Abstract
Studies on liquid-liquid extraction and bulk liquid membrane (BLM) technique-based metal ion separation by a previously published Pb2+-selective acridono-18-crown-6 ether selector molecule were performed. The effects of the stirring speed, the quality of apolar organic membrane, the counterions of Pb2+, the pH of the aqueous phase, the concentration of the source phase, the concentration of the carrier in the BLM and the temperature on the Pb2+-separation were investigated. Moreover, the effects of the competitive inhibition due to the presence of Ag+, Ca2+, Co2+, Cu2+, K+, Mg2+, Na+ and Zn2+ as competing ions in a multicomponent aqueous source phase of different ion-concentrations were also studied. After a proper dilution of the multicomponent aqueous source phase, excellent Pb2+-selectivity was achieved without a significant reduction in the efficiency compared to the liquid membrane transport of single-component systems. Based on the BLM-cell studies the applied selector molecule proved to be suitable for the development of liquid membrane-based Pb2+-selective separation methods, which can be greatly aided by the analysis of the effects on the separation and by the optimization of the parameters of the process discussed here.
Similar content being viewed by others
Introduction
Heavy metal ions have been in the focus of interest of many researchers worldwide since they are considered one of the most important environmental pollutants nowadays. Heavy metal ions often tend to accumulate in the body, which can be the basic cause of various serious illnesses. Lead compounds are the most common heavy metal contaminants as they are utilized by many industries such as metallurgy, paint-, building- and energy etc. industries. Heavy metals usually spread in the environment as dissolved ions in water. Several methods have been developed previously for the removal of dissolved heavy metals from surface or wastewater including precipitation [1], ion exchange [2], oxidation-reaction [3], sorption [4], extraction, filtration, reverse osmosis, electrochemical treatment [5], and evaporative recovery [6]. However, these processes typically have the disadvantages of the requirement of expensive equipment, utilization of high energy and the production of a large amount of waste [7]. Various liquid membrane (LM) techniques, i.e., organic solvent as a membrane in contact with separated aqueous phases and governed by a non-equilibrium mass transfer, can be suggested as an advantageous alternative to the solvent extraction methods, when other separation techniques cannot be applied efficiently. This technique is based on a simple liquid-liquid extraction with the difference that it has multiple advantages, like the simplicity in operation, the reduction of operation costs, the use of small amounts of materials due to the regenerability, the energy economy and the fact, that separation, preconcentration and regeneration procedures can take place simultaneously [8]. Among LM-technologies, the simplest design is served by the conventional bulk liquid membrane (BLM)-based methods. Although in the case of earlier publications similar techniques were carried out to model the selective ion-transport through biological membranes and to study the selectivity of synthetic supramolecular carriers [9, 10], it was later proved that BLMs were suitable for removing heavy metals from water with a significant efficiency especially in the case of small-scale applications [11, 12]. Moreover, the BLMs serve as useful models for several advanced structural devices in the developmental stages. These improved membrane-based heavy metal separation techniques, like emulsion liquid membranes (ELMs) [13], supported liquid membranes (SLMs) [14] and polymer inclusion membranes (PIMs) [15] have demonstrated their outstanding effectiveness in analytical practice and in most of the cases their properties showed a good correlation with the previous studies on conventional LM-systems.
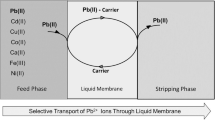
Transport studies were carried out in a BLM-cell reported first by Lamb and co-workers [9]. Since then, model devices with similar design (Fig. 1) proved to be capable of studying phase transfer processes in many cases [8, 16].
According to previous studies crown ether-type carriers have shown a great promise for Pb2+-recovery from water [17]. We used an acridono-18-crown-6 ether macrocycle as carrier, which showed high selectivity toward Pb2+ [18, 19] and its lipophilic analogue (Fig. 2) proved to be suitable for electrochemical determination of Pb2+ in multicomponent water samples [20].
Despite the large number of publications in the field of Pb2+-selective extraction and transport processes, selective recognition and removal of Pb2+ are still of great interest [21,22,23,24,25,26]. Although the number of the reported novel macrocycle-based liquid membrane applications has also increased recently [27, 28], there are only a few publications about investigating the LM-based separation methods under highly competitive conditions [29, 30], which can be limiting for the development of future applications.
The aim of our present research was to investigate the Pb2+-selective liquid-liquid extraction- and facilitated transport process of a macrocyclic carrier through BLMs using multicomponent samples with a large number of competing ions. The additional goal of the study was to provide a useful starting point for effective designing of novel neutral ionophore-based extraction- and phase transport process in the future. We hope that the described relationships could be generalized for other LM-based procedures applying similar type of carrier molecule.
Experimental
Chemicals, apparatus and procedure
The Pb2+-selective lipophilic macrocycle used as carrier was synthesized as reported [20]. Materials were purchased from Sigma-Aldrich Corporation (USA, owned by Merck) and used without further purification unless otherwise noted. Solvents were dried and purified according to well established methods [31].
