Abstract
Datura stramonium L. (Solanaceae) possesses a rich tropane alkaloids (TAs) spectrum. The plant contains, in particular, the allelopathic compounds scopolamine and atropine, which are poorly soluble in water, thus limiting their use in agrochemical formulations as biocidal and deterrent agents against herbivore insects. The efficacy of the hydrophobic TAs extracts could be increased with the improvement of their dissolution/leaching properties. This is important for improving screening and test performance and for elucidating the activity of environmentally friendly agricultural approaches, with new perspectives for the production and use of those biodegradable insecticidal products. The present study explores the aspects of atropine and scopolamine complexation with cyclodextrin (CDs) through FT-IR and UV–Vis spectroscopies. In addition, the structures of the inclusion complex of atropine, scopolamine and β-CD have been investigated by molecular modeling techniques. The results obtained indicate that β-CDs are a promising carriers for improving the properties of TAs, therefore increasing their application potential in agrochemical formulations.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The allelopathy, a phenomenon of biochemical interactions among plants, is considered one of the possible alternatives for reaching sustainable weed management (Cheng and Cheng 2015; Singh et al. 2001, 2003). Allelopathic compounds (ACs) are bioactive secondary metabolites produced by plants that exert ecological functions to counterbalance abiotic and biotic stressors (Cheng and Cheng 2015; Gross 2009; Mithofer and Boland 2012). They play a key role in the agroecosystems leading to a wide array of interactions between plants such as crop-crop, crop-weed, and tree-crops (Farooq et al. 2011). Allelopathy is consequently considered a valid option for controlling weeds, either through directly utilizing natural allelopathic interactions between plants or by using ACs as natural herbicides (Jabran et al. 2015; Singh et al. 2003). ACs play a major role in plant diversity, dominance, and success, as well as in the development of natural vegetation, plant productivity, and pest control (Einhellig 2018; Latif et al. 2017). Since ACs are safe, selective and active substances, their use in agricultural management can reduce the amount of synthetic herbicides, fungicides, and insecticides. This eco-friendly possibility reduces environmental damage and concerns for human health, in accordance with the sustainable development of green agriculture, as in the context of the Food Quality Protection Act (FQPA) (Batish et al. 2001; Ragsdale 2000). Tropane alkaloids (TAs) are a well-known class of AC molecules that play a role in plant defense, exerting insecticidal and deterrent effects against herbivore insects (Ali et al. 2019; Chowanski, 2016; Kupeli Akkol et al. 2020; Mairink et al. 2017). The mode of action of TAs is based on their ability to block acetylcholine receptors (Kohnen-Johannsen and Kayser 2019).
Datura stramonium L., known also with the common names of thorn apple, jimsonweed, or devil's snare, is a plant of the Solanaceae family which possesses a rich alkaloid spectrum (Berkov et al. 2006). The main TAs components are atropine and scopolamine (Fig. 1), and their proportions differ considerably between plant parts (leafs, roots, stems, capsules, petioles and seeds) and developmental stages, but also for environment, geographic location, temperature and season (Jakabová et al. 2012). Iranbakhsh et al. identified the highest concentration of atropine and scopolamine in leafs in a vegetative phase (0.127% dried-weight, with atropine:scopolamine ratio of 0.4:1), while their content has been showed to decrease rapidly in the generative phase (0.050% with a ratio of 1.5:1) (Iranbakhsh et al. 2006). In the petioles, the trend from the vegetative to the generative phase showed a decrease in the concentration of the two alkaloids from 0.122 to 0.082% and an increase in their ratio from 2:1 to 3:1 (Iranbakhsh et al. 2006). In addition to scopolamine and atropine, which account for about 80% of total ATs, D. stramonium L. also contains other alkaloids, including hyoscyamine, cuscohygrine, and toluidine (Steenkamp et al. 2004). All these components are poorly soluble in water, thus limiting their use in agrochemical formulations.
The cyclic oligosaccharides cyclodextrins (CDs) can be used to increase solubility, stability, and bioavailability of several classes of molecules, comprising ACs. CDs are cylinder-shaped nanostructure composed of six or more α-1,4-linked glucose units. In particular, CDs of six, seven, and eight α-d-glucose residues are known as α-, β-, and γ-CD, respectively (Fig. s1) (Crini 2014).
