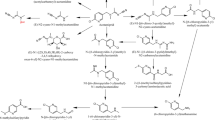
Abstract
Economically speaking, rodents possess a serious threat to the agriculture sector. One of these organisms that directly threaten agriculture, stocks, and others is the Norway rat, Rattus norvegicus (R. norvegicus). The 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide (1) was used as a precursor to give 2-cyano-3-(dimethylamino)-N-(1H-1,2,4-triazol-3-yl) acrylamide (2) and ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3). Infra-red, 1H-NMR, 13C-NMR, MS, and elemental analysis were done for the precise structure elucidation of the applied synthons. The prepared compounds were tested as potential rodenticides against the Norway rat, Rattus norvegicus. Toxicity analysis using four serial doses of both prepared compounds revealed that the LD50 values were 160.6 and 391.7 mg/kg body weight, for ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3) and 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide (1), respectively. Several biological variables, such as alanine transaminase (ALT), aspartate transaminase (AST), serum urea, creatinine, and total protein, have been assessed and evaluated as biological response indicators. Analysis revealed a highly significant increase in both AST, ALT, urea, and creatinine levels, while the total protein level showed a considerable reduction in treated rats exposed to 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide (1) and ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3) when compared to the control treatment. Liver histological examination showed structural changes in the form of congestion in the central vein, necrosis in some hepatic regions, and pyknotic nuclei, while kidney histological examination showed vacuolar degeneration of the epithelial cells of some convoluted tubules and the disappearance of some glomeruli and other marked atrophies. Necrosis in some areas was noticed. Field application through bait consumption took place with a satisfactory reduction of 68.4% for ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3), while it was 61.9% for 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide (1) when compared to the recommended Zinc phosphide commercial rodenticide that poses an 81% reduction.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rodents have earned a terrible reputation for being one of the most widespread vertebrate pests that seriously impact human populations. They cause economic problems as a result of the damage they can cause to agroecosystems (Neena and Babbar 2010) (Singla and Babbar 2012) environmental problems as a result of the chemicals used to control them (Naidu et al. 2021), social problems as a result of their close proximity to human habitation, and, of course, health problems as zoonotic carriers (Singla et al. 2008) (Singla and Babbar 2012) (Herbreteau et al. 2012). When grains are damaged by rodents in stores, the germ is often destroyed, resulting in germination failure when implantation takes place; because the grain has been contaminated with feces, hair, and even urine, it has a poorer grade and lower marketing value. Contaminated batches are frequently considered unfit for human consumption (Ognakossan 2017). Furthermore, rodents destroy various items, including building structures, packing materials, clothing, and furniture, as a result of their chewing and burrowing behaviors; they cause fires by eating through electrical lines, resulting in severe losses (Belmain et al. 2015). Rodenticides are not exempt from this ongoing change in the agriculture sector, especially with regard to pesticides and pesticide alternatives. In order to successfully reduce the number of these destructive pests, it is therefore needed to look for better alternatives and chemical groups. Despite the fact that triazole compounds were synthesized for the first time decades ago, they continue to attract the interest of organic chemistry scientists (Kumari et al. 2021), (Zhao et al. 2022). To assess the triazole in rat’s blood, Li and his team (Li et al. 2013) used ultrasound-enhanced temperature-controlled (UETC) ionic liquid dispersive liquid–liquid micro-extraction (IL-DLLME). The results demonstrated that the approach developed for determining target triazole groups in rat blood samples was effective. 3-Amino-l,2,4-triazole compound was evaluated as an agent of thyroid tumor prevalence in rats when treated with a subeffective dose of N-bis (2-hydroxypropyl) nitrosamine over a period of twenty weeks and using a triazole diet with a concentration of 2000 ppm, where the incidence of thyroid tumors was 91% in the injected rats (Hiasa et al. 1982). The toxicity effect in rats of hexaconazole, a commonly used fungicide containing a triazole moiety, was well illustrated. For 12 weeks, rats were given 100 mg/kg of hexaconazole orally via diet. A group of biochemical parameters were noticed; creatinine, bilirubin, ALAT, ASAT, and LDH levels were found to be significantly higher in the blood of treated rats, indicating toxicity in various organs. A histopathological examination of the liver was also performed. Several liver abnormalities were observed, including centrilobular vein congestion, necrosis, immune cell infiltration, and microvesicular steatosis. Exposure to hexaconazole causes kidney and liver damage, according to these biochemical and histopathological examinations (Jalal et al. 2020); furthermore, toxigenomic analysis was performed to verify the ability of the genomics to predict the potential toxicity; three compounds used as fungicides, all containing triazole nucleus, were used on male Sprung-Dawley rats via oral serum over one, three, and five days at doses of 300, 175, 20, or 10 mg/kg day. Genomic, clinical chemistry, and hematological analysis showed the induction of triazole compounds on pregnancy X receptors (PXR), the metabolism of xenobiotics, as well as oxidative stress genes (Martin et al. 2007). Biochemical variables like acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) were investigated, and the effects of 1H-1,2,3-triazole tacrine-chalcone derivatives against mice were well characterized. Additionally, investigations on oxidative stress' effects on behavior and neurochemistry have revealed decreased glutathione levels (GSH) and alterations in lipid peroxidation products (TBARS) in both the brain and hemocytes (Rani et al. 2021). When difenoconazole, a fungicide with a triazole moiety, was applied to the albino rat, many parameters were measured; lipid profile indices and glucose level, as well as thyroid hormonal level and renal parameters. Results revealed a significant increase in blood glucose levels and the lipid profile indices. In addition, a significant increase in renal markers (creatinine and urea) was well noticed (Mohamed El-Sayed et al. 2022).
The current study is an experiment with new synthetic triazole derivatives as novel materials that can be used in R. norvegicus control operations as one of the most widely distributed rodent species; it studies the influence of these compounds on biochemical parameters and histological alternations, as well as field experimentation evaluating their ability to reduce the population in the field environment.
