Abstract
In this study, an alternative application of supported liquid membranes (SLMs) containing specific ion carriers dissolved in supra molecular solvent (SUPRA) to the selective and simultaneous separation of Ag+ and Pb2+ ions from a dilute source solution is introduced. A two-membrane three-compartment cell was used. Two micro-porous polypropylene supported membranes loaded with dibenzyl-diaza-18-crown-6 (DBzDA18C6) and dicyclohexyl-18-crown-6 (DC18C6) dissolved in supra molecular solvent (SUPRA) were used for selective transport of silver (I) and lead (II) ions, respectively. At first, vesicles of decanoic acid (SUPRA), as green solvent, were synthesized. Then, each of two mentioned crown ethers was easily dissolved into the SUPRA phase and used as the selective membrane phase for facile and simultaneous transport of lead and silver ions through the designed cell. The DBzDA18C6-loaded membrane was placed between the first and the second compartments (MP1), whereas the DC18C6-loaded membrane was placed between the second and the third ones (MP2) of the transport cell. Using this cell assembly, simultaneous separation and transport of Ag+ and Pb2+ ions are possible from a 1.854 × 10–4 M picric acid source solution containing these two species and several other interfering ions into the two corresponding receiving compartments of the transport cell. The silver (I) and lead (II) ions quantitatively transport during 75 and 15 min of time into two individual receiving solutions, respectively. Sodium thiosulfate (0.6 M at pH 6.4) and sodium pyrophosphate (0.25 M at pH 7) were used as selective stripping agents for Ag+ and Pb2+ ions (RP1 and RP2), respectively.

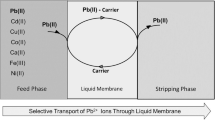
source solution compartment, 500 ml of picrate solution mixture of Ag+ and Pb2+ ions, and other cations; MP: membrane phases, MP1: DBzDaza18C6 in SUPRA (for silver transport) and MP2: DC18C6 in SUPRA (for lead transport); RP: receiving solution compartments, RP1: 100 ml of S2O32− aqueous solution (for silver ions) and RP2: 100 ml of P2O74− aqueous solution (for lead ions); 1: source sampling port; 2 and 3: receiving sampling ports


source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.854 × 10−4 M picric acid at pH = 3.2; MP1, 0.008 M of DBzDA18C6 and MP2, 0.008 M of DC18C6 in SUPRA solvent; RP1, 100 ml of varying concentration of Na2S2O3, (1) 0.6 M, (2) 0.7 M, (3) 0.5 M, at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH = 7. a %Transported into the receiving phase (RP1), b and c % Remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.854 × 10−4 M picric acid at pH = 3.2; MP1, 0.008 M of DBzDA18C6 and MP2, 0.008 M of DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of varying concentration of Na4P2O7, (1) 0.25 M, (2) 0.3 M, (3) 0.17 M, (4) 0.1 M, (5) 0.06 M at pH = 7. a %Transported into the receiving phase (RP2), b and c % Remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.854 × 10−4 M picric acid at pH = 3.2; MP1, 0.008 M of DBzDA18C6 and MP2, 0.008 M of DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at varying pH, (1) 7, (2) 6, (3) 8, (4) 9. a % Transported into the receiving phase; b and c % remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, varying concentration of picric acid, (1) 1.85 × 10−4 M at pH 3.2, (2) 3.85 × 10−4 M at pH 2.4, (3) 8.85 × 10−5 M at pH 4; MP1, 0.008 M DBzDA18C6 and MP2, 0.008 M DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH 7. a % Transported into the receiving phase (RP1); b and c % remaining in the source and membrane phases, respectively

