Abstract
A straightforward and efficient spectrum technique was created using Ortho-chloranil as the electron acceptor (-acceptor) in a charge transfer (CT) complex formation reaction to determine the concentration of famotidine (FMD) in solutions. Compared to the double-distilled blank solution, the reaction result detected a definite violet colour at a maximum absorption wavelength of 546 nm, For concentrations range 2—28 µg/ml, the technique demonstrated excellent compliance with Beer-Law and Lambert's, as evidenced by its molar absorptivity of 2159.648 L mol−1 cm–1. Lower detection limits of 0.3024 µg/ml and 1.471 µg/ml, respectively, were discovered. The complexes of famotidine and Ortho-chloranil were found to have a 2:1 stoichiometry. Additionally, the suggested approach effectively estimated famotidine concentrations in pharmaceutical formulations, particularly in tablet form.
Similar content being viewed by others
Introduction
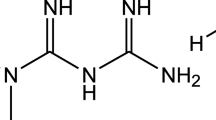
Famotidine, scientifically referred to as 3-[2-(diaminomethyleneamino) thiazol-4-ylmethylthio]-N-sulfamoylpropionamidine, is a type of medication that blocks H2 receptors and acts as an antihistamine. It is commonly prescribed to treat stomach and duodenal ulcers, as well as gastric reflux disease1,2,3. The therapeutic dosage of famotidine is typically 40 mg administered daily, and it is absorbed completely Through the gastrointestinal tract, the highest concentration of the substance in the blood is reached around 3 h after it is taken orally4,5. Figure 1 shows the chemical structure of famotidine.
The literature has documented several methods for analyzing famotidine in pharmaceutical formulations and biological fluids. Spectrophotometric methods, as well as chromatographic methods, have been widely employed6,7,8. Additionally, the flow injection analysis method using spectrophotometry has been utilised9,10. Electrochemical methods such as the voltammetric method11,12,13 and the polarography method have been explored, along with the use of ion-selective electrodes14,15.
Due to issues with reaction time, sensitivity, selectivity, efficacy, economic cost, and high detection rates, many of these approaches, however, have limits for famotidine screening16,17. In contrast, the flow-injection analysis (FlA) technology has proven to be a successful and promising approach for famotidine analysis. It has benefits, including little handling of reagent samples, quick reaction times, good prediction rates, and reproducibility18,19. Additionally, FlA can evaluate colourful and turbid solutions20.
This work aims to determine a straightforward, sensitive, and precise spectrophotometric method to estimate famotidine in pharmaceutical formulations. The study will concentrate on ideal circumstances and elements influencing electrostability.
Expermintal
Devices
In this study, spectrophotometric measurements were carried out using an Japanese SHIMADZU UV–Visible-1800 double-beam spectrophotometer. Furthermore, pH (3310, Jenway) values were measured, and sonicator (Labtech LUC-405, South Korea) and centrifuge (Hettich, Germany). A careful selection of this sophisticated equipment is intended to ensure the accuracy and reliability of data collection throughout the testing procedures.
Reagents and chemicals
The present study used a famotidine (20 mg/tablet) formulation which was obtained from Samra Drug of Iraq (SDI), a pharmaceutical company that complies with British Pharmacopoeia standards in its formulation. Methanol (CAS number: 67–56-1; BDH; UK) is used as a solvent chemical in experimental procedures, while Ortho-chloranil (CAS number: 2435–53-2; Fisher Scientific, US) was a significant reagent. Additional chemical substances, including NaOH (CAS number: 1310–73-2; Kishida Chemical Co., Ltd. Japan), Cetyltrimethylammonium Bromide (CTAB), CAS number 57–09-0, and Sodium dodecyl sulfate (SDS), CAS number 151–21-3—that you purchased from Sigma-Aldrich® in Germany, The CAS number 9036–19-5 Triton X-100 was acquired from BDH in the United Kingdom, Na2CO3 (CAS number: 497–19-8) and KOH (CAS number: 1310–58-3) obtained from Merack, USA, and double-distilled water were used in this study.