CDs possess a hydrophilic exterior, making them soluble in aqueous media, and a hydrophobic cavity that can host guest molecules of different sizes. Non-polar chemicals, such as ACs, can be encapsulated through hydrophobic interactions inside the cavity, forming nanoscale molecular inclusion complexes in solution, and in the solid-state (Bayomi et al. 2002; Kfoury et al. 2016; Nguyen et al. 2013). The main characteristics of CDs are bioadaptability, absorbability, interaction with bio-membranes, soil resistance, and biodegradability (Dodziuk 2006; Sharma and Baldi 2016). CD complexation leads to alterations in the physicochemical properties of guest molecules, such as solubility, chemical stability, dispersibility, flowability, wettability and dissolution rate. All these factors give to these complexes a considerable potential in sustainable agriculture (Mohapatra et al. 2018). For instance, host molecules entrap hydrophobic pesticides inside their cavity and enhance their solubility, protect them from hydrolysis, reduce their volatility, and increase their bioavailability (Villaverde et al. 2004,2005; Yanez et al. 2012). AC/CDs conjugations can be useful for accurate, ground-level administration, time-controlled–release, and time extended-release of ACs in combination with other substances/tools. Moreover, multifunctional CDs have the advantages of protecting sensitive compounds from the effect of heat- or light-induced degradation (Bayomi et al. 2002; Garcia et al. 2014).
Hydroxypropyl-β-cyclodextrin (HPβCD) is widely used as a solubilizing agent and carrier with rapid dissolution for much poorly water-soluble ACs (Perchyonok et al. 2014). Several inclusion complexes with CDs have been reported in the promising field of environmental friendly pesticides (Benfeito et al. 2013; Gao et al. 2019b; Geng et al. 2018; Kim et al. 2019; Villaverde 2007; Villaverde et al. 2004, 2005; Yanez et al. 2012).
In order to explore rationally new pesticide formulations with enhanced insecticidal or herbicidal activity, it is necessary to assess the influence of CDs on the properties of the guest molecules.
The present study explores the aspects of TAs complexation with CDs obtained by the “wet mixing method” described below. TAs have been obtained from D. stramonium L. extract. The increased TAs hydrosolubility, following the complexation with different types of CDs, have been followed through FT-IR and UV–Vis spectroscopies. Moreover, the structural characteristic of the inclusion complex has been also evaluated with the molecular docking method. The information presented in this research can prove useful in future trends in regard to biocides.
Materials
Plant material extraction
Datura Stramonium L. plants were harvested from Banat (Western Romania) in September 2019, at its physiological maturity (the fruits had ripened, and dispersal of the seeds has occurred).
Chemicals
Scopolamine, atropine, cyclodextrins, acetonitrile, methanol HPLC grade, ≥ 99.9% were purchased from Sigma–Aldrich (Germany). The water used in the study was double distilled and deionized with a Milli-Q RG (Millipore, Burlington, MA, USA) water purification system. The standard solution of scopolamine and atropine (10 mg) was dissolved in methanol (100 mL).
Experimental
Tropane alkaloids extraction procedure
Plant material, in powder (500 mg), obtained from D. stramonium L. species was subjected to extraction three times (30 min each), using chloroform (15 mL), methanol, ammonia (25%) (15: 15: 0.1 (v/v/v) and an ultrasound device (Sonicor Inc., West Babylon, NY, USA). The extract was kept at room temperature for one hour, filtered through filter paper, and washed twice with 1 mL CHCl3. The solvent was evaporated to dryness. The dry residue was dissolved in 5 mL of CHCl3 and 2 mL of H2SO4 (0.5 M). The CHCl3 organic fraction was removed, whereas the aqueous solution was adjusted to pH 10 by the addition of ammonium hydroxide (25%) in an ice-bath. From the solution, the alkaloids were extracted once with 2 ml and twice with 1 ml of CHCl3. Following the addition of anhydrous Na2SO4, the solution was filtered, and the filtrate washed again with CHCl3 (1–2 mL). The CHCl3 was further evaporated to dryness under vacuum at 40 °C (Kamada et al. 1986).
High-performance liquid chromatography (HPLC)
Scopolamine and atropine obtained from a raw extract of D. stramonium L. were simultaneously identified by HPLC–UV method (Perkin-Elmer Series 200 system with a UV/Vis detector). For the quantitative determination of the two compounds, HPLC was coupled with full-scan diode array spectrophotometry (DAD). HPLC method used a 100–5C8 Kromasil column (250 × 4.6 mm) with gradient elution and a working temperature of 25 °C in the column. The mobile phase was composed of acetonitrile (25%) and an aqueous solution (75%) (5 mM sodium 1–heptanesulfonate monohydrate, pH = 3.5). Detection was performed at UV (λ = 230 ± 4 nm with reference λ = 360 ± 8 nm). The calibration curve was linear between 0.13–13.75 mg/mL (r = 0.9951, n = 8) for scopolamine and 0.25–25.5 mg/mL (r = 0.9999, n = 8) for atropine (Hinescu 2011). The data were generated by ChromeGate, using atropine and (–) scopolamine as standard samples.
