Abstract
In this manuscript, efficiency of the used organic solvent for recovering, separating and producing highly purified zirconium oxide from zirconium chloride leach liquor of Rosetta white zircon concentrate was investigated. Zircon mineral was thermally decomposed with caustic soda, cooled, washed with distilled water and then dissolved in hydrochloric acid. Zirconium extraction efficiency reached 91.4% using 10% bis-(2-ethylhexyl) phosphate dissolved in kerosene, 15 min contact time and volume phase ratio O/A 1:1 after 3 stages. Zirconium re-extraction efficiency reached 99.95% using the following optimum conditions 4 molL−1 H2SO4 as an effective stripping agent, 15 min contact time and volume phase ratio O:A 1:1 after 2 stages. Finally, Zr (OH)4 was precipitated in presence and absence of H2O2 by NH4OH solution, filtrated, rinsed with double distilled H2O to remove any impurities several times and dehydrated at 200 °C for 2 h. From XRF and EDAX analysis the produced zirconium oxide in presence of H2O2 is purer than that produced in absence of H2O2.
Similar content being viewed by others
Introduction
Zirconia has excellent mechanistic, thermic, electrified, chemical and visual properties which made it attracted a great concern as a high-technological matter for processing use such as enamels, glazes, pigments in ceramic manufacture, refractory materials, foundry mold, piezoelectric crystals and catalyst in organic reactions (Mohamed and Daher 2002; Rajmane et al. 2006). Because of its superior rustiness resistance it is used as a structural material in nuclear industry and also used as a covering material for fuel rods in nuclear reactors due to its low neutron intake cross section. Also, zirconium can be used for synthesis of inorganic ion exchangers such as zirconium phosphate and zirconium silico-tungstate which were used for uranium sorption from nuclear waste solutions, (Ali 2018; Eliwa and Mubarak 2021). Due to its wide importance and applications in various fields, prompted the scientists for searching on different chemical methods for zirconium extraction from its resources.
Zirconium metal is naturally occurring in two minerals, zircon (ZrSiO4) is the most abundant source of zirconia and baddeleyite (ZrO2) which is the less abundance mineral (Elvers et al. 1989; Suriyachat 1992). Zircon mineral is vastly spread in ground as an ingredient of igneous, metamorphic, sedimentary rocks and heavy residual of rocks or beach sand which is a blend of heavy minerals. Zircon should be isolated from that blend using wet physical upgrading followed by magnetic and electrostatic separation (Abdel-Rahim 2005). Zircon mineral is chemically very stable. For extracting ZrO2 from zircon the bonds between ZrO2 and SiO2 should be cracked chemically or thermally. Several techniques were applied to separate ZrO2 from its sources such as, alkaline dissociation (MacDonald et al. 1982; Krishnan et al. 1986), treatment by chlorine (Manich et al. 1974), smelting in presence of reducing agents (Ballard and Marshall 1950), production of calcium zirconate and zirconium oxide or its salts by reacting zircon with a sufficient quantity of lime stone to produce calcium zirconate and tricalcium silicate (Schoenlaub 1955, 1974), hydrothermal treatments for zircon (Wikes 1972). Highly purified ZrO2 preparation was carried out by zircon thermal decomposition followed by wet chemical processing (Staumbaugh and Millier 1983), spray pyrolysis of zirconium-containing solutions (Zhang and Messing 1990), zirconium oxychloride separation by crystallization (McBerty 1946), and zirconium purification using a basic sulfate precipitation (Nielson and Govro 1956) and hydrated sulfate (Clabaugh and Gilchrist 1952). (Rolf 1961), used liquid–liquid extraction technique to produce zirconium free from impurities and hafnium.
Many solvent extraction investigations were carried out for extracting zirconium from different solutions as, organophosphorus compounds includes TBP (Aliakbari et al. 2014), TOPO (Banda et al. 2013), TRPO (trialkylphosphine oxide) (Xu et al. 2012), Cyanex301 and 302 (Saberyan et al. 2008, 2010), Cyanex272 (Taghizadeh et al. 2009), Cyanex923 (Taghizadeh et al. 2011), Cyanex921 (El Shafie et al. 2014), amines (Lakshmanan et al. 2014), D2EHPA (Taghizadeh et al. 2009; De beer et al. 2016) used Ionquest 801 to separate hafnium from zirconium these investigations showed a good results for extracting zirconium.