Materials and methods
Chemicals
3-Amino-1H-1,2,4-triazole, 2-cyanoacetic acid, and acetic anhydride were obtained from PubChem. N,N-dimethylformamide-dimethylacetal (DMF-DMA) was obtained from Sigma-Aldrich. DMF and ethyl cyanoacetate were obtained from Piochem. Zinc phosphide (94%) was obtained from KZ Pesticides Company, Egypt.
Synthetic procedures
Synthesis of 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide) (1)
A pre-prepared mixture of cyanoacetic acid (2.55 g, 0.03 mol) in acetic anhydride (25 mL) was heated in a water bath (80 oC) till an orange color appeared; 3-amino-1H-1,2,4-triazole (2.52 g, 0.03 mol) was added; then the mixture was heated for two hours. A faint yellow compound was formed and left to cool at room temperature. The obtained yellow precipitate was filtered, washed with ethanol, and dried to afford 3.6 g of compound (1) (79.4%). Yellow powder; mp 260–265 °C; IR [KBr] ν/cm−1: 3255 (NH), 2264 (CN), 1695 (C=O); 1H-NMR [DMSOــd6]: δ (ppm): 3.93 (s, 2H, CH2), 7.97 (br., s, 1H, CH-triazole), 11.40 (br., s, NH) and 13.40 (br., s, NH-triazole); 13C-NMR [DMSOــd6], δ (ppm): 26.56, 115.83, 156.37, 158.28, 163.65 ppm; MS (EI, 70 eV): m/z(%) = 151 (M+, 50.5), 84 (100), 68 (24.5), 57 (12.4); Anal. calculated for C5H5N5O (151.13): C, 39.74; H, 3.33; N, 46.34%. Found: C, 39.69; H, 3.37; and N, 46.40%.
Synthesis of 2-cyano-3-(dimethylamino)-N-(1H-1,2,4-triazol-3-yl) acrylamide (2)
A mixture of compound (1) (4.53 g, 0.03 mol) and (DMFـDMA) (3.6 mL, 0.03 mol) in dry DMF (30 mL) was stirred for 2 h at room temperature. The precipitated solid was filtered off and dried to afford 4.2 g of enaminonitrile (2) (68%).
White powder; mp 210–214 °C; IR [KBr] ν/cm−1: 3305 (NH), 2194 (CN), 1661 (C=O); 1H-NMR [DMSO-d6]: δ (ppm), 3.27 (s, 6H, N(CH3)2), 7.77 (s, 1H, α,β-unsaturated carbonyl proton), 7.88 (s, 1H, NH), 7.90 (s, 1H, CH-triazole), and 10.65 (s, 1H, NH); 13C-NMR [DMSO-d6], δ (ppm), 35.04 (2CH3), 117.30, 119.17, 147.86, 155.95, 156,94 and 164.70; MS (EI, 70 eV): m/z (%) = 205.96 (M+, 24.27), 160 (25.05), 50 (100), 40 (94.01); Anal. calculated for C8H10N6O (206.2): C, 46.60; H, 4.89; N, 40.76%. Found: C, 46.75; H, 4.97; and N, 40.79%.
Synthesis of ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3)
A mixture of the enaminonitrile (2) (2.06 g, 0.01 mol) and ethyl cyanoacetate (1.13. mL, 0.01 mol) in ethanol (15 mL) containing piperidine (0.5 mL) was refluxed for 3 h. The reaction mixture was left to cool and poured into iceــwater. The obtained precipitated solid was filtered, recrystallized from ethanol, and dried to afford 1.9 g the pyridine-carboxylate derivative (3) (69%). Yellow crystals; mp 290–292 °C; IR [KBr] ν/cm−1: 3201 (NH), 3343 (NH2), 2228 (CN), 1644 and 1699 (2C=O); 1HــNMR [DMSO-d6]: δ (ppm), 1.30 (t, J = 5.0 Hz, 3H, CH3); 4.26 (q, J = 5.0 Hz, 2H, CH2), 8.38 (s, 1H, triazole) and 8.77 (s, 1H, pyridine) and 8.99 (br, s, 1H, NH); 13CــNMR [DMSO-d6], δ (ppm), 14.57, 61.27, 86.94, 90.51, 116.97, 146.93, 149.56, 151.42, 157.83, 159.82 and 165.49 ppm.; MS (EI, 70 eV): m/z (%) = 274 (M+, 48.97), 110 (40.17), 83 (100); Anal. calculated for C11H10N6O3 (274): C, 48.18; H, 3.68; N, 30.65%. Found: C, 48.32; H, 3.75; and N, 30.78.
Instruments and general remarks
All melting points were determined on Gallenkamp electric melting point apparatus. Elemental analysis was carried out using Thermo Scientific CHNS/O Elemental Analyzer. IR spectra were recorded on a Mattson 5000 spectrometer with a model 550 spectrophotometers using a KBr wafer technique. The 13C- and 1H-NMR spectra were measured on Brucker Wpsy 125 and 500 MHz, deuterated dimethyl sulfoxide (DMSO-d6) as a solvent, using TMS as an internal reference. The chemical shift (δ) expressed in ppm. The acronyms: (s) singlet, (d) doublet, (t) triplet, (q) quartet, and (m) multiplet were used to define the type of protons. Mass spectra were determined on a Varian MAT 311 (70 eV).
Rodenticide activity test
Experimental animals
Fields in Aga, Dakahlia Governorate's Norway rat, R. norvegicus adults, were captured using traps and then transported to a laboratory. They were kept in individual cages with a diet consisting of 65% crushed maize, 25% ground wheat, 5% sugar, and 5% corn oil. Food and water were available ad libitum throughout the whole experimental period. Unhealthy and pregnant animals were not included. Two weeks before the start of the experiments, rats were suitably equipped to adapt to a laboratory environment; the animals under study lived in laboratory conditions of 25 °C in a 12-h light/dark cycle. Each animal weighs between 200 and 230 g. The animals were divided into groups, each group containing five adult rats, and there was also a matching group for the control.