source phase (SP) on Pb2+ transport into RP2 through the SLM. Conditions: source phase, 500 ml of 2 ppm Ag+ and Pb2+, varying concentration of picric acid, (1) 1.85 × 10−4 M at pH 3.2, (2) 3.85 × 10−4 M at pH 2.4, (3) 8.85 × 10−5 M at pH 4; MP1, 0.008 M DBzDA18C6 and MP2, 0.008 M DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH 7. a % Transported into the receiving phase; b and c % remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.85 × 10−4 M picric acid at pH = 3.2; MP1, varying concentration of DBzDA18C6, (1) 0.008 M, (2) 0.013 M, (3) 0.005 M, and MP2, 0.008 M DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH 7. a %Transported into the receiving phase (RP1), b and c %Remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.85 × 10−4 M picric acid at pH = 3.2; MP1, 0.008 M of DBzDA18C6 and MP2, varying concentration of DC18C6, (1) 0.008 M, (2) 0.013 M, (3) 0.005 M in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH 7. a % transported into the receiving phase (RP2); b and c % remaining in the source and membrane phases, respectively

source phase, 500 ml of 2 ppm Ag+ and Pb2+, 1.854 × 10−4 M picric acid at pH = 3.2; MP1, 0.008 M DBzDA18C6 and MP2, 0.008 M DC18C6 in SUPRA solvent; RP1, 100 ml of 0.6 M Na2S2O3 at pH = 6.4; RP2, 100 ml of 0.25 M Na4P2O7 at pH 7. 1 and 4, % transported of Pb2+ and Ag+ into RP2 and RP1, and, 3 and 6, % remaining of Pb2+ and Ag+ in MP2 and MP1, respectively. 2 and 5, % remaining of Pb2+ and Ag+ in SP, respectively

Similar content being viewed by others
References
Abdullah N, Yusof N, Lau WJ, Jaafar J, Ismail AF (2019) Recent trends of heavy metal removal from water/wastewater by membrane technologies. J Ind Eng Chem 76:17–38. https://doi.org/10.1016/j.jiec.2019.03.029
Alpoguz HK, Memon S, Ersoz M, Yilmaz M (2005) Transport of Hg2+ ions across a supported liquid membrane containing Calix[4]arene nitrile derivatives as a specific ion carrrier. Sep Sci Technol 40:2365. https://doi.org/10.1080/01496390500202605
Amini M, Rahbar-Kelishami A, Alipour M, Vahidi O (2018) Supported liquid membrane in metal ion separation: an overview. J Membr Sci 4:121–135. https://doi.org/10.22079/jmsr.2017.63968.1138
Amiri AA, Safavi A, Hasaninejad AR, Shrghi H, Shamsipur M (2008) Highly selective transport of silver ion through a supported liquid membrane using calix[4]pyrroles as suitable ion carriers. J Membr Sci 325:295. https://doi.org/10.1016/j.memsci.2008.07.041
Arghavani-Beydokhti S, Rajabi M, Asghari A (2017) Combination of magnetic dispersive micro solid-phase extraction and supramolecular solvent-based microextraction followed by high-performance liquid chromatography for determination of trace amounts of cholesterol-lowering drugs in complicated matrices. Anal Bioanal Chem 409:4395–4407. https://doi.org/10.1007/s00216-017-0383-x
Azzoug S, Arous O, Kerdjoudj H (2014) Metallic ions extraction and transport in supported liquid membrane using organo-phosphoric compounds as mobile carriers. J Environ Chem Eng 2:154–162. https://doi.org/10.1016/j.jece.2013.11.028
Ballesteros-Gomez A, Rubio S (2012) Environment-responsive alkanol-based supramolecular solvents: characterization and potential as restricted access property and mixed-mode extractants. Anal Chem 84(1):342–349. https://doi.org/10.1021/ac2026207
Bayou N, Arous O, Kerdjoudj H (2010) Elaboration and characterization of plasticized cellulose triacetate membrane containing trioctylphosphine oxide (TOPO): application to the transport of uranium and molybdenum ions. CR CHIM 13:1370–1376. https://doi.org/10.1016/j.crci.2010.04.015
Bhatluri KK, Manna MS, Saha P, Ghoshal AK (2014) Supported liquid membrane-based simultaneous separation of cadmium and lead from wastewater. J Membr Sci 459:256–263. https://doi.org/10.1016/j.memsci.2014.02.019
Bhatluri KK, Manna MS, Ghoshal AK, Saha P (2015) Supported liquid membrane based removal of lead(II) and cadmium(II)from mixed feed: Conversion to solid waste by precipitation. J Hazard Mater 299:504–512. https://doi.org/10.1016/j.jhazmat.2015.07.030
Caballero-Casero N, García-Fonseca S, Rubio S (2018) Restricted access supramolecular solvents for the simultaneous extraction and cleanup of ochratoxin A in spices subjected to EU regulation. Food Control 88:33–39. https://doi.org/10.1016/j.foodcont.2018.01.003
Caballo C, Sicilia MD, Rubio S (2017) The Application of Green Solvents in Separation Processes, Chapter 5-Supramolecular Solvents for Green . Elsevier, Spain, pp 111–137
Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M (2017) Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng 5(3):2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Chaudry MA, Bukhari N, Mazhar M (2008) Coupled transport of Ag (I) ions through triethanol amine-cyclohexanone-based supported liquid membranes. J Membr Sci 320:93–100. https://doi.org/10.1016/j.memsci.2008.03.036
de San Miguel ER, Vital X, de Gyves J (2014) Cr(VI) transport via a supported ionic liquid membrane containing CYPHOS IL 101 as carrier: system analysis and optimization through experimental design strategies. J Hazard Mater 273:253–262. https://doi.org/10.1016/j.jhazmat.2014.03.052
Falaki F, Berijani S (2016) Aspartic acid-modified magnetic nanoparticles as an ideal sorbent for solid phase extraction of Pb in water samples prior to ICP-OES determination. Desalin Water Treat 57:25765–25772. https://doi.org/10.1080/19443994.2016.1157706
Falaki F, Shemirani F, Shamsipur M (2016) Surfactant-assisted transport of lead ion through a bulk liquid membrane containing dicyclohexyl-18-crown-6: efficient removal of lead from blood serum and sea water. J Iran Chem Soc 13(7):1257–1263. https://doi.org/10.1007/s13738-016-0839-5
Feizi N, Yamini Y, Moradi M, Karimi M, Salamat Q, Amanzadeh H (2017) A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal Chim Acta 953:1–9. https://doi.org/10.1016/j.aca.2016.11.007
Jean E, Villemin D, Hlaibi M, Lebrun L (2018) Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep Purif 201:1–9. https://doi.org/10.1016/j.seppur.2018.02.033
Kubota F, Shimobori Y, Koyanagi Y, Shimojo K, Kamiya N, Goto M (2010) Uphill transport of rare-earth metals through a highly stable supported liquid membrane based on an ionic liquid. Anal Chem 26:289–290. https://doi.org/10.2116/analsci.26.289
Lozano LJ, Godinez C, de los Rios AP, Hernandez-Fernandez FJ, Sanchez-Segado S, Alguacil FJ (2011) Recent advances in supported ionic liquid membrane technology. J Membr Sci 376:1–14. https://doi.org/10.1016/j.memsci.2011.03.036
Luo X, He D, Ma M (2010) Simultaneous transport and separation of Cu(II) and Zn(II) in Cu-Zn-Co sulfate solution by double strip dispersion hybrid liquid membrane (SDHLM). Sep Sci Technol 45:2130–2140. https://doi.org/10.1080/01496395.2010.504442
Luri J (1975) Handbook of analytical chemistry. Mir Publisher, Moscow
Memon ZM, Yilmaz E, Soylak M (2017) Multivariate statistical design optimization for ultrasonic-assisted restricted access supramolecular solvent-based liquid phase microextraction of quercetin in food samples. J Iran Chem Soc 14:2521–2528. https://doi.org/10.1007/s13738-017-1187-9
Moradi M, Yamini Y, Rezaei F, Tahmasebi E, Esrafili A (2012) Development of a new and environment friendly hollow fiber-supported liquid phase microextraction using vesicular aggregate-based supramolecular solvent. Anlst 137:3549–3557. https://doi.org/10.1039/C2AN35304K
Moral A, Sicilia MD, Rubio S (2009) Determination of benzimidazolic fungicides in fruits and vegetables by supramolecular solvent-based microextraction/liquid chromatography/fluorescence detection. Anal Chim Acta 650:207–213. https://doi.org/10.1016/j.aca.2009.07.056
Padilla I, Diaz IG, Urien A, Rodríguez O, Lopez F, Alguacil FJ (2012) Membrane-based extraction with strip/organic dispersion methodologies for metals removal and recovery from wastewaters. Desalin Water Treat 40:282–297. https://doi.org/10.1080/19443994.2012.671265
Parhi PK (2013) Supported liquid membrane principle and its practices: a short review. J Chem 2012:1–11. https://doi.org/10.1155/2013/618236
Pospiech B (2014) Synergistic solvent extraction and transport of Zn(II) and Cu(II) across polymer inclusion membranes with a mixture of TOPO and aliquat 336. Sep Sci Technol 49:1706–1712. https://doi.org/10.1080/01496395.2014.906456
Pospiech B, Kujawski W (2015) Ionic liquids as selective extractants and ion carriers of heavy metal ions from aqueous solutions utilized in extraction and membrane separation. Rev Chem 31(2):179–191. https://doi.org/10.1515/revce-2014-0048