The current work deals with evaluating bis-(2-ethylhexyl) phosphate as a novel and a promising extractant for producing highly purified zirconium oxide (free from hafnium and any impurities) from prepared zirconium chloride leach liquor after alkaline fusion of Egyptian Rosetta white zircon. The different parameters controlling the separation process as volume phase ratio (O:A), solvent concentration, shaking time and diluents type were investigated. The feasibility of using bis-(2-ethylhexyl) phosphate for zirconium separation was evaluated by stripping studies. The other factors affecting on zirconium re-extraction from loaded organic like; stripping agent type, stripping agent concentration and contact time were studied to determine the optimal conditions for extraction, separation and purification of zirconium. In addition, construction of McCabe–Thiele diagrams from the experimental data of zirconium extraction and stripping respectively to determine the required stages of extraction and stripping in the countercurrent scheme. Finally, an experimental separation process flow chart for the whole process has proposed.
Experimental
All chemicals used as received without further purification like, xylenol orange (XO), octa-hydrated zirconium oxychloride (ZrOCl2·8H2O, 98%), HCl acid, NaOH and HNO3 acid, H2SO4 acid and bis-(2-ethylhexyl) phosphate were purchased from Merck. A typical analysis of Egyptian Rosetta white zircon mineral and the dried fused cake after water washing is given below in Table 1 and Fig. 1.
Preparation of zirconium chloride solution
In a stainless steel crucible; 250 g of white zircon was thermally decomposed with 312 g of sodium hydroxide (NaOH) pellets in an electric furnace at 650 °C, the produced smelt consists of water insoluble sodium zirconate (Na2ZrO3) and water soluble sodium silicate (Na2SiO3) as shown by equation:
The fused product cooled, and then mixed with excess distilled water at 60 °C with stirring for 2 h to dissolve sodium silicate in this step, Na2SiO3 is dissolved to a large extent and a small portion of Na2ZrO3 is converted to hydrated zirconia:
Filtration on hot, and then dissolve the solid residual precipitate (Na2ZrO3) in HCl at 90 °C, agitation for 2 h then directly filtered giving zirconium chloride solution and the solid residual, which at most consists of unreacted zircon, TiO2, Al2O3, ThO2 and H2SiO3 as given in the following equation:
Extraction of zirconium using bis-(2-ethylhexyl) phosphate
In 50 mL beaker 10 mL of prepared zirconium chloride solution (12.01 gL−1) was mixed with different volumes of diluted bis-(2-ethylhexyl) phosphate and vigorous agitation was achieved by magnetic stirrer at room temperature for set periods of time the extraction experiments were performed twice and sometimes triple. A separating funnel was used to separate the two phases after 5 min settling time and the aqueous samples were analyzed. Variables impacting on the extraction process were studied in details. Zirconium concentration in organic phase is calculated by subtracting its concentrations in the aqueous phases before and after extraction. The extraction efficiency (capacity) (%E) was calculated by Eq. (1). Distribution coefficient (DE) of metal ion (M = Zr+4) was calculated as represented in Eq. (2).
[M]in is zirconium initial concentration in aqueous phase; [M]aq. and [M]org. refer to zirconium ions concentrations in aqueous and organic phases at equilibrium respectively.
Zirconium stripping from loaded bis-(2-ethylhexyl) phosphate
Batch stripping experiments were performed twice and sometimes triplicate on loaded organic solution bis-(2-ethylhexyl) phosphate, in 50 mL beaker a mixture of 20 mL (10.974 gL−1) and certain volumes of stripping agent was shaked vigorously using magnetic stirrer and at room temperature for certain contact times. A separatory funnel was used to separate the mixture after 5 min settling time. Test portion of the aqueous samples were removed by pipet for analysis. The controlling factors of the re-extraction process were investigated in details as, type of stripping agent, stripping agent concentration and contact time. The stripping efficiency (%S) calculated using Eq. (3):
Determination of zirconium
Xylenol orange (XO) method was used to analyze zirconium concentration in the aqueous phases versus a series of standard Zr (IV) solutions by placing the sample solution its concentration not exceed than 30 µg of Zr in a 25 mL volumetric flask, add 1 mL of 1% ascorbic acid solution, and 1 mL of XO (0.05%) solution, complete the volume with 0.6 molL−1 HC1 to the mark, and mix well. After 10 min measure the absorbance at 532 nm using a Lambda 3 UV/VIS spectrophotometer (PerkinElmer, USA), using a reagent blank solution as reference (Marczenko 1976).