Determination of LD50 values
The Norway rat was fasted for approximately 12 h before treatment to estimate the acute oral LD50 of the two tested synthetic organic compounds. Serial doses of compound (1) were 150, 300, 600, and 800 mg/kg body weight, while those of compound (3) were 50, 100, 400, and 500 mg/kg body weight. They were given to the animals orally using stomach tubes, combined with an appropriate amount of vegetable oil for dissolution, and then given water and food two hours later. Plain vegetable oil was used for a comparable control experiment. Up to 7 days after organic synthesized compound dosing, mortality percentages were adjusted using Abbott’s method; the LD50 and LD90 values were calculated using a probit analysis statistical approach (Abbott 1925).
Biochemical analysis
The effects of the lethal dose LD50 of the two tested organic synthesized compounds on some biochemical parameters were studied as a physiological response. Animals were orally intubated for 24 h. Animals were sacrificed after 3 days of treatment, and blood samples were taken from each animal, dispensed in clean tubes. Among the results of biochemical analysis of serum samples from the control and treated groups were aspartate aminotransferase and alanine aminotransferase; enzyme activity was quantified colorimetrically for AST and ALT (Huang et al. 2006); serum urea (Wang et al. 2022); creatinine (Imasawa et al. 2021); and total protein (Eaton et al. 2013) of treated and untreated animals.
Histological investigation
After the animals were killed and blood samples were obtained, the animals were dissected to obtain the liver and kidney specimens from the control and treated groups. The specimens were fixed in 10% formalin-saline. The samples were then taken to the histopathology Laboratory at Mansoura University's Faculty of Medicine for analysis. Hematoxylin and eosin were used to stain tissue sections of 5μ thickness (Creasy et al. 2021) for illustration of the histological examination.
Field experiments
In the field conditions of Aga district, Dakahlia Governorate, a field evaluation of crushed maize bait containing 1% zinc phosphide, compound (1), and compound (3) treated groups was performed (Hinds et al. 2023). The whole area is infested with R. norvegicus. Each compound was treated in an area equal to one feddan (an area unit used in Egypt equivalent to 4200 m2), while a comparable area was left untreated as a control. The pre- and post-treatment rat population density was calculated using the food consumption method (Rennison 1977). Each plastic bag containing 100 g (1 g toxicant)synthetic compound /or zinc phosphide(+ 5 g corn oil and 94 g crushed maize) of the proposed bait weighed 2 kg and was placed in the selected plot for five successive days; each tested bait consumption amount was reported. Calculations were made to determine the population reduction as follows:\({\text{Population reduction}}\% = \frac{{{\text{Pre}} - {\text{treatment}}\;{\text{consumed}} - {\text{post}} - {\text{treatment}}\;{\text{consumed}}}}{{{\text{Pre}} - {\text{treatment}}\;{\text{consumed}}}} \times 100\)
Statistical analysis
LD50 values were expressed as mg/kg body weight unit. All data, presented as mean ± SE, were subjected to one way analysis of variance (ANOVA) (St and Wold 1989). Confidence intervals with a 95% simultaneous confidence level were created using Tukey’s method. Significant was considered to be a 0.05 probability. Cohort Software was used for all statistical analyses (Cho et al. 2004).
Results and discussion
Chemistry
In general, our synthons were prepared through the reaction of 3-amino-1H-1,2,4-triazole with cyanoacetic acid to give the 2-cyano-N-(1H-1,2,4-triazol-3-yl) acetamide (1), which reacts with N,N-dimethylformamide dimethyl acetal (DMF-DMA) to give the enaminonitrile (2), which in turn enters into a reaction with ethyl cyanoacetate through a non-isolable intermediate to afford the ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3).
The key starting material, (1), was prepared by cyanoacetylation of 1H-1,2,4-triazol-3-amine through a reaction with a pre-prepared mixture of 2-cyanoacetic acid and acetic anhydride (Scheme 1) (Ibrahim et al. 2011) (Tenor and Kröger 1964). The mechanism of this reaction was preceded via the initial formation of the acetylating agent, which was attacked by the exocyclic amino group of the aminotriazole (Bayazeed and Alnoman 2022), (Mohamed and Mahmoud 2019). The structure of (1) has been established via different analytical and spectral data. The IR spectrum showed absorption bands at ν = 3260, 2264 and 1696 cm−1 corresponding to (NH), (CN) and amidic carbonyl functions. The 1H-NMR spectrum (DMSO-d6) showed a singlet signal at δ 3.93 ppm for two protons of CH2, a singlet signal at δ 7.97 ppm for CH of the triazole ring, and two broad signals for two protons of 2NH groups at δ 11.40 and 13.40 ppm (S1). The 13C-NMR spectrum showed signals for methylene carbon at δ 26.56 ppm, a carbonyl carbon at δ 163.66 ppm, cyano carbon at δ 115.83 ppm, and two carbons at δ 158.28 and 156.37 ppm for C3, C5 triazole carbons (S2). The mass spectrum (S3) showed the molecular ion peak at m/z = 151 (M+), which is well-matched with the molecular formula (C5H5N5O).
The chemistry of enaminonitriles has received significant interest as a precursor to synthesize azoles, azines, and azoloazine ring systems (Madkour et al. 2008) (Bondock et al. 2011) (Fadda et al. 2013) (Fadda et al. 2021). The utility of enaminonitriles in organic synthesis as a reactive precursor for the production of diverse heterocyclic compounds has been reported (Bondock et al. 2009) (Fadda et al. 2012). Treatment of compound (1) with DMF-DMA by stirring in dry DMF afforded the predicted enaminonitrile. The enaminonitrile (2) structure was elucidated according to analytical and spectral data. The IR spectrum showed an absorption band at 3305 cm−1 due to (NH) group, 2194 cm−1 for (CN) group and 1661 cm−1 due to amidic carbonyl group. The 1H-NMR spectrum (DMSO-d6) revealed a singlet signal at δ 3.27 ppm analogous to N,N-dimethylamine protons. A characteristic singlet signal at δ 7.77 ppm attributed to β proton in α,β-unsaturated carbonyl system, singlet signal at δ7.88 ppm for the olefinic proton of triazole and two singlet signals (at δ 7.90 and broad at 10.65 ppm) for two NH protons (S4). The 13C-NMR spectrum showed signals at δ 35.04 for two [N–(CH3)2], and δ 117.30, 119.17, 147.86, 155.95, 156.94, 164.70 ppm (S5). The mass spectrum showed the molecular ion peak at m/z = 206 (M+) for the molecular formula (C8H10N6O) (Scheme 2).