In the case of liquid-liquid extraction procedures aqueous solutions of metal salts (6 mL) were extracted with equal volumes (6 mL) of crown ether in dichloromethane. In order to prevent the change in volumes of the liquid phases during the extraction, the solvents were saturated with each other before the measurements. In the aim of providing an equal efficiency of phase contact during the extraction procedures, the two-phase mixtures were placed in glass vials and then shaken at 300 rpm using an IKA KS 130 (IKA®-Werke GmbH & Co. KG, Germany) shaker. In order to quantify the metal salt content in the organic phase, the dichloromethane was separated from the aqueous phase using a separatory funnel, the organic phase was then shaken with 5 × 2 mL of 10 m/m% aqueous acetic acid solution to remove the cations and the resulted aqueous phase was evaporated to a constant weight under reduced pressure.
In the case of the transport studies, the source phase was an aqueous solution of Pb2+ (2 mL), while the receiving phase was 6 mL of double-distilled water or buffered aqueous solution. The BLM was formed by a solution of the crown ether dissolved in 12 mL of an apolar solvent with a relatively high density compared to water. Using an apolar organic membrane prevents membrane leakage and allows only the carrier-mediated transport of the metal salts. The metal ions were in a large excess during the transport. During the transport experiment the membrane phase was stirred continuously using a polytetrafluoroethylene-coated magnetic stirring bar with a length of 10 mm and a diameter of 4 mm. The material transfer surface area of the applied transport device was 7.07 × 10−4 m2. For preparation of the source phases the metal salts were dissolved in double-distilled water, unless otherwise indicated. For comparison, the experiments were also carried out with buffered aqueous phases (KH2PO4/Na2HPO4, pH = 7.0). No significant difference was observed, thus the different pH values of the solutions of the salts did not influence the results of the measurements. In order to regenerate the membrane and to determine the total amount of Pb2+ inside the membrane, the bulk phase was separated from the source phase with a separatory funnel, the organic phase was then shaken with 5 × 2 mL of 10 m/m% aqueous acetic acid solution to remove the cations. For regeneration, the organic phase was dried over magnesium sulphate, filtered and evaporated. The purity of the carrier was checked by TLC on silica gel using a mixture of methanol and dichloromethane 1:10 as an eluent (Rf = 0.75). For determining the total amount of Pb2+ inside the membrane the combined aqueous phases were evaporated and dried to a constant weight before the analysis. Measurements were carried out at an adjusted temperature of 10 ± 0.5 or 15 ± 0.5 or 20 ± 0.5 °C as indicated in each case using an air-tempering equipment. All data reported are derived from the average of at least three replicate measurements.
The extracted and transported quantities were determined by weight measurements. For preparing the samples, the receiving phase was evaporated under reduced pressure, dried to a constant weight and the mass of the solid residue was determined by a Mettler Toledo XS105 microanalytical balance (Mettler Toledo, USA) to the nearest 0.1 mg. The errors of the transported amounts of metal salts were smaller than ±1.0 mg. The errors were calculated from at least three independent measurements. The membrane leakage was less than 1.0% in all cases.
In the cases of multielement sample compositions the element masses of the residue were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES). From the residues 5 mL sample solutions were made. The solutions were acidified with 50 µL nitric acid (63%) and then diluted with distilled water before determining the element concentrations with ICP-OES method. The sample solutions were measured in simultaneous, multielement mode by a Labtest Plasmalab ICP-spectrometer (Labtest Equipment Company, USA) with a 40-channel Paschen-Runge vacuum polychromator with photomultiplier detectors, using 27 MHz argon plasma. Instrument settings were the following, forward power: 1.3 kW, sample introduction with a “OneNeb” nebulizer (Agilent Technologies, USA), cyclonic spay chamber and Gilson peristaltic pump (Gilson Company, USA) at 1 mL/min sample flow rate, observation height: 13.5 mm, integration time: 5 s. Limit of quantitation for Ag was 0.025 mg/L, (wavelength: 328.068 nm), for Ca was 0.03 mg/L, (wavelength: 422.673 nm), for Co was 0.019 mg/L, (wavelength: 238.892 nm), for Cu was 0.025 mg/L, (wavelength: 224.700 nm), for K was 0.05 mg/L, (wavelength: 766.491 nm), for Mg was 0.0005 mg/L, (wavelength: 279.553 nm), for Na was 0.19 mg/L, (wavelength: 589.592 nm), for Pb was 0.13 mg/L, (wavelength: 220.353 nm), and for Zn was 0.005 mg/L, (wavelength: 213.856 nm).
Evaluation of the results
The calculations were performed using Microsoft Excel 2019 (Microsoft Corporation, USA). OriginPro 8.6 (OriginLab Corporation, USA) software was used for the graphical interpretation of the results. The randomization of the experimental design and the statistical evaluation were carried out using STATISTICA 13.4.0.14 (TIBCO Software Inc., USA) software. During the statistical investigations a confidence level of 95% was used in all the cases.