Famotidine standard solution (100 µg.ml)
0.01g was dissolved using a small quantity of double-distilled water that created a solution. Subsequently, the volume in the 100 ml volumetric flask was incrementally raised to the intended level using the identical solvent.
Solution for ortho-chloranil (0.005M)
The Ortho-chloranil powder (0.123 g) was mixed with the amount of methanol. Following this, the volume in a 100 ml volumetric flask was carefully set to the mark.
Pharmaceutical solutions (tablets)
Ten tablets of 20 mg each were used in this study. FMD tablets were crushed into a fine powder, after which the equivalent of 0.1 g was prepared. The obtained powders were dissolved in a proportionate amount of double-distilled water. The dissolution process was performed by ultrasonication for 15 min and centrifuging for 5 min. The filtrate was separated using Whatman filter paper No. 41. The resulting mass was transferred to a100 ml volumetric flask, and the volume was completed to the mark.
Approximate NaOH solution (0.1M)
Dissolve 0.400 g of NaOH with the appropriate amount of double-distilled water in a 100 ml flask and completing to the mark.
Solution of surface active compounds (0.1%)
Dissolve 0.100 g of each surfactant compound CTAB and SDS and Triton X-100 were added to a 100 ml volumetric flask filled with hot double-distilled water to dissolve the compounds.
The general principle of the procedure
The method is mostly based on the interesting way that famotidine and Ortho-chloranil work together as π-acceptors. The absorbance at 546 nm shows that this interaction occurs in an alkaline medium compared to a double-distilled blank solution. For future studies and applications in this chemical system, this analysis tool acts as a source of vital information regarding the formation and quantification of these complexes.
Results and discussion
Optimisation conditions
1.3 ml of Ortho-chloranil at a concentration of 0.005 M, 0.5 ml of FMD at 100 μg/ml, and 0.5 ml of NaOH at a concentration of 0.1 M, respectively, in a 5 mL volumetric flask. The spectrophotometric readings were carefully made using the maximum absorption wavelength at 546 nm and compared to double-distilled blank solutions. The accuracy of experimental results is ensured by this careful procedure, so that it is reliable even further, making it possible to have a better understanding of how these compounds’ components interact with one another in detail.
The influence of the base solution
A solution with a concentration of 0.1 M was used to make the alkaline solution. A fixed amount of 0.3 ml of each chemical was mixed with the famotidine solution, The absorbance was measured at 546 nm , and Table 1 shows the results.
As seen in Table 1, different bases affect famotidine dye absorption at 546 nm. The data indicates that NaOH displayed the most intense color, with an absorbance value of 0.07. When comparing, Na2CO3 and KOH, 0.02 and 0.03 were the respective low absorption values that were observed. Table 1 indicates that famotidine is strongly absorbed and reacts well with NaOH, making it the best choice for further research.
Effect of sodium hydroxide volume
Varying amounts of NaOH have been studied to determine their ability for absorption by the FMD. The volumes ranged from 0.1 ml to 1.9 ml. They were added to a volumetric flask that already contained 0.5 ml of FMD solution with a concentration of 100 µg /ml, as well as 1 ml of Ortho-chloranil reagent solution with a concentration of 5.0 × 10–3 M. The final volume was decreased to 5 ml using double distilled water, as shown in Table 2.
After doing data analysis, it was determined that the best concentration for FMD absorption, which attained its maximum value of 0.084, was 0.5 ml of sodium hydroxide (1 × 10−1M) at pH 10.5. Hence, this particular quantity of sodium hydroxide was considered ideal and then was utilized for further studies. The increased absorbance at this volume indicates a positive interaction between famotidine and the prescribed quantity of sodium hydroxide, which is an essential factor for obtaining dependable and precise outcomes in subsequent analyses and applications.