The reaction of enaminonitrile (2) with ethyl cyanoacetate afforded ethyl 2-amino-5-cyano-1,6-dihydro-6-oxo-1-(1H-1,2,4-triazol-3-yl) pyridine-3-carboxylate (3) through a non-isolable intermediate(Alnajjar et al. 2018) (Bondock et al. 2009). The mechanism of the reaction is proposed through the initial addition of the active methylene group of ethyl cyanoacetate to β-carbon of enaminonitrile to form the non-isolable intermediate, followed by the nucleophilic attack of the NH group on the cyano functional group and the elimination of the dimethylamine molecule (Al-Mousawi et al. 2008) (Scheme 3).
The structure of (3) was well confirmed by different elemental analysis and spectral data. The IR spectrum of the pyridine derivative showed absorption bands at ν = 1699, 1664 cm−1 due to two carbonyl groups, a band at ν = 3343 cm−1 corresponding to the amino functional group, and a band corresponding to the cyano group at ν = 2228 cm−1.
The 1H-NMR spectrum (DMSO–d6) revealed a singlet signal at δ 8.77 ppm for the CH proton of the triazole ring, a singlet signal at δ 8.38 ppm for the C4-H proton of the pyridine ring (S6), a quartet at δ 4.26 ppm for the CH2CH3 protons, and a triplet signal at δ 1.30 ppm for the CH2CH3 protons of the ester group (Fig. 1). The 13C-NMR spectrum revealed signals at δ 14.57, 61.27, 86.94, 90.51, 116.97, 146.93, 149.56, 151.42, 157.83, 159.82, and 165.49 ppm, which agree with the proposed structure (S7). The mass spectrum showed the molecular ion peak at m/z = 274 (M+), which is well matched with the molecular formula (C11H10N6O3).
Photomicrographs of sections in livers of R. norvegicus. a: Control, showing a central vein (CV), radiating cords of liver cells separated by blood sinusoids (S) and Kupffer cells (arrow). b: After treatment with compound (1), displaying sinusoidal hemorrhage (arrow). c: After treatment with compound (3), demonstrating congested central vein (CV), necrosis (N) in some hepatic regions and pyknotic nuclei (arrow). (H & E X400)
Toxicity assessment and LD values
We elucidated the rodenticidal activities of (1) and (3) against the Norway rat; the results are summarized in Table 1. Compound (3) shows a higher mortality rate for R. norvegicus, followed by compound (1); the LD50 and LD90 values were (160.6 and 391.7 mg/kg w) and (665.6 and 943.1 mg/kg w) with a 2.07 and 3.35 slope for compounds (3) and (1), respectively.
The obtained results showed a considerable toxicity of our synthetically applied compounds against R. norvegicus; it obviously shows that (1) and (3) are toxic to rats in general. Triazoles are a type of fungicide that has recently been evaluated in investigations as a potential rodenticide. Conazoles are a category of azole-based fungicides utilized in pharmaceutical and agricultural applications. Some members of this class have been reported to be hepatotoxic and to cause thyroid follicular cell tumors in Wister rats as well as mice hepatocellular cancers (Allen et al. 2006). Recent studies applying synthetic chemicals or pesticides with triazole nuclei (mefentrifluconazole) on rats revealed LD50 values of 2000 mg/kg body weight orally and > 5000 mg/kg body weight by dermal doses; aerosol formulation via inhalation was also used to produce > 5.314 mg/L (Tesh et al. 2019).
Biochemical parameters
Table 2 shows several biological variables (ALT, AST, serum urea, creatinine, and total protein) that were assessed and evaluated as a biological response to compound (1) and, (1) as potential rodenticidal compounds. The study indicated a significant increase in AST, ALT, serum urea, and creatinine concentration when both (1) and (3) were applied compared to the control group in the R. norvegicus rat. A significant decrease in total protein in treated compound groups is well noticed while compared to the control group in rats.
Amino transferases are a series of enzymes that are contained in the cytoplasm of living cells, with the liver possessing the highest levels of ALT and slightly lower amounts in other tissues such as the brain and muscle (Azimi et al. 2022). AST is a transaminase enzyme that is extensively located in the liver, kidneys, and muscles. Its release into the bloodstream results from an attack on these organs; an increase in its release into the bloodstream signifies hepatic toxicity (Nirmal et al. 2021). Analysis revealed a highly significant increase (P = 0.000) in AST levels in treated rats exposed to (1) and, (3); (47.1 ± 0.23, 72.8 ± 0.86 U/ml), respectively, compared to the control (41.1 ± 0.56U/ml); furthermore, ALT enzyme showed a considerable increase (P = 0.000) in its level (43.3 ± 1.27 and 60.9 ± 1.8 U/ml) for both prepared (1) and (3), respectively, when compared to the control treatment (30.6 ± 0.53U/ml). When animals were treated with LD50 values, the AST and ALT, as well as other parameters such as urea and creatinine increasing values, may be interpreted as a reflection of what happens inside the body during breakdown and malfunctioning, and an immediate result of organ damage, particularly liver and kidney damage (Elhamalawy et al. 2022). A clear increase in the concentration of both the ALT and AST enzymes in male albino rats is well considered when liver tissue undergoes histological analysis after exposure to an acetaminophen synthetic compound. There were obvious histological changes and oxidative stress, as well as inflammatory parameters like interleukin-1 beta (IL-1 beta), myeloperoxidase (MPO), and tumor necrosis factor-alpha (TNF-α) (Mohamed Kamel et al. 2022).
