Abstract
An inexpensive, simple, sensitive and validated approach is developed for estimation of fingolimod through production of colored charge transfer complexes of fingolimod with different electron acceptor reagents, including a reaction of fingolimod as n-donor with 7,7,8,8-tetracyanoquinodimethane, tetrachloro 1,4-benzoquinone and tetracyanoethylene and as n-acceptors, yielding colored and stable anions which were measured spectrophotometrically. The range that obeyed Beer’s law is 50–300 µg mL−1 for fingolimod with all the studied reagents. The various parameters that affect the reaction were studied and optimized. The results were statistically compared with a reported method showing equal precision and accuracy. The researched approaches were utilized to determine the cited drug in its pharmaceutical form and spiked human plasma with accepted accuracy and precision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
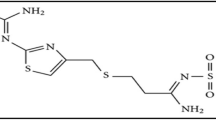
Fingolimod is an immune modulating medication which is utilized to treat multiple sclerosis, that is considered as the most familiar inflammatory anarchy of the central nervous system (Brinkmann et al. 2010, 2002; Chiba 2005; Salm et al. 2006) with nomenclature of 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3- diol (Fig. 1). Fingolimod metabolism is phosphorylated, that can cause a decline in lymphocytes in the periphery, while increasing sphingosine-1-phosphate (SIP) receptors on lymphocytes (Brinkmann et al. 2002). SIP receptors activation is required for the dispersion of 99 of leukocytes to other organs from lymph nodes (Chiba 2005). In the literature survey for the determination of fingolimod, few methods are reported, including LC- (Salm et al. 2006; Zollinger et al. 2011; Ferreiros et al. 2012; Emotte et al. 2012), HPLC (Chhabda et al. 2013; Narasimha et al. 2014; Effat et al. 2015; Somsubhra and Ravikumar 2015; Islam and Emad 2016; Ravi et al. 2016; Suneethaa and Rajeswari 2016), only three spectrophotometric methods (Zhila et al. 2016; Jian-Rong et al. 2015; Somsubhra et al. 2014) and only one spectrofluorimetric method (Salem et al. 2021). In terms of the availability of regents and handy and relatively inexpensive apparatus, one of the simplest and most cost-effective approaches of chemical analysis is colorimetric assay (Fernandes 2020). Colorimetric estimation of chemicals and pharmaceuticals has recently resurfaced as a point of interest for researchers, as it can be utilized for the detection and the quantification of dosage forms, forensic and environmental purposes (Shrivas et al. 2017; Lantam et al. 2020; Kailasa et al. 2019; Son et al. 2021). That charge transfer reaction, in which the analyte interacts with various reagents to yield a charge transfer complex, which is stable with absorption in a range of 400–800 nm (visible area) is the most infamous colorimetric interactions (El-Adl et al. 2019).
In this work, four colorimetric approaches for the estimation of fingolimod were designed and validated, through the reaction with the charge acceptor reagents, including tetrachloro 1,4-benzoquinone (CL), tetracyanoethylene (TCNE), 7,7,8,8-tetracyanoquinodimethane or (TCNQ), (Fig. 1) in pharmaceutical form and biological specimens.
Experimental
Apparatus
Shimadzu – UV 1800 double beam UV–Visible spectrophotometer (Japan) having a glass cuvettes of 1 cm (a pair) was utilized for required operations. An Eppendorf micropipette (Germany) that was calibrated was utilized for the spiking of plasma.
Materials
The reagents utilized were of analytical grade. Fingolimod of 99.98% purity (Salem et al. 2021) applied form Novartis Pharmaceutical Corp (USA). Gilenya® Capsules (batch No: 0385) manufactured by Novartis pharmaceutical dorp, USA, that is defined to have 0.5 mg fingolimod for one capsule. TCNQ, CL, TCNE and methanol were bought from Sigma Chemical Co. USA. Human collective plasma was taken from VACERA (Egypt).
Preparation of stock and standard solutions and reagents
50 mg of fingolimod, TCNQ, CL and TCNE was precisely weighed and quantitatively transferred into a set of different volumetric flask of 100-mL, dissolved in methanol and finished to the line utilizing same solvent. 50 mg of fingolimod was dissolved in DMSO (dimethylsulfoxide) and sonicated for 15 min. for plasma spiking.
Preparation of pharmaceutical samples
Twenty Gilenya® capsules content was unfilled and mixed well; the powder equal to 25 mg was weighed and blended in methanol via stirring by a magnetic stirrer for 20 min.; then, the solution was filtered via a filter paper, that has been cleaned with methanol. After that the filtrate, in volumetric flask of 50 mL, was finished to the line with same solvent. The finished solution contained 500 mg mL−1 of fingolimod was obtained.