Influence of the quantity of ortho-chloranil reagent
An investigation was conducted to examine the impact of different quantities of Ortho-chloranil reagent on the absorbance of the famotidine solution. Adding different amounts (0.1 ml to 1.9 ml) of a reagent solution with a concentration of 5 × 10−3M to volumetric flasks that already had 0.5 ml of a 100μg/ml famotidine solution was done. Afterwards, 0.5 ml of a solution containing 1 × 10−1M sodium hydroxide was introduced, and the total volume was modified to 5.0 ml by adding double-distilled water. These studies show how the Ortho-chloranil reagent's volume affects absorbance values in Table 3.
Data analysis revealed that famotidine absorbance peaked at 0.124 with 1.3 ml of Ortho-chloranil reagent (5 × 10−3M). The increased absorbance at this volume signifies a positive interaction between famotidine and the given quantity of Ortho-chloranil, giving it a critical parameter for obtaining dependable and precise outcomes in subsequent experiments and applications.
Effect of surface-active substances
Different metabolites were tested to see how they affected famotidine-Ortho-chloral complex absorption.The study focused on three metabolites: SDS, Triton X-100, and CTAB. Investigations were carried out, According to Table 4, these surfactants reduced water absorption, making them unsuitable for future use.
Table 4 shows that the surfactant-free solution possessed the maximum absorption value, measuring at 0.124. Nevertheless, the use of SDS resulted in an average decrease in absorbance of 0.117. However, the use of Triton X-100 and CTAB led to a turbid solution, suggesting an important interference with the absorbance measurements.
They were determined to be unsuitable for further testing because of reduced absorption or contaminants resulting from their reactivity. The absence of synthesized surfactants yielded consistent outcomes that were particularly resilient for the famotidine- Ortho-chloranil complex, providing precise data acquisition and analysis in subsequent experimental investigations.
Effect of temperature
An investigation was conducted to examine the impact of temperature (ranging from 15°C to 65°C) on the reaction of charge transfer complexes, using settings that were optimized. According to the findings, the rate at which absorption occurred changed depending on the temperature. Table 5 shows that absorption was maximum at 30 °C.
The data analysis revealed that the charge transfer complex had the highest level of adsorption at a temperature of 30° C. As a result, this temperature was regarded as appropriate and chosen for the following experiments. The absorbance values demonstrated a clear and consistent relationship with temperature. At elevated temperatures, the tensile strength indicated an initial increase until it exceeded 30 °C, after which it began to decrease. The result is highly valuable for comprehending the kinetics and thermodynamics associated with the formation of charge transfer complexes.
Effect of order of addition
A series of extensive experiments were conducted to assess the impact of various modification strategies on the absorption properties of the violet material. The results obtained from the three distinct methodologies are thoroughly described and displayed in Table 6.
Upon comprehensive data analysis, it is evident that the additive structure has an important influence on the absorption of the violet material. The absorption level varies depending on the reagent (Reag.), NaOH, and drug that are added.
-
1.
The absorbance was 0.09 after adding the reagent, NaOH, and drug subsequently.
-
2.
When the drug, reagent, and NaOH were added in order, the absorbance increased to 0.122.
-
3.
Lastly, the absorbance was comparatively lower at 0.05 when the medication was added first, then NaOH, and finally the reagent.
The results of the study demonstrate the significant effect of additive structure on the creation of violet products. As a result, the second combined system (Drug + Reag. + NaOH) had the highest absorption, indicating its preference for the following tests. An appropriate configuration of this setting is required.
Effect of reaction time
The experiment attempted to examine the effects of time on the stability of the pigment complex that is created when the reactants are mixed under optimum experimental conditions. The stability of pigment absorption takes a minimum of 40 min, as seen in Table 7.
Data show that solid absorbance was stable and constant for at least 40 min after mixing the active ingredients, and the absorbance values were observed to be between 0.120 and 0.124 at this point, indicating that the composition remains available colors. However, after 40 min, the adsorption capacity decreased gradually, indicating a gradual decrease in color intensity.
The obtained result suggests that the color complexity, resulting from the combination of reaction components under optimum experimental conditions, remains constant for roughly 40 min. Experimental design and study reliability and consistency require this information when using complex color schemes in complex environments.