Rehman SU, Akhtar G, Chaudry MA (2012) Coupled transport of Tl3+ through triethanolamine–xylene–polypropylene supported liquid membranes. J Ind Eng Chem 18:492–498. https://doi.org/10.1016/j.jiec.2011.11.130
Rezaei F, Yamini Y, Moradi M, Daraei B (2013) Supramolecular solvent-based hollow fiber liquid phase microextraction of benzodiazepines. Anal Chim Acta 804:135–142. https://doi.org/10.1016/j.aca.2013.10.026
Safari M, Yamini Y, Tahmasebi E, Ebrahimpour B (2016) Magnetic nanoparticle assisted supramolecular solvent extraction of triazine herbicides prior to their determination by HPLC with UV detection. Microchim Acta 183:203–210. https://doi.org/10.1007/s00604-015-1607-4
Scheel GL, Tarley CRT (2017) Feasibility of supramolecular solvent-based microextraction for simultaneous preconcentration of herbicides from natural waters with posterior determination by HPLC-DAD. Microchem 133:650–657. https://doi.org/10.1016/j.microc.2017.03.007
Shamsipur M, Azimi G, Mashhadizadeh MH, Madaeni SS (2001) Selective Transport of Silver Ion through a Supported Liquid Membrane Using Hexathia-18-Crown-6 as Carrier. Anal Sci 17:491–494. https://doi.org/10.2116/analsci.17.491
Shamsipur M, Kazemi SY, Azimi G, Madaeni SS, Lippolis V, Isaia F (2003) Selective transport of silver ion through a supported liquid membrane using some mixed aza-thioether crowns containing a 1,10-phenanthroline sub-unit as specific ion carriers. J Membr Sci 215:87–93. https://doi.org/10.1016/S0376-7388(02)00604-X
Shamsipur M, Hashemi OR, Lippolis V (2006) A supported liquid membrane system for simultaneous separation of silver (I) and mercury (II) from dilute feed solutions. J Membr Sci 282:322–327. https://doi.org/10.1016/j.memsci.2006.05.034
Shamsipur M, Falaki F, Shemirani F (2016) Highly facile supported liquid membrane transport and removal of silver ion using dibenzyldiaza-18-crown-6 dissolved in a supramolecular solvent as selective ion carrier. Desalin Water Treat 57:25705–25717. https://doi.org/10.1080/19443994.2016.1162204
Sharma S, Panja S, Ghosh SK, Dhami PS, Gandhi PM (2016) Efficient transport of Am(III) from nitric acid medium using a newconformationally constrained (N, N, Nˊ, Nˊ-tetra-2-ethylhexyl)7-oxabicyclo[2.2.1]heptane-2,3-dicarboxamide across a supported liquid membrane. J Hazard Mater 305:171–177. https://doi.org/10.1016/j.jhazmat.2015.11.045
Suren S, Wongsawa T, Pancharoen U, Prapasawat T, Lothongkum AW (2012) Uphill transport and mathematical model of Pb(II) from dilute synthetic lead-containing solutions across hollow fiber supported liquid membrane. Chem Eng J 191:503–511. https://doi.org/10.1016/j.cej.2012.03.010
Wang D, Hu J, Liu D, Chen Q, Li J (2017) Selective transport and simultaneous separation of Cu(II), Zn(II) and Mg(II) using a dual polymer inclusion membrane system. J Membr Sci 524:205–213. https://doi.org/10.1016/j.memsci.2016.11.027
Yang D, Li X, Meng D, Wang M, Yang Y (2017) Supramolecular solvents combined with layered double hydroxide-coated magnetic nanoparticles for extraction of bisphenols and 4-tert-octylphenol from fruit juices. Food Chem 237:870–876. https://doi.org/10.1016/j.foodchem.2017.06.063
Zaheri P, Abolghasemi H, Maraghe MG, Mohammadi T (2015) Intensification of Europium extraction through a supported liquid membrane using mixture of D2EHPA and Cyanex272 as carrier. Chem Eng Process 92:18–24. https://doi.org/10.1016/j.cep.2015.03.004
Acknowledgements
The support of this work Research Councils of Shahr-e-Qods Branch, Islamic Azad University, and Tehran University and Razi University is gratefully acknowledged. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Falaki, F., Shamsipur, M. & Shemirani, F. Simultaneous selective separation of silver (I) and lead (II) ions from a single dilute source solution through two supported liquid membranes composed of selective crown ethers in supra molecular solvent. Chem. Pap. 75, 5489–5502 (2021). https://doi.org/10.1007/s11696-021-01734-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01734-4