Preparation of spiked plasma samples
A spiked plasma of 0.2 mL combined with 50 mL of the stock solution of fingolimod dissolved in DMSO, mixed well with vortex for 20 s before being held in a 37 °C water bath for 10 min. For protein precipitation and liberation of drug, 0.5 mL of methanol was added. The mixture was centrifuged for 10 min at 4000 rpm, then 4 mL of the centrifuge was transferred to volumetric flask of 10 mL and processed as previously described down general procedure. The blank was made in the same design, and except instead of fingolimod solution, DMSO was utilized.
General procedure
Certain volumes of each of the following—standard solution, dosage form solution or biological sample—were relocated to a volumetric flask of 10-mL, then 3, 4 and 6 mL of each of the reagents; TCNQ, CL and TCNE, respectively, were mixed well with the sample, after that the volume finished with methanol to obtain the desired concentration. The mixture left for 30 s and the resulted color measured at 843, 640 and 418 nm for TCNQ, CL and TCNE, respectively. The blank was made under the same condition. The blank of spiked plasma was previously cited.
Effect of diluting solvent
The reaction of CL, TCNE and TCNQ with the examined drug was carried out in several solvents as ethanol, chloroform, acetonitrile and methanol, to determine the best for charge transfer reaction. According to the solubility of the drug and reagents as well as the increased absorbance reported in comparison to the other solvents, it was found that methanol is the optimum solvent in those reactions.
Effect of reaction time
The optimum time is established by spectrophotometrically examining the intensity of produced color developed over TCNQ, TCNE and CL reagents at specific time intervals at room temperature (25 °C).
Effect of reagent volume
The reagent volume is examined by utilizing diverse volumes of 50 mg% CL and 100 mg% of either TCNQ or TCNE reagents that combined with a rigid concentration of the researched drug in methanol to study its effect and to know the select the proper reagent volume.
Determination of reaction stoichiometry
To define the ratio of different reagents and the cited drug in each complex, the equimolar solutions of TCNQ, CL or TCNE and fingolimod were produced, and Job’s method of continuous variation (Basavaiah and Zenita 2011) was utilized.
Results and discussion
Drug complexes spectra
TCNQ reacts with fingolimod, at room temperature (25 ± 5 °C) giving bluish green colored complex that absorb maximally at 843 nm; CL reacts with fingolimod, at room temperature (25 ± 5 °C) giving color complex that absorb at 640 nm maximally although TCNE acts with fingolimod yielding a brownish yellow colored complex that absorb at new 418 nm maximally. The spectra of fingolimod-complex with either TCNQ, CL or TCNE are displayed in Fig. 2. Peaks of the generated complexes are put in the visible range where it noticed that there will be no interference with the medication or reagents spectra. Colors produced are simply detectable by the naked eye, making them valuable in the creation of portable quantification or detection probes.
Effect of the reaction time
The colorful complexes are generated instantly with the three reagents, and they stay for 30 min, stable at room temperature at least, according to the reaction time research. This rapid production of the colored complex could be advantageous in using this method to determine the cited drug in quality control or pharmacokinetics researches, as well as if it is employed as a base of a portable analysis or detection purposes. Time effect on TCNQ, TCNE and CL complexes with the chosen drug is shown (Fig. 3).
Effect of reagent volume
By increasing the volume of TCNQ, CL and TCNE till 3, 4 and 6 mL, respectively, the intensity of the absorbance of the produced complex is gradually increased, and after that nothing changed in the intensity (Fig. 4). It is clear that 3, 4 and 6 mL of TCNQ, CL and TCNE reagent solutions were satisfactory for the formation of maximum intensity of reproducible color with the studied drug solutions.
Effect of temperature
The disintegration of the generated complexes induced color fading at temperatures over 40 °C when the ensuring charge transfer complex of TCNQ, TCNE or CL was heated in a water bath. This suggest that at high temperatures, the complex dissociates back into its constituents. The complexes, however, were stable at ambient temperature and up to 30 °C. As a result, lower temperature is preferred for those reactions. For quantitative work, room temperature was determined to be sufficient.
Reaction stoichiometry between drug and TCNQ, TCNE and CL
Job’s continuous variation approach was used to determine the stoichiometry of the reactions, yielding the drug reagent ratios1:1 for each of the cited reagents (Fig. 5).
Analytical method validation
The proposed approaches were validated in accordance with the guidelines of International Conference of Harmonization (ICH) (Moffat et al. 2000). The validation results were as follows.