Final absorption spectrum
Synthesized colour material absorption spectrum was measured by combining 10 µg/ml of FMD with Ortho-chloranil reagent (5 × 10−3M) in a starting solution of 1 × 10–1 M NaOH at a pH of 10.5 and a temperature of 30 °C. Max absorbance (λ max) was measured at 546.0 nm. See Fig. 2.
According to the data, Table 8 shows the best FMD Ortho-chloranil complex creation conditions.
The optimum conditions involve measuring the absorbance at 546 nm, using 0.5 ml of 0.1 M sodium hydroxide and 1.3 ml of 5 × 10–3 M Ortho-chloranil reagent. The temperature of the reaction should be maintained at 30 °C, and water serves as the solvent under a basic medium with a pH of 10.5. These optimal conditions ensure the highest sensitivity and stability of the formed colored complex, providing accurate and consistent results in further analytical studies involving the FMD—Ortho-chloranil complexes.
Methodology for constructing the calibration curve
Add increasing amounts of FMD (100 μg/ml) to a series of solutions ranging from 0.1 ml to 2.0 ml. Combine these solutions with 1.3 ml of 0.005M Ortho-chloranil and 0.5 ml of 0.1 M NaOH in a 5.0 ml volumetric flask. Finally, fill the flask to the mark with distilled water and measure the absorption of all solutions versus a double-distilled blank solution at 546.0 nm. A linear calibration curve for FMD with a concentration of 2–28 μg/ml, R2 = 0.9977, a molar absorption coefficient of range 2159.648 /mol.cm, and a Sandell Index of 0.15625 μg/cm2, are shown in Fig. 3.
Accuracy and precision
For each concentration of 100 μg/ml, the recovery percentage (Rec%) and standard deviation (RSD) were calculated to confirm these results. By taking an average of six readings for each, the recovery rate was 99.93%, and the relative standard deviation (0.781–0.928%) and results in Table 9 are shown, meaning that the method is highly accurate and has a good agreement.
Limit of detection (LOD) and limit of quantification (LOQ)
The limit of detection (LOD) was determined by assessing the absorption of the lowest concentration (2 μg/ml) in the calibration curve under the optimal conditions. The LOD and LOQ values of the suggested approach are presented in Table 10.
The reaction's stoichiometry
The stoichiometry of the reaction between FMD and Ortho-chloranil is the quantitative relationship between the amounts of reactants and products involved in the chemical reaction. The concentration of the FMD and Ortho-chloranil was set at 5 × 10–3 M in both the Job's method and the molar ratio method, Used a set of volumetric flasks with a capacity of 5.0 ml to contain varying amounts of the drug solution ranging from 0.2 to 1.8 ml, as well as variable volumes of the reagent solution ranging from 1.8 to 0.2 ml. Added 0.5 ml of a 0.1 M NaOH solution, and then filled the flasks to the mark with double-distilled water. The absorbance was quantified at a wavelength of 546.0 nm relative to the blank reagent. The figures in Fig. 4 indicate a ratio of 2:1.
The molar ratio method involves adding 0.6 ml of a standard FMD solution to a series of volumetric flasks, each containing 5.0 ml. Different volumes of a reagent solution ranging from 0.2 to 2 ml are then added, along with 0.5 ml of NaOH 0.1 M. The flasks are then filled to the mark with double-distilled water, and the absorbance is measured at 546.0 nm, using the blank reagent as a reference. The data presented in Fig. 5 indicates a ratio of 2:1.