Tropane alkaloids quantification
TAs (atropine and scopolamine) quantification was carried out through the external standard method (Ashtiania and Sefidkonb 2011). Standard solutions of atropine and scopolamine at different concentrations (4, 10, 25, 50, 100, 200, 400 ppm) were dissolved in methanol. Aliquots of 20 µL from each standard solution were injected into HPLC.
Preparation of the inclusion complex
The inclusion complexes of TAs in β–CD were prepared by the wet mixing method according to the following procedure. From the extract of D. stramonium L., one system was made by using the raw alkaloids tropane (mainly composed by atropine and scopolamine), whereas the purchased purified forms have been considered for preparing an additional system.
For each system, five mixtures were prepared, each containing approximately 500 mg TAs (brought to a final volume of 25 mL, far beyond its hydrosolubility) and different β–CD quantities weighed with the accuracy of ± 0.1 mg.
Weight was performed with a semi-micro analytical balance (resolution ± 0.1 mg) (Sartorius). Mixtures were placed in an agate mortar, wetted, and kneaded every 10 min. Subsequently, mixtures were further transferred in a 25 mL volumetric flask and filled to the mark with distilled water and mixed for one hour.
In addition, a saturated solution of TAs in water (revealing some solubility in the absence of CD) was prepared. All solutions were filtered through disposable filters (0.2 µm pore size).
FT-IR and UV–Vis spectroscopy
The infrared absorption spectra were recorded with a Fourier transform infrared spectroscopy (FT-IR), model “460 Plus”, Jasco Products Co. Detector: DLATGS with Peltier element (KRS-5), MCT-N (- 750 cm−1), resolution of 0.9 cm−1 and S/N 15.000:1. Apodization function: Happ-Genzel. Samples were included in a potassium bromide tablet (potassium bromide of spectral purity "Uvasol," Merck, was used). Data processing was done with "Jascow" software (Jasco Company). The FT-IR spectrum of the inclusion complex was compared with those of TAs and hydroxypropyl β–CD (HPβCD). Since water traces from samples interfere with FT-IR spectra, after the mixing operation, the product has been kept for 10 h at 120 °C in an oven. Solubility changes were followed by tracking the absorption spectrum in the ultraviolet domain with a double beam spectrophotometer, model PG Instruments, stray light 0.015% T (220 nm and 340 nm), wavelength accuracy 0.3 nm (automatic wavelength correction), photometric range 0.2–3 Abs, using a UV–Vis, UV WIN 5.05 software. Samples were placed in quartz cuvettes with a 1 cm length path.
Compound design and optimization
The three-dimensional structures of atropine, scopolamine and β-cyclodextrin (β-CD) were designed using USCF Chimera 1.11.2 software (Pettersen et al. 2004). The optimization process for all three compounds was performed with the GAMESS software (Barca 2020; Gordon and Schmidt 2005). For detailed information, see the Supporting Information.
Molecular dynamics (MD) and docking simulations
The molecule of β-CD was submitted to MD simulation with AMBER14 (Case 2014). The optimized conformation of β-CD was employed for the derivation of atomic charges with the RESP methodology (Bayly et al. 1993). Cyclodextrin was solvated in water and periodic boundary conditions were applied with a cutoff of 10.0 Å. The system was heated to 300 K, followed by pressure equilibration at 1 atm. A 150 ns production run was performed using the pmemd implementation in AMBER. All docking calculations were carried out with the AutoDock Vina (Trott and Olson 2010). The structure of β-CD was considered rigid while the docked molecules of atropine and scopolamine were defined as flexible. All molecular graphics were generated using the USCF Chimera 1.11.2 (Pettersen et al. 2004). For further details, see the Supporting Information.
Results and discussion
FT-IR characterization of HPβCD-tropane alkaloids inclusion complex
The comparison of FT-IR spectra of the three systems: TAs, extract of D. stramonium L., HPβCD and the inclusion complex of the two components obtained with the wet mixing method is depicted in Fig. 2 and S2. The changes in shift, shape, and intensity of the FT-IR absorption peaks providing clear evidence for the occurrence of the inclusion (Ge et al. 2012).
FT-IR spectrum of alkaloids tropane is characterized by typical absorption peaks for the stretching vibrations of various functional groups; 3700–3000 cm−1 (O–H alcohol, N–H amine), 3104 cm−1 (aromatic C–H), 1738 cm−1 (C = O ester), 1650 cm−1 (N–H bending), 1496 cm−1 (C = C aromatic), 1600–1300 cm−1 (C–H bending alkane), 1200 (C–O ester), 1050 cm−1 (C–O primary alcohol), 900–700 cm−1 (C–H aromatic bending vibrations) (Baranska and Schulz 2009; Christen et al. 2009).
FT-IR spectrum of HPβCD showed the main peaks related to the stretching vibrations of the functional groups: 3460 cm−1 (O–H), 3000 cm−1 (C-H), 1160 cm−1 (O–H of CHOH) 1050 cm−1 (C–O–C ether linkage) 1021 cm−1 (C–O of CH2OH) 800–650 cm−1 (C–H bending vibrations) (Darekar et al. 2016; Gao, 2019a; Su et al. 2012).