Linearity and range
By plotting each produced complex's absorbance, at 843, 418 and 640 nm for TCNQ, TCNE and CL, respectively, versus the selected concentration, calibration curves for colored complexes generated between TCNQ, TCNA and CL with fingolimod were obtained (Fig. 6). Table 1 summarizes the parameters of the derived calibration curves. The high correlation coefficient obtained greatly aided linearity. The determination coefficient (r2) ranged from 0.9997 to 0.9999, suggesting good linearity.
Linearity and range
Three-time analyses of three distinct concentrations of pure sample were utilized to assess the accuracy. The results demonstrate a high degree of agreement between the measured values and true ones, showing that the proposed approaches are accurate. Three replications of three concentrations were used to test intraday and inter-day precision. The standard deviation was detected to be small under 2, indicating that the proposed approaches are repeatable and reliable. Table 1 summaries the findings and their statistical analysis.
Robustness
During the development phase, the robustness of the proposed approaches was evaluated by examining the impact of small variations in various variables, as time spent standing or volume of the reagent, on the researched approaches. With each change in each single parameter, the % recovery was recorded. The recovered standard deviations and % recoveries show that any tiny change in any researched variables has no major impact on the suggested procedure's results, Table 2. This evaluation revealed the proposed charge transfer approach's reliability in everyday operation, making them acceptable for quality control laboratories or for biological samples.
Application to pharmaceutical dosage form
The proposed approaches were successfully implemented for the examination of certain dosage forms of the researched drug, and the recovered data were compared with the reported method (Salem et al. 2021) for validation, using student’s t test at a confidence level of 95%. The outcome is shown in Table 3. There was no discernible contrast among the results produced using the researched and reported approaches. Excipients and additives had no effect on the analysis, and the results were as accurate, precise and confident as the reported and validated approaches. By applying the standard addition technique, the validity of the proposed approaches regarding handling, extraction and so on was tested. A certain quantity of each single concentration was combined to the researched pharmaceutical form, powder Gilenya® capsules, for comparing the acquired data from the proposed approaches to the for-real combined standard. The standard addition technique data, shown in Table 3, ensure the validity of the proposed approach for determining the cited drug in its dosage form without any interference from utilized additives. The approaches demonstrated to be advisable for determining fingolimod in its dosage form with sufficient precision and accuracy.
Determination of fingolimod in spiked plasma
After adding organic solvent to plasma for precipitation of its proteins and liberation of the bound drug, fingolimod was measured in spiking plasma samples. The proposed approaches were successful in accurately and precisely determining the cited drug in plasma Table 3. To reduce the impact of the plasma matrix on the findings, a blank plasma solution was treated as the same.
Conclusion
The charge transfer reaction offers a suitable foundation for sensitive, cost-effective and practical analysis approaches for determining the anticancer agent, fingolimod. TCNQ, CL and TCNE, three charge transfer reagents, were profitably utilized to determine the researched drug in its pharmaceutical dosage form and spiked plasma in a speedy, accurate and precise manner. These methods of determination can be employed in quality control for various dosage forms of the drug, as well as in pharmacokinetics and preclinical researches. Colorimetric assays are utilized as an acceptable tool for the yielding of estimation kit or automated determination probes.
References
Basavaiah K, Zenita O (2011) Spectrophotometric determination of famotidine using sulphonphthalein dyes. Quim Nova 34:735–742
Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Burns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR (2002) The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277:21453–21457. https://doi.org/10.1074/jbc.C200176200
Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9:883–897
Chhabda PJ, Balaji M, Srinivasarao V (2013) Development and validation of a stability indicating RP-HPLC method for quantification of fingolimod in bulk and pharmaceutical dosage form. Pharmanest 4:1206–1218
Chiba K (2005) FTY720, a new class of immonomodulator, inhibits lymphocyte egres from secondry lymphoid issues and thymus by agonistic activity at sphingosine 1- phosphate receptors. Pharm Ther 108:308–319
Effat S, Mohammad Z, Siavash M, Reza A, Abbas K (2015) A stability-indicating HPLC method for the determination of fingolimod in pharmaceutical dosage forms. Sci Pharm 83:85–93