The biochemical assay showed a remarkable increase (P = 0.017) in urea levels (28.5 ± 0.34 and 30.1 ± 1.7 mg/dl) for (1) and (3) compounds, respectively, compared to the control treatment (24.4 ± 1.1 mg/dl). Creatinine levels in blood serum (P = 0.047) also had a noticeable rise (0.74 ± 0.06 and 0.88 ± 0.05 mg/dl) for (1) and (3), respectively, when compared to control (0.68 ± 0.05 mg/dl). Compounds (1) and (3) in the current investigation cause a considerable rise in serum urea and creatinine levels, which is a result of the body's inability to excrete the metabolic end products of proteins. The byproduct of creatine breakdown; creatinine, is largely filtered out by the kidneys. Creatinine levels rising in the bloodstream are a reliable sign of nephrotoxicity (Wyss and Kaddurah-Daouk 2000). Total protein levels showed a considerable reduction (P = 0.029) in their amounts (5.9 ± 0.59 and 4.8 ± 0.42 g/dl) for compounds (1) and (3), respectively, when compared to the control (7.44 ± 0.75 g/dl). In general, increasing ALT and AST values were accompanied by a decrease in total protein levels (Helmy et al. 2022). The overall animal metabolism is obviously affected by the toxin’s impact; the ALP decrease is well related to the protein synthesis stopping process and is automatically accompanied by a decrease in total protein amount (Anand et al. 2012) (Aryaeian et al. 2021); change in the endoplasmic reticulum that develops the cell membrane is a reasonable factor affecting metabolism through receiving toxins (Zhang et al. 2020a, b) (Zhang et al. 2020a, b).
Histopathology
Liver
Examination of histological sections of the liver of control R. norvegicus showed that the classic hepatic lobule was composed of a central vein and masses of liver cells (hepatocytes) arranged in the form of liver cords radiating from the central vein. The hepatocytes were polygonal or rounded in shape, with central and vesicular nuclei. The liver cords were separated from each other by narrow blood sinusoids lined by endothelial cells and Von Kupffer cells (Fig. 1a). Histological examination of a liver section from animals treated with compound (1) revealed sinusoidal hemorrhage (Fig. 1b). The histological liver evaluation of the animals treated with compound (3) was characterized by structural changes in the form of congestion in the central vein, necrosis in some hepatic regions, and pyknotic nuclei (Fig. 1c).
The most essential organ for the excretion of poisons or any other metabolites is generally agreed to be the liver. It performs a variety of tasks, including transporting and accumulating metabolites, assisting in food digestion, regulating glucose synthesis, and storing glucose. The liver's primary function is to neutralize and remove harmful chemicals from the body (Sun et al. 2021).
Triazole chemicals are used in agricultural control operations, particularly as fungicides. Additionally, these substances have undergone extensive rodenticide evaluation. The hepatotoxic properties of numerous triazole compounds applied to rat control processes have been analyzed in a number of standard toxicity tests (Ku et al. 2021) (Jalal et al. 2020).
Similar investigations were carried out against rodents with high dosages of cyproconazole and epoxiconazole, which definitely increased liver size in addition to causing tumors to appear in sizable portions of hepatocytes (SA 2010); additionally, for cyproconazole, histopathological findings have already been presented (Heise et al. 2015). Treatment-related effects in the liver were mostly limited to the highest dose level (NOAELx10), with cyproconazole displaying the most severe effects. These effects included vacuolization and hypertrophy of hepatocytes. Hepatocyte hypertrophy was noticed in 80–100% of the animals given the highest doses of cyproconazole, whereas vacuolization was detected in 100% of the rats given cyproconazole.
Kidney
Hematoxylin- and eosin-stained sections showed the normal histological structure of the kidney (renal cortex). The cortex showed Malpighian renal corpuscles, proximal convoluted tubules, and distal convoluted tubules. Malpighian renal corpuscles consisting of a normal glomerulus with a thin glomerular basement membrane and Bowman’s capsules with normal cellularity and patent capsular space; surrounding tubules (proximal and distal); interstitium; and blood vessels were normal (Fig. 2a). The histological changes in the kidney after treatment with compound (1) showed atrophy in some glomeruli capsules and rupture in Bowman’s capsules (Fig. 2b). Histological examination of the kidney section after treatment with compound (3) showed vacuolar degeneration of the epithelial cells of some convoluted tubules. Disappearance of some glomeruli and others was markedly atrophied. Necrosis in some areas was noticed (Fig. 2c). When toxic substances are utilized, the kidney is one of the organs that gets attention. The kidney's main tasks include detoxifying metabolic waste products and external substances, including body pigments (Effendy et al. 2006). Pathological alterations and disruptions, including both glomerular and renal tubular activities, will result from exposure to circulation toxins (Wannang, et al. 2005).
Photomicrographs of sections in kidney (renal cortex) of R. norvegicus. a: Control, showing Malpighian renal corpuscles with normal glomerulus (G) and Bowman’s capsules (BC), normal proximal convoluted tubules (PCTs) with brush border and distal convoluted tubules (DCTs). b: After treated with compound (1), displaying atrophied glomerular capsule and rupture of BC (arrow). c: After treated with compound (3), showing atrophied glomerular capsule, vacuolar degeneration (D) of some convoluted tubules and disappearance of some glomeruli (G) and necrosis (N) in some regions. (H & E X400)
At low doses, tebuconazole (TEB), a triazole moiety fungicide, caused an alteration in the biochemical parameters of renal functions, a lesion throughout the renal tissues, and an induced renal toxicity in the male Wistar rat (Othmène et al. 2020).
Field application and population diminution
Data in Table 3 demonstrate the relative effectiveness of our synthons field assessment through bait consumption technique against the Norwegian rat, Rattus norvegicus, under natural field conditions. For (1) and (3) compounds, respectively, the average consumption of untreated crushed maize during the pre-treatment was 210 and 225 g, while it was 80 and 70 g during the post-treatment. When compared to the recommended rodenticide zinc phosphide treatment, there is a significant difference in the consumption of untreated crushed maize (post-treatment). Compound (3) bait is highly effective at reducing the rat population in natural field conditions, according to the 68.4% population reduction it achieved, followed by compound (1), and finally an 81% reduction by the recommended rodenticide zinc phosphide. For compounds (1) and (3), the average amount of treated bait that was consumed was 191 and 198 g, respectively (Fig. 3). The results from the field and the laboratory were in agreement. Previous data showed an acceptable population diminution under field conditions.