Spectral comparison (Fig. 2 and S2) indicated that the two components are not physically mixed but have undergone a significant alteration of their vibration properties (mainly atropine and scopolamine) following their inclusion in the CD cavity with the subsequent restricted dynamic motion. This observation is in accordance with other studies that have been performed in CD inclusion complexes and by a MD and docking simulations performed in this study (Asztemborska et al. 2019; Mansouri et al. 2013; Wszelaka-Rylik and Gierycz 2015).
In the inclusion complex, the characteristic FT-IR absorption peaks of alkaloid tropane and HPβCD changed. The FT-IR spectrum of the inclusion complex showed no features similar to pure tropane alkaloids. In particular, the absorption peak of alkaloid tropane at 1738 cm−1 (C = O ester) strongly reduce its intensity whereas the peaks at 1650 cm−1 (N–H bending), 1496 cm−1 (C = C aromatic) together with the signals around 1400 cm−1 (C–H bending alkane) almost disappeared, while other bands were obscured by very intense and broad HPβCD bands. Other apparent changes in terms of shift and shapes in several absorption peaks evidence that alkaloids tropane have been included in the HPβCD to form an inclusion complex. Some changes observed in the O–H stretching vibration (absorption peak at 3460 cm−1), could allow probing the alteration of the H-bonded environments upon complexation. The O–H bending vibrations of CH2OH and C–H stretching vibrations of HPβCD got distorted due to the inclusion of guests to the host cavity, as has been reported in other researches (Darekar et al. 2016).
UV–Vis evaluation of tropane alkaloids water solubility following CDs complexation
To outline the effect of β–CD to enhance atropine and scopolamine water solubility, the absorption spectrum of TAs saturated aqueous solution (sample n° 1, Fig. s3) was compared to some solutions in which, in addition to TAs (atropine and scopolamine from purified extract of D. stramonium L.), increasing quantities of β–CD have been introduced (sample no. 2–6, Fig. s3).
Table 1 collects the β–CD solution concentrations (samples no 1–6) and the related optical absorbance values recorded for purified atropine and scopolamine extract of D. stramonium L and for the raw TAs.
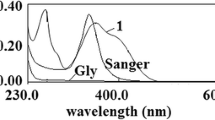
Figure 3a shows the relative experimental UV–Vis spectra and the relationship between the concentrations of β–CD (m/v %) and the absorbance of the saturated solution of purified atropine and scopolamine from the extract of D. stramonium L..
The solubility of pure tropane alkaloids in the presence of β–CD: UV–Vis spectra of tropane alkaloids with different β–CD concentration (a) and the correlation between the absorbance (at 278 nm) and β–CD concentration (b). The solubility of tropane alkaloids raw mixture in the presence of β–CD: UV–Vis spectra of tropane alkaloids mixture with different β –CD concentration (c) and the correlation between the absorbance (at 254 nm) and β –CD concentration (d)
The mixture presents low solubility even in the absence of CD (spectrum no. 1) according to absorbance results (Fig. 3a). Increasing concentration of β–CD, in turn, enhances the absorbance of TAs, hence contributing to their water solubility.
The absorption plot (Fig. 3b) shows a second-degree polynomial correlation (R2 = 0.9996) with increasing β–CD concentration. This correlation points in the direction of saturation phenomena, which is probably because the complex purified TAs–CD has a certain upper limit of solubility.
Experimental UV–Vis spectra and the relationship between the concentrations of β–CD and the absorbance of the saturated solution of raw TAs recorded at 254 nm are shown in Fig. 3c, d (Table 1).
UV–Vis absorption spectra were recorded on filtered solutions in 220–300 nm range. This system reflects better the natural tropane alkaloid components of the extract of D. stramonium L.. As atropine and scopolamine are insoluble in water, their spectra practically coincide with the baseline (spectrum no. 1) (Fig. S3), while in the presence of β–CD, an increase in absorbance was observed (no. 2–6). As can be seen, β–CD increases tropane alkaloid solubility significantly. Unlike pure compounds, an excellent linear correlation (R2 = 0.9995) was observed between β–CD concentration and TAs mixture solubility in water (in the range tested). This difference can be attributed to the higher solubility of TAs–β–CD complexes compared to allelochemical (pure mixed atropine and scopolamine)–β–CD complexes (at the reported concentrations). This interpretation is fairly in agreement with the β–CD solubilization effect, being higher with raw natural TAs mixture than with pure atropine and scopolamine mixture (Castagne et al. 2010).