Characterization of ZrO2 product
The produced zirconia ZrO2 was analyzed by EDAX model Philips XL 30 ESEM (25–30 k eV accelerating voltage, 1–2 mm beam diameter and 60–120 s counting time, BSE detector) and X-ray fluorescence technique (XRF Model geol-gsx3222), was applied for the quantitative determination of the trace elements to determine its purity.
Results and discussion
Diluents type effect
Five diluents (Benzene, Toluene, xylene, 2-octanol and kerosene) were used to study its effect on the extraction efficiency and distribution coefficient; while the other variables were kept constant like 10% bis-(2-ethylhexyl) phosphate, volume phase ratio O/A 1:1 and 15 min contact time. Figure 2 showed those zirconium extraction capacity and distribution coefficient were affected by diluents type. Maximum extraction capacity and distribution coefficient (E% = 91.40%, D = 10.63) were reached when kerosene used. Benzene being the closer in efficiency (83.1%, D = 4.91).
Effect of organic solvent concentration
Experiments were carried out to study the effect of organic solvent concentration on zirconium extraction efficiency and distribution coefficient using bis-(2-ethylhexyl) phosphate as organic solvent its concentration was changed from (2.5–15%) while other conditions were fixed as; volume phase ratio O:A is 1:1, kerosene and contact time 15 min. The obtained results illustrated on Fig. 3 showed that; the maximal zirconium extraction efficiency and distribution coefficient (E% increased from 38.71 to 91.4% and D increased from 0.63 to 10.63) were achieved at 10%. By increasing concentration over 10% there is no noticeable change in either extraction efficiency or distribution coefficient, so that the optimal solvent concentration is 10%.
Contact time effect
The influence of contact time on zirconium extraction efficiency and distribution coefficient from chloride acid medium was studied in the interval 1–30 min, keeping the other extraction conditions constant as 10% bis-(2-ethylhexyl) phosphate dissolved in kerosene and volume phase ration (O:A) 1:1. Figure 4 showed that; extraction efficiency reaches 91.4% (E% increased from 7.64 to 91.4%), and distribution coefficient reached 10.63 (D increased from 0.083 to 10.63) in 15 min and did not considerably changed at longer contact times. Finally, 15 min was taken as the optimal time for extracting zirconium from chloride media.
Effect of volume phase ratio organic solvent (O): aqueous solution (A)
The influence of volume phase ratio (O/A) on extraction capacity and distribution coefficient was evaluated by varying volume phase ratio O:A (from 4:1 to 1:4) using 10% bis-(2-ethylhexyl) phosphate dissolved in kerosene at contact time 15 min. Figure 5 showed that (E% slightly increased from 91.4 to 95.07%, D increased from 10.63 to 19.3) by increasing O:A ratio from 1:1 to 4:1 on the other hand, (E% highly reduced from 91.4 to 5%, D reduced from 10.63 to 0.058) with increasing aqueous volume from 1:1 to 1:4, so that the optimum volume phase ratio for zirconium extraction from its acidic medium by bis-(2-ethylhexyl) phosphate was 1:1 (O: A).
Distribution isotherm equilibrium curve
The diagram illustrating the relationship between zirconium concentrations in organic layer versus its concentrations in aqueous layer after extraction (raffinate) at a given temperature is called distribution isotherm. Distribution isotherm can be plotted for either extraction process (extraction isotherm) or stripping process (stripping isotherm).
