the conserved amino acid residue ASN46 which might make them potent inhibitors of DNA gyrase B. MD simulation studies also suggested that the ligand-receptor complex between compounds AM1, AM5, and DNA gyrase would be stable as supported by the RMSD and H–bond plots. ADME studies also reinforced the findings that compounds AM1 and AM5 have an acceptable pharmacokinetic profile and are drug-like molecules. Based on the above studies, herein we report compounds AM1 (1R,3S)-1-(2-((3-(ammoniomethyl)phenyl)amino)-2-oxoethyl)-3-carbamoylpiperidin-1-ium and AM5 (1'S,2 s,4R)-4-ammonio-6-ethyl-1'-methylspiro[chromane-2,4'-piperidin]-1'-ium as potential DNA gyrase inhibitors which can be further developed as a potential lead against the menace of antibiotic resistance.
References
Alfonso EE, Deng Z, Boaretto D et al (2022) Novel and structurally diversified bacterial DNA gyrase inhibitors discovered through a fluorescence-based high-throughput screening assay. ACS Pharmacol Transl Sci 5(10):932–944. https://doi.org/10.1021/acsptsci.2c00113
Amorim JC, Cabrera Bermeo AE, Vásquez Urgilés VE et al (2022) An in-silico evaluation of anthraquinones as potential inhibitors of DNA gyrase B of mycobacterium tuberculosis. Microorganisms 10:2434. https://doi.org/10.3390/MICROORGANISMS10122434/S1
Azman M, Sabri AH, Anjani QK et al (2022) Intestinal absorption study: challenges and absorption enhancement strategies in improving oral drug delivery. Pharmaceuticals 15(8):975. https://doi.org/10.3390/PH15080975
Barthe L, Woodley J, Houin G (1999) Gastrointestinal absorption of drugs: methods and studies. Fundam Clin Pharmacol 13:154–168. https://doi.org/10.1111/J.1472-8206.1999.TB00334.X
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys Commun 91:43–56. https://doi.org/10.1016/0010-4655(95)00042-E
Berman H, Henrick K, Nakamura H (2003) Announcing the worldwide protein data bank. Nat Struct Biol 10:980. https://doi.org/10.1038/NSB1203-980
Boutin JA, Altieri F, Dibavar AS et al (2023) DNA gyrase as a target for quinolones. Biomedicines 11:371. https://doi.org/10.3390/BIOMEDICINES11020371
Carpio Arévalo JM, Amorim JC (2021) An in-silico analysis reveals 7,7′-bializarin as a promising DNA gyrase B inhibitor on gram-positive and gram-negative bacteria. Comput Biol Med 135:104626. https://doi.org/10.1016/J.COMPBIOMED.2021.104626
Champoux JJ (2003) DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413. https://doi.org/10.1146/annurev.biochem.70.1.369
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 71(7):1–13. https://doi.org/10.1038/srep42717
Dutta R, Inouye M (2000) GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci 25:24–28. https://doi.org/10.1016/S0968-0004(99)01503-0
Elseginy SA, Anwar MM (2022) Pharmacophore-based virtual screening and molecular dynamics simulation for identification of a novel DNA gyrase B inhibitor with benzoxazine acetamide scaffold. ACS Omega 7:1150–1164. https://doi.org/10.1021/acsomega.1c05732
Friesner RA, Banks JL, Murphy RB et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. https://doi.org/10.1021/jm0306430
Friesner AR, Murphy BR, Repasky PM et al (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein−ligand complexes. J Med Chem 49:6177–6196. https://doi.org/10.1021/JM051256O
Genheden S, Ryde U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449. https://doi.org/10.1517/17460441.2015.1032936
Govender P, Müller R, Singh K et al (2022) Spiropyrimidinetrione DNA gyrase inhibitors with potent and selective antituberculosis activity. J Med Chem 65(9):6903–6925. https://doi.org/10.1021/acs.jmedchem.2c00266
Hasan MR, Chowdhury SM, Aziz MA et al (2021) In silico analysis of ciprofloxacin analogs as inhibitors of DNA gyrase of staphylococcus aureus. Inform Med Unlocked 26:100748. https://doi.org/10.1016/J.IMU.2021.100748
Holdgate GA, Tunnicliffe A, Ward WHJ et al (1997) The entropic penalty of ordered water accounts for weaker binding of the antibiotic novobiocin to a resistant mutant of DNA gyrase: a thermodynamic and crystallographic study. Biochemistry 36:9663–9673. https://doi.org/10.1021/bi970294+
Jakhar R, Khichi A, Kumar D et al (2022) Discovery of novel inhibitors of bacterial DNA gyrase using a QSAR-based approach. ACS Omega 7:32665–32678. https://doi.org/10.1021/acsomega.2c04310