El-Adl SM, El-sadek ME, Hassan MH (2019) spectrophotometric analysis of azithromycin and clarithromycin in tablets and human plasma using p-chloranilic acid. Anal Chem Lett 9(3):362–372
Emotte C, Deglave F, Heudi O, Picard F, Kretz O (2012) Fast simultaneous quantitative analysis of FTY720 and its metabolite FTY720-P in human blood by on-line solid phase extraction coupled with liquid chromatography-tandemmass spectromertry. J Pharm Biomed Anal 58:102–112. https://doi.org/10.1016/j.jpba.2011.09.021
Fernandes GM et al (2020) Novel approaches for colorimetric measurements in analytical chemistry–a review. Anal Chim Acta 1135:187–203
Ferreiros N, Labocha S, Schroder M, Radeke HH, Geisslinger G (2012) LC-MS/MS determination of FTY720 and FTY 720- phosphate in murine intracellular ompartments and human plasma. J Chromatoogr B 887–888:122–127. https://doi.org/10.1016/j.jchromb.2012.01.023
Islam A, Emad B (2016) Method development and validation for fingolimod by HPLC/UV in immediate-release oral capsule and study the effect of excipients on solubility and dissolution behavior. J Anal Pharm Res 2(1):00008
Jian-Rong W, Sha L, Bingqing Z, Xuefeng M (2015) Insight into the conformational polymorph transformation of ablock-buster multiple sclerosis drug fingolimod hydrochloride(FTY 720). J Pharm Biomed Anal 109:45–51
Kailasa SK, Park T-J, Koduru JR (2019) Metal nanoparticles-based colorimetric methods for drug analyses. Nanoarchitectonics in Biomedicine. Elsevier, New York, pp 619–641
Lantam A et al (2020) A portable optical colorimetric sensor for the determination of promethazine in lean cocktail and pharmaceutical doses. Microchem J 159:105519
Moffat AC et al (2000) Meeting the International Conference on Harmonisation’s Guidelines on Validation of Analytical Procedures: Quantification as exemplified by a near-infrared reflectance assay of paracetamol in intact tabletsThe opinions expressed in the following article are entirely those of the authors and do not necessarily represent the views of either The Royal Society of Chemistry or the Editor of The Analyst. Analyst 125(7):1341–1351
Narasimha RK, ChVS N, Rajan ST, Eshwaraiah S, Sampath Kumar RG, Rakesh M, Seetha Rama SP, Kishore M, Chakavarthy IE (2014) Stability indicating HPLC method for the quantification of fingolimod hydrochloride and its related substances. Der Pharma Chem 6:335–342
Ravi V, Madhavi P, Darshana P, Anamik K, Ranjan C (2016) Development and validation of immunomodulating drug fingolimod by RPHPLC method with detailed force degradation study. Chem Biol Interface 6(4):210–223
Salem H, Elsoud FAA, Heshmat D (2021) Spectrofluorimetric analysis of fingolimod via complex formation with eosin Y in its pure form, pharmaceutical preparation and biological samples. Spectrochim Acta Part A Mol Biomol Spectrosc 250:119331
Salm P, Warnholtz CR, Lynch SV, Taylor PJ (2006) Measurement and stability of FTY720 in human whole blood by high-performance-atmospheric pressure chemical ionization-tandem mass spectrometry. J Chromatogr B 843:157–163. https://doi.org/10.1016/j.jchromb.2006.05.026
Shrivas K et al (2017) Label-free selective detection of ampicillin drug in human urine samples using silver nanoparticles as a colorimetric sensing probe. New J Chem 41(14):6685–6692
Somsubhra G, Ravikumar B (2015) Development of stability indicating RP-HPLC method for the estimation of Fingolimod in its bulk dosages form as per ICH guideline. J Chem Pharm Res 7(2):23–29
Somsubhra G, Laxmi P, Sowjanya B, David B (2014) Analytical method development and validation of fingolimod in bulk drug by spectrophotometric method as per ICH guidelines. Asian J Res Chem 7(1):55–57
Son SU et al (2021) Colorimetric paper sensor for visual detection of date-rape drug c-hydroxybutyric acid (GHB). Sens Actuat B 347:130598
Suneethaa S, Rajeswari K (2016) A high throughput flow gradient LC–MS/MS method for simultaneous determination of fingolimod, fampridine and prednisone in ratplasma, application to in vivo perfusion study. J Pharm Biomed Anal 120:10–18
Zhila A, Eslam P, Abolghasem B (2016) Mixed hemimicelles solid-phase extraction based on sodium dodecyl sulfate (SDS)-coated nano-magnets for the spectrophotometric determination of fingolomid in biological fluids. Spectrochim Acta A Mol Biomol Spectrosc 15(153):599–604. https://doi.org/10.1016/j.saa.2015.09.013
Zollinger M, Gshwind HP, Jin Y, Sayer C, Zecri F, Hartmann S (2011) Absorption and disposition of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in healthy volunteers: a case of xenobiotic biotrans formation following endogenous metabolic pathways. Drug Metab Dispos 39:199–207. https://doi.org/10.1124/dmd.110.035907
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, H., Mazen, D.Z., Heshmat, D. et al. Development and validation of three colorimetric charge transfer complexes for estimation of fingolimod as an antineoplastic drug in pharmaceutical and biological samples. Chem. Pap. 76, 6675–6681 (2022). https://doi.org/10.1007/s11696-022-02334-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02334-6