Figure 4 represents a comparison of the solubilization effects for TAs in complex with different types of CDs: α–cyclodextrin (α–CD), β–cyclodextrin (β–CD), γ–cyclodextrin (γ–CD), hydroxypropyl β–cyclodextrin (HPβCD) and randomly methylated β–cyclodextrin (RAMEB).
The effect of various CDs on tropane alkaloids solubility. UV–Vis spectra of tropane alkaloids with different types of CDs: α–cyclodextrin (α–CD), β–cyclodextrin (β–CD), γ–cyclodextrin (γ–CD), hydroxypropyl β–cyclodextrin (HPβCD) and randomly methylated β–cyclodextrin (RAMEB) mixture with β-CD (a) and the enlarged scale (b)
Because of the difference in the number of glucopyranose units, each CD has a distinct molecular weight, cavity size, mobility and water solubility. Since α-CD has the fewest glucopyranose units (six), they have the smallest cavity size of 0.57nnm, followed by β-CD which has seven glucopyranose units and a cavity size of 0.78 nm, while, γ-CD, which has eight glucopyranose units, has a cavity size of 0.95 nm. Water solubility depends mainly from the hydroxyl groups interacting much less with each other and much more with water. Since γ-CD ring is more strained compared to α-CD and β-CD, its hydroxyl groups are more distant and more able to interact with water, providing greater solubility. In summary due to the larger size and hence more hydroxyl groups available, the aqueous solubility of γ-CD is greater than α-CD, while β-CD possess strong intramolecular bonding between its hydroxyl groups and thus possess the lowest water solubility. However, the modification of β-CD in HPβCD leads to a large increase in its water solubility (> 60% at 25 °C).
The comparison between the different CDs (Fig. 4) shows a clear superiority effect of RAMEB, respect to all other CDs, in the enhancement of the aqueous solubility of TAs. However, RAMEB possesses poor permeability and high cytotoxicity (Kiss 2010) and its use should be avoided. Therefore, compared to the remaining CDs, the dimensions of the inner cavity of the safer β-CD and HPβCD appear the best to include ATs molecules which lead to their improved hydrosolubility.
To exclude the hypothesis that the solubilization effect is due to a solvotropic effect generated by the CDs and not to the inclusion of TAs in the hydrophobic cavity, a solubilization test was carried out with an equivalent quantity of glucose. The glucose employed contained the same amount of glucopyranose units as the CDs without having a cyclic structure, therefore being deprived of the signature characteristics of CDs. The comparison indicated (Fig. 4b) that the presence of glucose virtually does not have any effect in the solubility of TAs. The outcome is that the solubilization effect can be attributed on the inclusion properties of the cyclic structure of CDs.
Molecular modeling study
The initial conformations of atropine, scopolamine and β-CD were optimized in order to obtain the conformation with the lowest potential energy. The results are presented in Fig. S4 and S5 in Supporting information. The optimized structures do not present any substantial difference compared to the initial conformation (Fig. S5). For β-CD, this observation is more pronounced due to the circular nature of the molecule (Fig. S5c) that does not allow great flexibility.
The analysis of the MD simulation showed that the circular conformation of the molecule impedes the movement of the atoms. The RMS changes observed during the production run are more pronounced in the first 60 ns of the simulation whereas between 70 and 150 ns the conformation of cyclodextrin does not present high RMS fluctuations (Fig. S6a). The mean RMS over the whole simulation process is 2.25 ± 0.424 Å, a measure that suggests small conformational changes from its starting conformation. The small changes of β-CD show that the solvent molecules may interact favorably with the solute due to the presence of ester and –OH groups in β-CD. The number of –OH groups in cyclodextrin may also lead to increased interactions with other organic molecules via formation of hydrogen bonds. Thus, β-CD proves to be an excellent drug delivery system. The clustering analysis of the MD simulation revealed the presence of three representative conformations for β-CD (Fig. S6b). The superimposition of the structures revealed only small conformational changes (Fig. S6b and Table S1).
The dominant conformation of β-CD (Fig. S6b, black) was employed in the molecular docking experiments. The results of the docking simulations showed that the atropine molecule presents higher binding affinity to β-CD in contrast to scopolamine. The calculated docking score for atropine and scopolamine is − 4.5 and − 4.0 kcal mol−1, respectively. The docking scores show that atropine presents a better binding affinity than scopolamine. The analysis of the docking conformations presents the hydrogen bonds (HBs) forming between atropine/ scopolamine and β-CD (Fig. 5a-magenta and 5b-cyan). As expected, the –OH groups of β-CD are involved directly with the formation of HBs. Additionally, atropine and scopolamine are positioned in the cavity of β-CD (Fig. 5c and d).