The suggested chemical reaction
Ortho-chloranil bonds strongly with chemical atoms having electron pairs, especially nitrogen21. Ortho-chloranil and nitrogen form a 2:1 complex (nitrogen: Ortho-chloranil), demonstrating the participation of the nitrogen atoms in the making of the complex. The complex displays a clear peak caused by charge transfer, which is distinct from the absorbance peaks of both the donor and acceptor molecules. The occurrence of this new peak indicates the creation of an n-π charge transfer (CT) complex. When this combination is exposed to visible or UV radiation, it undergoes further changes, resulting in the creation of two free radical ions: D + and A-22. NaOH expedites the quick synthesis of free radicals. The complexation reaction, which involves the transfer of charge and the production of free radicals, can be represented as follows:
Charge transfer complexes and free radicals form when Ortho-chloranil interacts with the hydroxyl group, is interesting in many chemical processes and analytical applications. This study highlights the importance of Ortho-chloranil as a π-acceptor reagent in studying complex formation mechanisms and free radical reactions.
Applications
The procedure was applied to pharmaceutical preparations containing FMD 20 mg/Tablet.
Direct method
Three various concentrations (4, 12 and 20) μg/ml were used. The solution passed the identical procedures as the calibration curve and was assessed at a wavelength of 546.0 nm. The outcomes are displayed in Table 11, indicating the efficacy of the proposed approach in evaluating FMD in pharmaceutical formulation.
Standard additions
The standard additions method was applied in order to prove that the developed method is free of interference based on the concentration of the drug by taking 0.8 ml of pharmaceutical preparation solution at a concentration of 100 μg/ml of FMD to six 5.0 ml volumetric flasks, then adding an increasing volume of standard solution FMD (0.2–1.0) ml with the six bottles remaining without addition. After that, it was measured at 546.0 nm, like the calibration curve. Figure 6 and Table 12 illustrate typical addition results. Spectrophotometric and suggested approaches for famotidine determination in pharmaceutical formulations are compared in Table 13.
Conclusions
A fast and accurate method for assessment was developed by using a charge transfer complex reaction based on the method of reduction FMD with Ortho-chloranil and NaOH 0.1 M, and the complex formed had a violet color, was soluble in water, and was stable for at least 40 min at a wavelength of 546 nm (R2 = 0.9977). The standard calibration curve shows that the linear range is 2–28 μg/ml, the recovery is 99.56%, the value of the standard deviation was less than the mean 1.22%, the molar absorptivity is 2159.648 L mol−1 cm−1, and the Sandell Index is 0.15625 μg.cm−2.
Data availability
The data used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References.
Al-Khesraji, T. O. & Al-Hayawi, A. Y. Five new taxa records for the macromycota of Iraq from suliamaniya and tikrit provinces (Iraq). Plant Arch. 19, 4067–4072 (2019).
Al-Hayawi, A. Y. & Al Jubori, M. H. S. The inhibitory effect of garlic and onion root exudates on escherichia coli from urinary tract infection and molecular detection of hlyA virulence gene. Drug Delivery. 10, 131–135 (2020).
MS Eissa, A. Elsonbaty, K. Attala, R.A.A. Salam, G.M. Hadad, M.A. Abdelshakour, A.E. Mostafa, Innovative and sustainable deconvoluted amplitude factor spectrophotometric method for the resolution of various severely overlapping pharmaceutical mixtures: Applying the complex-GAPI-tool. Heliyon. 9 (2023)
Elsonbaty, A., Madkour, A. W., Abdel-Raoof, A. M., Abdel-Monem, A. H. & El-Attar, A.-A.M. Computational design for eco-friendly visible spectrophotometric platform used for the assay of the antiviral agent in pharmaceutical dosage form. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 271, 120897 (2022).
Abood, S. J. & AlHayawi, A. Y. Detection of AGTX gene mutations of kidney stone patients in Tikrit city, Iraq. Ann. Trop. Med. Public Health. 22, 187 (2019).
Tsvetkova, B., Maslarska, V. & Peikova, L. An overview of determination of famotidine by different analytical methods. Pharmacia. 62, 12–24 (2015).
Walash, M., El-Brashy, A., El-Enany, N. & Wahba, M. Spectrofluorimetric determination of famotidine in pharmaceutical preparations and biological fluids through ternary complex formation with some lanthanide ions: application to stability studies. Int. J. Biomed. Sci. IJBS. 5, 158 (2009).