The issue of bait shyness, in addition to the toxicity of zinc phosphide, was one of the guiding and persuasive reasons for carrying out this investigation. Many researchers have dealt with, in not a few studies, the disadvantages of using zinc phosphide in general and its use in rodent control in particular. Studies have been conducted on the toxicity of zinc phosphide on human (Ghasempouri et al. 2022), and others have been conducted to assess its impact on wildlife (Bildfell et al. 2013), as well as investigations that talk about the harm of this substance to the environment in acidic media (Knight 2013). This is in addition to the phenomenon of bait shyness when using zinc phosphide, which would limit its use (Horak et al. 2018).
Bait consumption is regarded as a valuable and effective rodent management technique, as well as a reliable indicator for assessing reduction ratios, particularly in field conditions when different environmental parameters (predators and food competitors) are taken into account (Patergnani et al. 2010). In order to achieve the quality of the bait that achieves the highest percentage of population reduction and also within the control operations in different environmental conditions, the relationship between baits (color, additives, and the percentage of moisture in the total content) and the behavior of four species of rodents, including R. norvegicus, toward these different variables has been thoroughly studied (Clapperton 2006). Rattus sordidus canefield rats were treated using zinc phosphide bait, which resulted in population declines of 80% and 86%, respectively, for two treatments, while there was no reduction in the control treatment. The results demonstrate that zinc phosphide is effective at suppressing canefield rat populations (Rivera et al. 2008). Through the bait consumption technique, the experiment was done in a clothing store, and the reduction rate was evaluated. Acetylsalicylic acid (0.04%) reduces the rat population by 72% (Kandil et al. 2022).
Conclusion
The present investigation describes the synthesis of cyanoacetamide (1) and triazolylpyridine (3) derivatives derived from the triazole nucleus compounds. Different analysis techniques confirmed the successful synthesis of cyanoacetamide (1) and triazolylpyridine (3) derivatives. Both compounds (1) and (3) were applied as newly synthesized potential rodenticides against R. norvegicus. The LD50 values were 391.7 and 160.6 mg/kg body weight for (1) and (3), respectively. Both (1) and (3) instigate a considerable increase in ALT, AST, serum urea, and creatinine enzymes but a significant decrease in TP content activity in treated rats compared to the control group. Histopathological assessment of the liver showed structural changes in the form of congestion in the central vein, necrosis in some hepatic regions, and pyknotic nuclei, while kidney examination showed vacuolar degeneration of the epithelial cells of some convoluted tubules and the disappearance of some glomeruli and other marked atrophies. Necrosis in some areas was noticed. Field application through bait consumption took place with an adequate reduction of 68.4% for compound (3), while it was 61.9% for compound (1) when compared to the recommended Zn phosphide commercial rodenticide that poses an 81% reduction; these compounds can be employed in rodent control operations due to their impact on biochemical parameters, histopathological effects, and population reduction rates, according to our results obtained. Among the main reasons for the restrictive use of Zn phosphide, as well as the search for new synthetic acceptable alternatives in rodent control operations, are its high toxicity, the phenomenon of bait shyness, and the fact that Zn phosphide can reach wildlife and the environment.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Allen JW, Wolf DC, George MH, Hester SD, Sun G, Thai S-F, Delker DA, Moore T, Jones C, Nelson G (2006) Toxicity profiles in mice treated with hepatotumorigenic and non-hepatotumorigenic triazole conazole fungicides: propiconazole, triadimefon, and myclobutanil. Toxicol Pathol 34(7):853–862
Al-Mousawi S, Moustafa MS, Elnagdi MH (2008) Studies with enamines: functionally substituted enamines as aldehyde equivalents in Gewald reaction. ARKIVOC 10:17–25
Alnajjar A, Abdelkhalik MM, Riad HM, Sayed SM, Sadek KU (2018) Regioselectivity in the reaction of 2-aminobenzothiazoles and 2-aminobenzimidazoles with enaminonitriles and enaminones: synthesis of functionally substituted pyrimido [2, 1-b][1, 3] benzothiazole and pyrimido [1, 2-a] benzimidazole derivatives. J Heterocycl Chem 55(12):2760–2765
Anand R, Kumari P, Kaushal A, Bal A, Wani WY, Sunkaria A, Dua R, Singh S, Bhalla A, Gill KD (2012) Effect of acute aluminum phosphide exposure on rats—A biochemical and histological correlation. Toxicol Lett 215(1):62–69
Aryaeian N, Amiri F, Rahideh ST, Abolghasemi J, Jazayeri S, Gholamrezayi A, Motevalian M, Solaymani-Dodaran M, Taghizadeh M, Heshmati E (2021) The effect of Cornus mas extract consumption on bone biomarkers and inflammation in postmenopausal women: a randomized clinical trial. Phytother Res 35(8):4425–4432
Azimi M, Mehrzad J, Ahmadi E, Orafei M, Aghaie F, Ahmadi A, Rahimi M, Ghorbani Ranjbary A (2022) The effect of thymus vulgaris on hepatic enzymes activity and apoptosis-related gene expression in streptozotocin-induced diabetic rats. Evid-Based Comp Altern Med 2022:1–11
Bayazeed AA, Alnoman RB (2022) Synthesis of polyheterocyclic ring systems included triazolo [1, 5-a] pyrimidine as antioxidant agents. Polycyclic Aromat Compd 42(3):735–748
Belmain SR, Htwe NM, Kamal NQ, Singleton GR (2015) Estimating rodent losses to stored rice as a means to assess efficacy of rodent management. Wildl Res 42(2):132–142
Bildfell RJ, Rumbeiha WK, Schuler KL, Meteyer CU, Wolff PL, Gillin CM (2013) A review of episodes of zinc phosphide toxicosis in wild geese (Branta spp.) in Oregon (2004–2011). J Vet Diagn Invest 25(1):162–167
Bondock S, Fadaly W, Metwally MA (2009) Enaminonitrile in heterocyclic synthesis: synthesis and antimicrobial evaluation of some new pyrazole, isoxazole and pyrimidine derivatives incorporating a benzothiazole moiety. Eur J Med Chem 44(12):4813–4818
Bondock S, El-Gaber Tarhoni A, Fadda AA (2011) Recent progress in the synthesis and applications of heterocycles derived from enaminonitriles. Curr Org Chem 15(5):753
Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E (2004) Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 96(13):1015–1022
Clapperton, B. K. (2006). A review of the current knowledge of rodent behaviour in relation to control devices, Science & Technical Pub., Department of Conservation Wellington, New Zealand.