Two methods were used for obtaining extraction isotherm data: (i)"phase ratio variation" i.e. a fixed volume of aqueous feed (input leach liquor) is contacted with different volumes of organic solvent for only one time, (ii)- or by "saturation process" i.e. the same a liquot of organic solvent is contacted several times with several a liquots of fresh input leach liquor. Composite plot of the distribution isotherm and the operating line is called Mc-Cabe Thiele diagram. The operating line could be established by only one point, which corresponds to the final raffinate composition and the ratios of the aqueous to organic phases that determines the slope of the line, as it is a straight line. The diagram can be used to evaluate the extraction results and to approximate the number of theoretical stages (trays) required to achieve a desired degree of separation process. In this study, organic solvent (10% bis-(2-ethylhexyl) phosphate dissolved in kerosene) were contacted with fresh aqueous solution [i.e. saturation method keeping volume phase ratio (O/A) constant], at the optimum conditions previously determined; contact time 15 min and at room temperature, the mixture was separated by separating funnel and the aqueous phase was analyzed. The equilibrium line is then established by plotting zirconium concentration in organic phase against its concentration in aqueous phase as represented on Fig. 6 (Robbins, 1981).The next step in the construction of Mc-Cabe Thiele diagram was to try a number of operating lines at slope equal to the ratio of aqueous / organic volumes. At each operating line, the theoretical number of stages could be stepped off upon the diagram as in Fig. 6. It is clear that, three theoretical stages are quite suitable for zirconium extraction using bis-(2-ethylhexyl) phosphate. Mc-Cabe Thiele diagram was constructed to define the required stages of extraction in the countercurrent scheme.
Zirconium re-extraction from loaded bis-(2-ethylhexyl) phosphate
Batch experiments were carried out to study the factors affecting on zirconium stripping efficiency from loaded bis-(2-ethylhexyl) phosphate. Different stripping agents were contacted with the loaded organic solvent (10.974 gL−1 of Zr+4); with extraction efficiency 91.4%. The re-extraction efficiency calculated relative to the initial amount of Zr+4 loaded onto organic solvent. The pertinent factors controlling zirconium stripping were studied, which include:
Effect of stripping agent type
Different aqueous solutions of 1molL−1 (HCl, HNO3 and H2SO4 and double distilled water) were tested, while other factors kept fixed as volume phase ratio A: O 1:1 and contact time 15 min. Figure 7 represents the results, it is clear that, the maximal stripping percentage (S% was 31.4%) was reached with H2SO4, HCl being the next in performance (27%).
Effect of H2SO4concentration on zirconium stripping efficiency from loaded organic
The influence of stripping agent (H2SO4) concentration on zirconium stripping from loaded organic was studied using different concentrations varied from (0.5–5 molL−1), while other factors kept fixed, volume phase ratio 1:1, at room temperature and contact time 15 min. The obtained results are represented in Fig. 8; the results showed that the maximum stripping efficiency (%S 99.95%) was reached at 4 molL−1H2SO4 concentration after that it remains constant, so 4 molL−1 was chosen as the optimum concentration.
Effect of contact time
The impact of contact time on zirconium re-extraction from loaded organic was studied by varying time from 5 to 30 min while other factors were kept fixed as, volume phase ratio A:O 1:1 and 4 molL−1 H2SO4 stripping agent. By increasing contact time from 5 to 15 min the stripping efficiency (S% increased from 45.9 to 99.95%) increased till reached its maximum value at 15 min, after that it was almost fixed as shown by Fig. 9. It is clear that the best contact time for zirconium stripping from loaded bis-(2-ethylhezyl) phosphate is 15 min.
Distribution isotherm equilibrium curve
A certain volume of aqueous phase (4 molL−1 H2SO4) was contacted for 15 min with various aliquots of fresh loaded organic until equilibrium is reached keeping volume phase ratio fixed (A:O) at 1:1. A measured test aqueous sample was taken for zirconium analysis. This process was carried out until saturation of the aqueous phase with zirconium is obtained. The results represented on Fig. 10, by construction of Mc-Cabe Thiele diagram for zirconium stripping from loaded bis-(2-ethylhexyl) phosphate, it is clear that two stages are sufficient for zirconium stripping from loaded organic.