Abdulredha, M. et al. Electrochemical defluorination of water: an experimental and morphological study. J. Water Sanit. Hygiene Dev. 12, 394–404 (2022).
Elsonbaty, A., Hasan, M. A., Eissa, M. S., Hassan, W. S. & Abdulwahab, S. Synchronous spectrofluorimetry coupled with third-order derivative signal processing for the simultaneous quantitation of telmisartan and chlorthalidone drug combination in human plasma. J. Fluorescence. 31, 97–106 (2021).
Azmi, S. N. H. et al. Box-Behnken design based development of UV-reversed phase high performance liquid chromatographic method for determination of ascorbic acid in tablet formulations. Separations. 9, 361 (2022).
Trojanowicz, M. & Kołacińska, K. Recent advances in flow injection analysis. Analyst. 141, 2085–2139 (2016).
Trojanowicz, M. & Pyszynska, M. Flow-injection methods in water analysis—recent developments. Molecules. 27, 1410 (2022).
Felix, F.S., & Angnes, L. Flow-injection analysis with electrochemical detection. Agric. Food Electroanal. (2015) 49.
Elsonbaty, A., Eissa, M. S., Hassan, W. S. & Abdulwahab, S. Separation-free spectrophotometric platforms for rapid assessment of combined antiplatelet therapy in complex matrices. Bioanalysis. 12, 335–348 (2020).
AlSamarrai, S. Y., Abdoon, F. M. & Hashim, K. K. A simple method to determine tramadol using a coated-wire electrode as a detector in the flow injection analysis. Microchem. J. 146, 588–591 (2019).
Vernekar, P. R., Shanbhag, M. M., Shetti, N. P. & Mascarenhas, R. J. Silica-gel incorporated carbon paste sensor for the electrocatalytic oxidation of famotidine and its application in biological sample analysis. Electrochem. Sci. Adv. 2, e2100093 (2022).
DeVault, K. R. & Castell, D. O. Gastroenterology, updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Off. J. Am. Coll. Gastroenterol ACG 94, 1434–1442 (1999).
Armenta, S., Alcala, M. & Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chimica Acta. 703, 114–123 (2011).
Al-Samarrai, S., Determination, Manufacturing and application of a nanoparticle lead ion-selective electrode as a sensor in flow injection systems. Sensors Mach. Learn. Appl. 3 (2024)
Al Samarrai, S.Y., Manufacturing a new electrochemical sensor for the determination of silver ions and its application to X-ray waste, Sensors Mach. Learn. Appl. 2 (2023)
Fulton, A. & Lyons, L. E. The ionisation energies of some phenothiazine tranquillisers and molecules of similar structure. Aust. J. Chem. 21, 873–882 (1968).
Kochi, J. K. Charge-transfer excitation of molecular complexes in organic and organometallic chemistry. Pure Appl. Chem.. 63, 255–264 (1991).
Zuhri, A. Z. A., Shubietah, R. M. & Badah, G. M. Extractional-spectrophotometric determination of famotidine in pharmaceutical formulations. J. Pharmaceut. Biomed. Anal. 21, 459–465 (1999).
Kesur, B. R., Salunkhe, V. & Magdum, C. Development and validation of UV spectrophotometric method for simultaneous estimation of ibuprofen and famotidine in bulk and formulated tablet dosage form. Int. J. Pharm. Pharm. Sci. 4, 271–274 (2012).
Funding
Open access funding provided by Lulea University of Technology.
Author information
Authors and Affiliations
Contributions
SYA: Methodology, investigation, formal analysis, and writing—review and editing. RA: Validation, formal analysis, and writing—review and editing. NA: Validation, formal analysis, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Samarrai, S.Y., AlZubaidi, R. & Al-Ansari, N. Charge transfer complex-based spectrophotometric analysis of famotidine in pure and pharmaceutical dosage forms. Sci Rep 14, 3661 (2024). https://doi.org/10.1038/s41598-024-54402-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54402-4
- Springer Nature Limited