Creasy DM, Panchal ST, Garg R, Samanta P (2021) Deep learning-based spermatogenic staging assessment for hematoxylin and eosin-stained sections of rat testes. Toxicol Pathol 49(4):872–887
Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, Gillingwater TH, Wishart TM (2013) Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE 8(8):e72457
Effendy A, Siti-Nurtahirah J, Hussin Z, Zamri-Saad M (2006) The side effects of Kacip Fatimah extract on liver and kidney of white rats. J Sustain Sci Manag 1(1):40–46
Elhamalawy OH, Al-Anany FS, El Makawy A (2022) Thiamethoxam-induced hematological, biochemical, and genetic alterations and the ameliorated effect of Moringa oleifera in male mice. Toxicol Rep 9:94–101
Fadda AA, Etman HA, El-Seidy MY, Elattar KM (2012) Utility of enaminonitriles in heterocyclic synthesis: synthesis of some new pyrazole, pyridine, and pyrimidine derivatives. J Heterocycl Chem 49(4):774–781
Fadda AA, El-Mekabaty A, Elattar KM (2013) Chemistry of enaminonitriles of pyrano [2, 3-c] pyrazole and related compounds. Synth Commun 43(20):2685–2719
Fadda AA, Abd El Salam M, Tag Y, Selim YA (2021) Role of enaminonitriles in heterocyclic synthesis: synthesis of some new aminothiazole derivatives against prostate carcinoma. Polycyclic Aromatic Compd 3(2):1068–1080
Ghasempouri SK, Zakariaei Z, Hoseininejad SM, Chinian F, Soleymanii M, Pashaei SM, Sadeghi M (2022) Clinical manifestations and treatment management of hospitalized patients with zinc phosphide poisoning, Mazandaran Province, Northern Iran. BMC Emerg Med 22(1):104
Heise T, Schmidt F, Knebel C, Rieke S, Haider W, Pfeil R, Kneuer C, Niemann L, Marx-Stoelting P (2015) Hepatotoxic effects of (tri) azole fungicides in a broad dose range. Arch Toxicol 89(11):2105–2117
Helmy ET, Ali MA, Ayyad MA, Mohamedbakr H, Varma RS, Pan JH (2022) Molluscicidal and biochemical effects of green-synthesized F-doped ZnO nanoparticles against land snail Monacha cartusiana under laboratory and field conditions. Environ Pollut 308:119691
Herbreteau V, Bordes F, Jittapalapong S, Supputamongkol Y, Morand S (2012) Rodent-borne diseases in Thailand: targeting rodent carriers and risky habitats. Inf Ecol Epidemiol 2(1):18637
Hiasa Y, Ohshima M, Kitahori Y, Yuasa T, Fujita T, Iwata C (1982) Promoting effects of 3-amino-1, 2, 4-triazole on the development of thyroid tumors in rats treated with N-bis (2-hydroxypropyl) nitrosamine. Carcinogenesis 3(4):381–384
Hinds LA, Henry S, Van de Weyer N, Robinson F, Ruscoe WA, Brown PR (2023) Acute oral toxicity of zinc phosphide: an assessment for wild house mice (Mus musculus). Integrative Zoology 18(1):63–75
Horak K, Hofmann N, Kimball B (2018) Assessment of zinc phosphide bait shyness and tools for reducing flavor aversions. Crop Prot 112:214–219
Huang X-J, Choi Y-K, Im H-S, Yarimaga O, Yoon E, Kim H-S (2006) Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors 6(7):756–782
Ibrahim HM, Behbehani H, Makhseed S, Elnagdi MH (2011) Acylation of heteroaromatic amines: facile and efficient synthesis of a new class of 1, 2, 3-triazolo [4, 5-b] pyridine and pyrazolo [4, 3-b] pyridine derivatives. Molecules 16(5):3723–3739
Imasawa T, Claverol S, Lacombe D, Amoedo ND, Rossignol R (2021) Proteomic study of low-birth-weight nephropathy in rats. Int J Mol Sci 22(19):10294
Jalal M, Nchioua Z, Chouham S, Ez-Zaher L (2020) Triazole fungicides induce hepatic lesions and metabolic disorders in rats. GSC Biol Pharm Sci 10(2):040–047
Kandil RA, Elgohary FM, Mobark SA (2022) Can acetylsalicylic acid be used As a rodenticide? Egypt Acad J Biol Sci F Toxicol Pest Control 14(1):35–46
Knight MW (2013) Zinc phosphide. Elsevier, Small animal toxicology, pp 853–864
Ku T, Zhou M, Hou Y, Xie Y, Li G, Sang N (2021) Tebuconazole induces liver injury coupled with ROS-mediated hepatic metabolism disorder. Ecotoxicol Environ Safety 220:112309
Kumari M, Tahlan S, Narasimhan B, Ramasamy K, Lim SM, Shah SAA, Mani V, Kakkar S (2021) Synthesis and biological evaluation of heterocyclic 1, 2, 4-triazole scaffolds as promising pharmacological agents. BMC Chem 15(1):1–16
Li Y, Zhang J, Peng B, Li S, Gao H, Zhou W (2013) Determination of triazole pesticides in rat blood by the combination of ultrasound-enhanced temperature-controlled ionic liquid dispersive liquid–liquid microextraction coupled to high-performance liquid chromatography. Anal Methods 5(9):2241–2248
Madkour H, Afify A, Elsayed G, Salem M (2008) Synthetic utility of enaminonitrile moiety in heterocyclic synthesis. Bulg Chem Comm 40:147–159
Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, Wood CR, Corton JC, Kavlock RJ, Dix DJ (2007) Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 97(2):595–613
Mohamed Kamel GA, Harahsheh E, Hussein S (2022) Diacerein ameliorates acetaminophen hepatotoxicity in rats via inhibiting HMGB1/TLR4/NF-κB and upregulating PPAR-γ signal. Molecular Biol Rep 49(7):5863–5874
Mohamed MS, Mahmoud AM (2019) Believes versus evidence-based regio-orientation in the structure assignment of pyrazolo [1, 5-a] pyrimidines. J Appl Pharm Sci 9(11):126–144
Mohamed RE, s., M. M. El-Sayed, M. M. Arief and A. A. Mahmoud, (2022) Evaluation of the ameliorative effect of Cinnamon cassia against metabolic disorder and thyroid hormonal disruption following treatment with difenoconazole fungicide in the male albino rats. Egypt J Chem 65(1):55–67
Naidu R, Biswas B, Willett IR, Cribb J, Singh BK, Nathanail CP, Coulon F, Semple KT, Jones KC, Barclay A (2021) Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ Int 156:106616
Neena S, Babbar B (2010) Rodent damage and infestation in wheat and rice crop fields: district wise analysis in Punjab State. Indian J Ecol 37(2):184–188
Nirmal NK, Awasthi KK, John PJ (2021) Hepatotoxicity of graphene oxide in Wistar rats. Environ Sci Pollut Res 28(34):46367–46376
Ognakossan, K. E. (2017). Assessment of rodents’ postharvest losses in on-farm maize storage in Kenya, Jomo Kenyatta university of Agriculture and Technology.