Precipitation stage
Precipitation of Zirconia
After stripping process, zirconium-loaded solution was put in a beaker for precipitation by adding ammonium hydroxide (in presence and absence of H2O2 to evaluate its ability for separating zirconium from hafnium and reducing impurities in the produced zirconia). Zirconium hydroxide was precipitated at pH 9, filtered and washed several times with double distilled water. And this precipitates were dehydrated in an electric oven at 200 °C for 2 h. This temperature will not affect on zirconia crystallization and conductivity this agreed with (Graham King et al 2018) who investigated the effect of calcination temperature, local structure of Zr(OH)4 and its thermal decomposition into ZrO2. It was found that Heating up to 125 °C results in significant water loss but does not alter the network of Zr and bridging O atoms and the Zr connectivity is unaffected. Additional water loss caused by heating to 250 °C causes a transition to a new amorphous phase with a similar correlation length. The produced zirconium oxides were used for complete characterization. Table 2 shows XRF analysis; Fig. 11 shows EDAX analysis for the purified products.
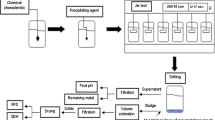
From EDAX and XRF analysis of the produced zirconium oxide it is clear that; the produced zirconium oxide in presence of H2O2 is highly purified than that produced in absence of H2O2. The investigation allows the proposal of a general flow sheet for the production of highly purified zirconium oxide from Egyptian zircon as shown in Fig. 12.
Conclusions
Bis-(2-ethylhexyl) phosphate was used for zirconium extraction from its chloride solution, various factors affecting this process as; solvent concentration, contact time, diluents type used and volume phase ratio (O:A) were studied. It was found that the optimum conditions for extracting zirconium were 10% of bis-(2-ethylhexyl) phosphate dissolved in kerosene and 15 min contact time at volume phase ratio (1:1) which achieved maximum zirconium ions extraction efficiency 91.4%. Additionally 4 molL−1 H2SO4 was chosen to be the best stripping agent for re-extracting zirconium at 15 min from loaded organic with efficiency 99.95%. The produced zirconium oxide in presence of H2O2 is more pure than that produced in absence of H2O2.
Data and materials availability
All the data used for this work are publicly available.
References
Abdel-Rahim AM (2005) A new technique for extracting zirconium from Egyptian zircon concentrate. Int J Miner Process 76:234–243
Ali AH (2018) Potentiality of zirconium phosphate synthesized from zircon mineral for uptaking uranium. Sep Sci Technol 53(14):2284–2296. https://doi.org/10.1080/01496395.2018.1445115
Aliakbari M, Saberyan K, Noaparast M, Abdollahi H, Akcil A (2014) Separation of hafnium and zirconium using TBP modified ferromagnetic nanoparticles: effects of acid and metals concentrations. Hydrometallurgy 146:72–75
Ballard A, Marshall AW (1950) US Patent 2,535,536
Banda R, Lee H, Lee M (2013) Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants. J Radioanal Nucl Chem 298(1):259–264
Clabaugh WS, Gilchrist R (1952) Method for Freeing Zirconium of Common Impurities and for Preparing Zirconium Sulfate and Oxide. J Am Chem Soc 74(8):2104–2105. https://doi.org/10.1021/ja01128a507
De Beer L, van der Westhuizen DJ, Krieg HM (2016) Solvent extraction and separation of hafnium from zirconium using Ionquest 801. J S Afr Inst Min Metall 116:93–99
El Shafie AS, Daher AM, Ahmed IS, Sheta ME, Moustafa MM (2014) Extraction and separation of nano-sized zirconia from nitrate medium using cyanex921. Int J Adv Res 2(11):647–659
Eliwa AA, Mubark AE (2021) Effective sorption of U(VI) from chloride solutions using zirconium silico-tungstate matrix. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.1921762
Elvers B, Hawkins S, Ravenscroft M, Rounsaville JF, Sculz G (1989) Ulmann’s Encyclopedia of industrial chemistry. VCH, Weinhein, Germany