The two molecules are anchored in the cavity of cyclodextrin via hydrogen bonds forming with the respective sides of β-CD. The positioning of the molecules inside the cavity of cyclodextrin may improve their water solubility as described in previous sections and thus make β-CD an ideal carrier molecule. Moreover, the HBs formed between the alkaloids and β-CD may improve the bioavailability of the alkaloids and further explain the stability of both the carrier molecule and the substances attached, as reported in the experimental sections above.
Conclusions
A number of complexes of CDs, CD polymers, and conjugations of CDs have been designed and evaluated for practical use in the field of sustainable agriculture. Because of their multifunctional characteristics and bioadaptability, CDs can mitigate the undesirable properties of AC molecules through the formation of inclusion complexes or as AC/CDs conjugates. One of the characteristics of CDs is their tendency to include in their hydrophobic cavity active molecules with hydrophobic properties (partial to complete hydrophobicity). The experimental intermolecular complex presented in this study shows an increased water solubility of tropane alkaloids (TAs) obtained from D. stramonium L. extract due to the hydrophilicity of the external surface of CDs. The increased TAs hydrosolubility has been evaluated, through UV–Vis spectroscopy, following the complexation with different types of CDs. The degree of solubilization effect is dependent on the nature of TAs mixture, the size and characteristics of the cyclic molecule (α–, ß–, HPβ- or \(\gamma\)–CD) and the CDs concentration. The dimensions of the inner cavity of the safer β-CD and its hydroxyled derivative HPβCD, appear the best to include ATs molecules which lead to their improved hydrosolubility respect to other CDs. FT-IR analysis demonstrated that TAs can be effectively encapsulated in HPβCD forming inclusion complexes with enhanced hydrosolubility. In addition, molecular docking gives evidences of the better binding affinity of atropine than scopolamine with β-CD.
The results obtained indicate that β-CD and HPβCD are the most efficient carriers for improving the properties of tropane alkaloids. The information gained can prove useful in future trends concerning biocides for the application of tropane alkaloids in agrochemical formulations as natural and biodegradable pesticide products.
References
Ali AH, Abdelrahman M, El-Sayed MA (2019) Alkaloid role in plant defense response to growth and stress. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense signaling in growth and stress. Springer, Cham, pp 145–158. https://doi.org/10.1007/978-3-030-27165-7_9
Ashtiania F, Sefidkonb F (2011) Tropane alkaloids of Atropa belladonna L. and Atropa acuminata Royle ex Miers plants. J Med Plant Res 5:6515–6522
Asztemborska M, Ceborska M, Pietrzak M (2019) Complexation of tropane alkaloids by cyclodextrins. Carbohydr Polym 209:74–81. https://doi.org/10.1016/j.carbpol.2019.01.011
Baranska M, Schulz H (2009) Chapter determination of alkaloids through infrared and Raman Spectroscopy. In: Cordell GA (ed) The alkaloids: chemistry and biology, 67th edn. Academic Press, Cambridge, pp 217–255. https://doi.org/10.1016/S1099-4831(09)06704-2
Barca GMJ et al (2020) Recent developments in the general atomic and molecular electronic structure system. J Chem Phys 152:154102. https://doi.org/10.1063/5.0005188
Batish DR, Singh HP, Kaur S (2001) Crop allelopathy and its role in ecological agriculture. J Crop Prod 4:121–161. https://doi.org/10.1300/J144v04n02_03
Bayly CI, Cieplak P, Cornell W, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280
Bayomi MA, Abanumay KA, Al-Angary AA (2002) Effect of inclusion complexation with cyclodextrins on photostability of nifedipine in solid state. Int J Pharm 243:107–117. https://doi.org/10.1016/s0378-5173(02)00263-6
Benfeito S, Rodrigues T, Garrido J, Borges F, Garrido EM (2013) Host-guest interaction between herbicide oxadiargyl and hydroxypropyl-beta-cyclodextrin. Sci World J 2013:825206. https://doi.org/10.1155/2013/825206
Berkov S, Zayed R, Doncheva T (2006) Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia 77:179–182. https://doi.org/10.1016/j.fitote.2006.01.002
Case DA et al (2014) Amber 14. University of California, San Francisco
Castagne D, Dive G, Evrard B, Frederich M, Piel G (2010) Spectroscopic studies and molecular modeling for understanding the interactions between cholesterol and cyclodextrins. J Pharm Pharm Sci 13:362–377. https://doi.org/10.18433/j3bs34