Othmène YB, Hamdi H, Salem IB, Annabi E, Amara I, Neffati F, Najjar MF, Abid-Essefi S (2020) Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem Biol Interact 330:109114
Patergnani M, Mughini Gras L, Poglayen G, Gelli A, Pasqualucci F, Farina M, Stancampiano L (2010) Environmental influence on urban rodent bait consumption. J Pest Sci 83(3):347–359
Rani A, Singh A, Kaur J, Singh G, Bhatti R, Gumede N, Kisten P, Singh P, Kumar V (2021) 1H-1, 2, 3-triazole grafted tacrine-chalcone conjugates as potential cholinesterase inhibitors with the evaluation of their behavioral tests and oxidative stress in mice brain cells. Bioorg Chem 114:105053
Rennison BD (1977) Methods of testing rodenticides in the field against rats. Pestic Sci 8(4):405–413
Rivera DF, Smith M, Staples L, Leung LK-P (2008) Effect of zinc phosphide baiting on canefield rat populations in teak. Crop Prot 27(3–5):877–881
SA, E. (2010) Conclusion on the peer review of the pesticide risk assessment of the active substance cyproconazole. EFSA 8(11):1897
Singla N, Babbar BK (2012) Critical timings of rodenticide bait application for controlling rodents in sugarcane crop grown in situations like Punjab, India. Sugar Tech 14(1):76–82
Singla LD, Singla N, Parshad VR, Juyal PD, Sood NK (2008) Rodents as reservoirs of parasites in India. Integrative Zool 3(1):21–26
St L, Wold S (1989) Analysis of variance (ANOVA). Chem Intell Lab Syst 6(4):259–272
Sun M, Zhang J, Liang S, Du Z, Liu J, Sun Z, Duan J (2021) Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol Environ Safety 208:111496
Tenor E, Kröger CF (1964) Über 1.2. 4-Triazole, VII. Synthese und Reaktivität von 7-Amino-s-triazolo [1.5-a] pyrimidonen-(5). Chem Ber 97(5):1373–1382
Tesh SA, Tesh JM, Fegert I, Buesen R, Schneider S, Mentzel T, van Ravenzwaay B, Stinchcombe S (2019) Innovative selection approach for a new antifungal agent mefentrifluconazole (Revysol®) and the impact upon its toxicity profile. Regulatory Toxicol Pharmacol 106:152–168
Wang K, Wu S, Zhao J, Zhou M, Li G, Wang D, Lin L (2022) Quantitative analysis of urea in serum by synchronous modulation and demodulation fluorescence spectroscopy. Spectrochimica Acta Part a: Mol Biomol Spectrosc 268:120645
Wannang, N., J. Anuka, L. Bichi and L. Dapar (2005). "Aqueous root extract of Sercuridaca longepedunculata linn alters haematological indices in rats." Journal of Phytomedicine Therapeutics 10.
Wyss, M. and R. Kaddurah-Daouk (2000). "Creatine and creatinine metabolism." Physiological reviews
Zhang C, Wang L-L, Cao C-Y, Li N, Talukder M, Li J-L (2020a) Selenium mitigates cadmium-induced crosstalk between autophagy and endoplasmic reticulum stress via regulating calcium homeostasis in avian leghorn male hepatoma (LMH) cells. Environ Pollut 265:114613
Zhang H, Vidonish J, Lv W, Wang X, Alvarez P (2020b) Differential histological, cellular and organism-wide response of earthworms exposed to multi-layer graphenes with different morphologies and hydrophobicity. Environ Pollut 263:114468
Zhao F, Liu Y, Qin Z, Wu Y, Xiao Y, Li JQ (2022) Synthesis and insecticidal activity of novel 1, 2, 4-triazole containing amidine moiety. J Hetero Chem 59(10):1723–1735
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MAA, ETH, UAS, and MAA: Investigation, Data Collection and Analysis, Methodology, Writing–Original Draft, Conceptualization, Supervision, Review, and Editing. The manuscript was written with the contributions of all authors. All authors have given their approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayyad, M.A., Ali, M.A., Helmy, E.T. et al. Novel triazole derivatives as potential rodenticides against the Norway rat, R. norvegicus: histology, biochemical alternations, and field application. Chem. Pap. 77, 5947–5959 (2023). https://doi.org/10.1007/s11696-023-02912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02912-2