Graham K, Jennifer RS, Wesley OG (2018) Local structure of Zr(OH)4 and the effect of calcination temperature from x-ray pair distribution function analysis. Inorg Chem 57(5):2797–2803. https://doi.org/10.1021/acs.inorgchem.7b03137
Krishnan TS, Babu RS, Gupta CK (1986) Extended abstracts, zirconia 86. Ceram Soc Jpn, 200–201
Lakshmanan VI, Sridhar R, Jankovic Z, Halim MA (2014). Separation and purification of zirconium with amines from sulphuric acid system: a review. International solvent extraction conference
MacDonald DJ, Guidotti RA, Henry HG (1982). Investigation of methods for producing reactor-grade zirconium oxide from a zirconium-bearing chloride-sulfate strip liquor solution. US Bur Mines, Rep Invest, 8718
Manich AA, Scott DS, Spink DR (1974) Electrothermal fluidized bed chlorination of zircon. Can J Chem Eng 52:507
Marczenko Z (1976) Spectrophotometric determination of elements. Ellis Horwood Ltd., Halsted Press, New York, pp 474–482
McBerty FH (1957) FIAT Rev Germ Sc, Final Re. 774, 1946; cited in Miller [13]. Zirconium, Butterworth Scientific Pub, London, p 35
Mohammed NA, Daher AM (2002) Preparation of high-purity zirconia from Egyptian zircon: an anion-exchange purification process. Hydrometallurgy 65(2–3):103–107. https://doi.org/10.1016/s0304-386x(02)00042-7
Nielson RH, Govro RL (1956) Zirconium purification: using a basic sulfate precipitation. US Bur Mines Rep Invest 5214:36
Rajmane MM, Sargar BM, Mahamuni SV, Anuse MA (2006) Solvent extraction separation of zirconium(IV) from succinate media withN-n-octylaniline. J Serb Chem Soc 71(3):223–234
Robbins LA (1981) Liquid–liquid extraction. In: Schwitzer A (ed) Hand book of separation techniques for chemical engineers. McGraw-Hill, New York
Rolf RF (1961) Ann Chem 33:149
Saberyan K, Meysami AH, Rashchi F, Zolfonoun E (2008) Proposal of a new Hf(IV)/Zr(IV) separation system by the solvent extraction method. Chin J Chem 26(11):2067–2072
Saberyan K, Vahedian-Donyaparast P, Noparast M, Zolfonoun E, Nemati A (2010) Solvent extraction of zirconium from zircon leach liquor using triphenylphosphine oxide. Miner Metall Process 27(3):129–132
Schoenlaub RA (1955) US Patent 2,271,115
Schoenlaub RA (1974) Method of manufacturing zirconium oxide and salts. US Patent 3,832,441
Staumbaugh EP, Millier JF (1983) In: proceeding of 1st international symposium on hydrothermal research, p 859
Suriyachat D (1992) zirconium solvent extraction using organophosphorous compounds. Master thesis, McGill University, Montreal, Quebec, Canada
Taghizadeh M, Ghasemzadeh R, Ashrafizadeh SN, Ghannadi M (2009) Stoichiometric relation for extraction of zirconium and hafnium from acidic nitrate solutions with cyanex272. Hydrometallurgy 96(1–2):77–80
Taghizadeh M, Ghanadi M, Zolfonoun E (2011) Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923. J Nucl Mater 412(3):334–337
Wikes PH (1972) chemical Engineering progress. Chem Eng Prog 68:82
Xu C, Wang C, Wang J, Chen J (2012) Third phase formation in the extraction of zirconium(IV) by TRPO in kerosene. Sep Sci Technol 48(1):183–191
Zhang S, Messing GI (1990) Synthesis of solid, spherical zirconia particles by spray pyrolysis. J Am Ceram Soc 73:61. https://doi.org/10.1111/j.1151-2916.1990.tb05091.x
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation, data collection and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that I have no conflict of interest.
Ethical approval
The author confirms that the manuscript has been read and approved. The author declares that this manuscript has not been published and not under consideration for publication elsewhere.
Consent to participate
All of the authors consented to participate in the drafting of this manuscript.
Consent for publication
All of the authors consent to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.H. Production of highly purified zirconium oxide from zircon mineral leach liquor using bis-(2-ethylhexyl) phosphate as a promising extractant. Chem. Pap. 76, 6723–6734 (2022). https://doi.org/10.1007/s11696-022-02339-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02339-1