Cheng F, Cheng Z (2015) Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front Plant Sci 6:1020. https://doi.org/10.3389/fpls.2015.01020
Chowanski S et al (2016) A review of bioinsecticidal activity of solanaceae alkaloids. Toxins (basel). https://doi.org/10.3390/toxins8030060
Christen P, Bieri S, Munoz O (2009) Characterization of positional and configurational tropane alkaloid isomers by combining GC with NPD, MS and FTIR. Nat Prod Commun 4:1341–1348
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. https://doi.org/10.1021/cr500081p
Darekar T, Aithal KS, Shirodkar R, Kumar L, Attari Z, Lewis S (2016) Characterization and in vivo evaluation of lacidipine inclusion complexes with β-cyclodextrin and its derivatives. J Incl Phenom Macrocycl Chem 84:225–235. https://doi.org/10.1007/s10847-016-0600-9
Dodziuk H (2006) Cyclodextrins and their complexes: chemistry, analytical methods, applications. Wiley
Einhellig FA (2018) Allelopathy—a natural protection, allelochemicals. Handbook of natural pesticides: methods. CRC Press, Cambridge, pp 161–200
Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KH (2011) The role of allelopathy in agricultural pest management. Pest Manag Sci 67:493–506. https://doi.org/10.1002/ps.2091
Gao S et al (2019a) Preparation and characterization of cyanazine–hydroxypropyl-beta-cyclodextrin inclusion complex. RSC Adv 9:26109–26115. https://doi.org/10.1039/c9ra04448e
Gao S, Jiang JY, Liu YY, Fu Y, Zhao LX, Li CY, Ye F (2019b) Enhanced solubility, stability, and herbicidal activity of the herbicide diuron by complex formation with beta-cyclodextrin. Polymers (basel). https://doi.org/10.3390/polym11091396
Garcia A, Leonardi D, Salazar MO, Lamas MC (2014) Modified beta-cyclodextrin inclusion complex to improve the physicochemical properties of albendazole complete in vitro evaluation and characterization. PLoS ONE 9:e88234. https://doi.org/10.1371/journal.pone.0088234
Ge X, Huang Z, Tian S, Huang Y, Zeng C (2012) Complexation of carbendazim with hydroxypropyl-beta-cyclodextrin to improve solubility and fungicidal activity. Carbohyd Polym 89:208–212. https://doi.org/10.1016/j.carbpol.2012.02.072
Geng Q, Xie J, Wang X, Cai M, Ma H, Ni H (2018) Preparation and characterization of Butachlor/(2-Hydroxypropyl)-beta-cyclodextrin Inclusion complex: Improve soil mobility and herbicidal activity and decrease fish toxicity. J Agric Food Chem 66:12198–12205. https://doi.org/10.1021/acs.jafc.8b04812
Gordon MS, Schmidt MW (2005) Advances in electronic structure theory: GAMESS a decade later. In: Theory and applications of computational chemistry. Elsevier, pp 1167–1189
Gross EM (2009) Allelochemical Reactions. In: Likens GE (ed) Encyclopedia of inland waters. Academic Press, Oxford, pp 715–726. https://doi.org/10.1016/B978-012370626-3.00106-X
Hinescu LG et al (2011) HPLC method for the simultaneous determination of the components of an aqueous antidote solution. Farmacia 59:97–105
Iranbakhsh A, Oshaghi, Majd A (2006) Distribution of atropine and scopolamine in different organs and stages of development in Datura stramonium L. [Solanaceae]. Structure and ultrastructure of biosynthesizing cells. Acta Biologica Cracoviensia Series Botanica 48
Jabran K, Mahajan G, Sardana V, Chauhan BS (2015) Allelopathy for weed control in agricultural systems. Crop Prot 72:57–65. https://doi.org/10.1016/j.cropro.2015.03.004
Jakabová S, Vincze L, Farkas Á, Kilár F, Boros B, Felinger A (2012) Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J Chromatogr A 1232:295–301. https://doi.org/10.1016/j.chroma.2012.02.036
Kamada H, Okamura N, Satake M, Harada H, Shimomura K (1986) Alkaloid production by hairy root cultures in Atropa belladonna. Plant Cell Rep 5:239–242. https://doi.org/10.1007/BF00269811
Kfoury M et al (2016) Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem 196:518–525. https://doi.org/10.1016/j.foodchem.2015.09.078
Kim Y, Shinde VV, Jeong D, Choi Y, Jung S (2019) Solubility enhancement of atrazine by complexation with cyclosophoraose isolated from Rhizobium leguminosarum biovar trifolii TA-1. Polymers (basel). https://doi.org/10.3390/polym11030474
Kiss T et al (2010) Evaluation of the cytotoxicity of beta-cyclodextrin derivatives: evidence for the role of cholesterol extraction. Eur J Pharm Sci 40:376–380. https://doi.org/10.1016/j.ejps.2010.04.014
Kohnen-Johannsen KL, Kayser O (2019) Tropane alkaloids: chemistry, pharmacology, biosynthesis and production. Molecules. https://doi.org/10.3390/molecules24040796