Kasinathan N, Jagani HV, Alex AT et al (2015) Strategies for drug delivery to the central nervous system by systemic route. Drug Deliv 22:243–257. https://doi.org/10.3109/10717544.2013.878858
Khan T, Sankhe K, Suvarna V et al (2018) DNA gyrase inhibitors: progress and synthesis of potent compounds as antibacterial agents. Biomed Pharmacother 103:923–938. https://doi.org/10.1016/J.BIOPHA.2018.04.021
Kumar A, Rai S, Rathi E et al (2020) Pharmacophore-guided fragment-based design of novel mammalian target of rapamycin inhibitors: extra precision docking, fingerprint-based 2D and atom-based 3D-QSAR modelling. J Biomol Struct Dyn 39:1155–1173. https://doi.org/10.1080/07391102.2020.1726816
Lafitte D, Lamour V, Tsvetkov PO et al (2002) DNA gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5′-methyl group of the noviose. Biochemistry 41:7217–7223. https://doi.org/10.1021/BI0159837
Lewis RJ, Singh OMP, Smith CV et al (1996) The nature of inhibition of DNA gyrase by the coumarins and the cyclothialidines revealed by X-ray crystallography. EMBO J 15:1412–1420. https://doi.org/10.1002/J.1460-2075.1996.TB00483.X
Li MH, Luo Q, Xue XG, Li ZS (2011) Molecular dynamics studies of the 3D structure and planar ligand binding of a quadruplex dimer. J Mol Model 17:515–526. https://doi.org/10.1007/S00894-010-0746-0
Madhavi Sastry G, Adzhigirey M, Day T et al (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234. https://doi.org/10.1007/s10822-013-9644-8
Maxwell A (1997) DNA gyrase as a drug target. Trends Microbiol 5:102–109. https://doi.org/10.1016/S0966-842X(96)10085-8
Meena MK, Kumar D, Kumari K et al (2021) Promising inhibitors of nsp2 of CHIKV using molecular docking and temperature-dependent molecular dynamics simulations. J Biomol Struct Dyn 40(13):5827–5835. https://doi.org/10.1080/07391102.2021.1873863
Mohammed HHH, Ali DME, Badr M et al (2022) Synthesis and molecular docking of new N4-piperazinyl ciprofloxacin hybrids as antimicrobial DNA gyrase inhibitors. Mol Divers 1:1–15. https://doi.org/10.1007/s11030-022-10528-z
Mukherjee S, Abdalla M, Yadav M et al (2022) Structure-based virtual screening, molecular docking, and molecular dynamics simulation of VEGF inhibitors for the clinical treatment of Ovarian cancer. J Mol Model 284(28):1–21. https://doi.org/10.1007/S00894-022-05081-3
Pakamwong B, Thongdee P, Kamsri B et al (2022) Identification of potent DNA gyrase inhibitors active against mycobacterium tuberculosis. J Chem Inf Model 62:1680–1690. https://doi.org/10.1021/acs.jcim.1c01390
Rathi E, Kumar A, Kini SG (2020) Computational approaches in efflux pump inhibitors: current status and prospects. Drug Discov Today 25:1883–1890. https://doi.org/10.1016/J.DRUDIS.2020.07.011
Roos K, Wu C, Damm W et al (2019) OPLS3e: extending force field coverage for drug-like small molecules. J Chem Theory Comput 15:1863–1874. https://doi.org/10.1021/acs.jctc.8b01026
Saleh NM, Moemen YS, Mohamed SH et al (2022) Experimental and molecular docking studies of cyclic diphenyl phosphonates as DNA gyrase inhibitors for fluoroquinolone-resistant pathogens. Antibiot 11:53. https://doi.org/10.3390/ANTIBIOTICS11010053
Webber MA, Ricci V, Whitehead R et al (2013) Clinically relevant mutant DNA gyrase alters supercoiling, changes the transcriptome, and confers multidrug resistance. MBio 4(4):e00273. https://doi.org/10.1128/mBio.00273-13
Acknowledgements
The authors are also grateful to the Manipal-Schrodinger Centre for Molecular Simulations, Manipal College of Pharmaceutical Sciences, MAHE, Manipal, for providing facilities to carry out in silico studies.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection and analysis were performed by AK, CP and ER. The first draft of the manuscript was written by Avinash Kumar, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, A., Prasun, C., Rathi, E. et al. Identification of potential DNA gyrase inhibitors: virtual screening, extra-precision docking and molecular dynamics simulation study. Chem. Pap. 77, 6717–6727 (2023). https://doi.org/10.1007/s11696-023-02971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02971-5