Kupeli Akkol E, Ilhan M, Kozan E, Guragac Dereli FT, Sak M, Sobarzo-Sanchez E (2020) Insecticidal activity of Hyoscyamus niger L. on Lucilia sericata causing myiasis. Plants (basel). https://doi.org/10.3390/plants9050655
Latif S, Chiapusio G, Weston LA (2017) Chapter Two - allelopathy and the role of allelochemicals in plant defence. In: Becard G (ed) Advances in botanical research, vol 82. Academic Press, Cambridge, pp 19–54
Mairink SZ, Barbosa LC, Varejao EV, Farias ES, Santos ML, Picanco MC (2017) Larvicidal activity of synthetic tropane alkaloids against Ascia monuste orseis (Lepidoptera: Pieridae). Pest Manag Sci 73:2048–2053. https://doi.org/10.1002/ps.4565
Mansouri M, Pirouzi M, Saberi MR, Ghaderabad M, Chamani J (2013) Investigation on the interaction between cyclophosphamide and lysozyme in the presence of three different kind of cyclodextrins: determination of the binding mechanism by spectroscopic and molecular modeling techniques. Molecules 18:789–813. https://doi.org/10.3390/molecules18010789
Mithofer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450. https://doi.org/10.1146/annurev-arplant-042110-103854
Mohapatra S, Sapra O, Paroha S, Dubey RD (2018) Impact of cyclodextrin in drug delivery system. In: Lichtfouse E (ed) Sustainable Agriculture Reviews 27. Springer, Cham, pp 271–293. https://doi.org/10.1007/978-3-319-75190-0_10
Nguyen TA, Liu B, Zhao J, Thomas DS, Hook JM (2013) An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem 136:186–192. https://doi.org/10.1016/j.foodchem.2012.07.104
Perchyonok VT, Grobler SR, Zhang S (2014) IPNs from cyclodextrin: Chitosan antioxidants: bonding, bio-adhesion, antioxidant capacity and drug release. J Funct Biomater 5:183–196. https://doi.org/10.3390/jfb5030183
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Ragsdale NN (2000) The Impact of the food quality protection act on the future of plant disease management. Annu Rev Phytopathol 38:577–596. https://doi.org/10.1146/annurev.phyto.38.1.577
Sharma N, Baldi A (2016) Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 23:739–757. https://doi.org/10.3109/10717544.2014.938839
Singh HP, Batish DR, Kohli RK (2001) Allelopathy in agroecosystems. J Crop Prod 4:1–41. https://doi.org/10.1300/J144v04n02_01
Singh HP, Batish DR, Kohli RK (2003) Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit Rev Plant Sci 22:239–311. https://doi.org/10.1080/713610858
Steenkamp PA, Harding NM, van Heerden FR, van Wyk BE (2004) Fatal Datura poisoning: identification of atropine and scopolamine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci Int 145:31–39. https://doi.org/10.1016/j.forsciint.2004.03.011
Su J, Chen J, Li L, Li B, Shi L, Zhang H, Ding X (2012) Preparation of natural borneol/2-hydroxypropyl-beta-cyclodextrin inclusion complex and its effect on the absorption of tetramethylpyrazine phosphate in mouse. Chem Pharm Bull (tokyo) 60:736–742. https://doi.org/10.1248/cpb.60.736
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Villaverde J (2007) Time-dependent sorption of norflurazon in four different soils: use of beta-cyclodextrin solutions for remediation of pesticide-contaminated soils. J Hazard Mater 142:184–190. https://doi.org/10.1016/j.jhazmat.2006.08.005
Villaverde J, Morillo E, Perez-Martinez JI, Gines JM, Maqueda C (2004) Preparation and characterization of inclusion complex of norflurazon and beta-cyclodextrin to improve herbicide formulations. J Agric Food Chem 52:864–869. https://doi.org/10.1021/jf0350358
Villaverde J, Maqueda C, Morillo E (2005) Improvement of the desorption of the herbicide norflurazon from soils via complexation with beta-cyclodextrin. J Agric Food Chem 53:5366–5372. https://doi.org/10.1021/jf0502449
Wszelaka-Rylik M, Gierycz P (2015) Isothermal titration calorimetry (ITC) study of natural cyclodextrins inclusion complexes with tropane alkaloids. J Therm Anal Calorim 121:1359–1364. https://doi.org/10.1007/s10973-015-4658-1
Yanez C, Canete-Rosales P, Castillo JP, Catalan N, Undabeytia T, Morillo E (2012) Cyclodextrin inclusion complex to improve physicochemical properties of herbicide bentazon: exploring better formulations. PLoS ONE 7:e41072. https://doi.org/10.1371/journal.pone.0041072
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement. This research received no external funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Butnariu, M., Peana, M., Sarac, I. et al. Analytical and in silico study of the inclusion complexes between tropane alkaloids atropine and scopolamine with cyclodextrins. Chem. Pap. 75, 5523–5533 (2021). https://doi.org/10.1007/s11696-021-01742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01742-4