For the analysis of the extraction studies, the results were characterized using the extraction percentages (E, %), the distribution coefficients (D, -), the extraction capacities of the membranes (Cm, µmol/g) and the selectivity coefficients (α, -).
The extraction percentages represent the proportion of the extracted amount of metal ions in the organic phase after the separation process.
The distribution coefficients were determined as the ratio of ion concentration in the organic phase (corg) to ion concentration in the aqueous phase (caq) (Eq. 1).
The selectivity for Pb2+ over other ions is expressed as the ratio of the distribution coefficients of the initial ions (Di) to the distribution coefficient of Pb2+ (Eq. 2).
The extraction capacity of the membranes represents the total retained metal salt quantity per gram of LM and was calculated according to Eq. 3, where c0 and c are the Pb2+ concentrations in the aqueous solution before and after the extraction, respectively, V is the volume of the aqueous phase, mm is the total mass of the liquid membrane and M is the molar mass of Pb2+-salt.
In the case of studies on BLM-cell the transported weight-percentage values of the metal salt contents in the source phase were chosen as output parameter as well as fluxes (J, mol/(s × m2)), since they include information about both the transport time and the geometry of the applied device. Flux values were calculated as a transported amount of metal salt ((c0-c) × V, where c0 and c represent the concentration of the source phase at the starting point and after the transport, respectively and V is the volume of the source phase) divided by the product of transport time (ttr) and the effective surface area of the applied BLM-cell (Am) (Eq. 4).
For quantification of the separation properties of the BLM-technique the selectivity coefficient was used (Eq. 5).
The selectivity coefficient was defined as the ratio of the fluxes of competing ions (Ji) to the initial flux of Pb2+.
Results and discussion
Liquid-liquid extraction
Initially, we investigated the liquid-liquid phase simple extraction method. The effect of the concentration of the carrier in the membrane and the metal salt concentration of the source phase were examined together by expressing them as the molar ratio of these two concentrations (cr, -), respectively. An aqueous solution of Pb2+-acetate (6 mL, 1 mol/L) was extracted with an equivalent volume of a solution of the host molecule in dichloromethane, using a molar ratio of 1 : 25, 1 : 100 and 1 : 1000 = crown ether : Pb2+, respectively. Changes in cr were adjusted by varying only the content of the carrier in the membrane. We found that the organic phase was saturated with Pb2+ after 30 min. This period of time was sufficient to reach the equilibrium distribution ratio of the ions. After a longer phase contact, no change in the metal salt content of the organic phase was observed. The percentage of metal salt in the organic phase related to the amount of metal salt of the starting aqueous phase was determined after a 1 h-long shaking in all the cases. The results are shown in Fig. 3, where theoretical values refer to the maximum amount of Pb2+ in the organic phase estimated from the amount of crown ether in the membrane suggesting 1 : 1 metal ion - selector molecule complex-stoichiometry [20].
It can be seen that the theoretical and the measured values are close to each other, which means that the molar amount of Pb2+-acetate found in the organic phase was nearly equivalent to the molar amount of crown ethers dissolved in dichloromethane. This observation is consistent with the fact that the host molecule forms a complex with Pb2+ with a 1 : 1 complex-stoichiometry and suggests that the majority of metal ions within the membrane were coordinated by the host molecules. The amount of the free, solvated metal salt in the organic phase was negligible, so the separation was caused by the presence of the crown ether. Consistently, the theoretical Pb2+-binding capacity of the organic phase in Fig. 3 shows a direct proportion to the measured values, which are also along a line as a function of the molar ratio of the host molecule concentration (linear regression, R2 > 0.99).
The large standard deviation of the measured values indicates that the extraction separation was poorly reproducible. This may be related to the fact that the host molecules could form different associations within the organic phase and were present in different binding states at the time of the termination of the experiment due to their reversible binding properties. The average of the results still shows the expected correlation. The reproducibility of the separation can be improved by more advanced separation methods, such as the BLM-system, which served as the basis of further studies reported here.
Following studies focused on the efficiency of liquid-liquid extraction and on the Pb2+-selectivity in multicomponent systems. In this case the aqueous phase contained a solution of 9 different competing metal ion acetate salts in comparable amounts. The total ion concentration in the resulting aqueous solution was 860 mmol/L. The exact composition of the ion mixture is given in Table 1.
The separation of the multicomponent aqueous solution was studied by the analysis of the ions in the organic phase after 1 h of phase contact, consistently to the previous extraction experiments. The measured ion concentrations were corrected with the results obtained by using a host molecule-free dichloromethane phase (blank sample) by subtracting them from the measured data. The results are summarized in Table 2.
Based on the theoretical binding capacity of the organic phase (calculated from the molar amount of the host molecule), the organic phase was able to bind only 0.5% of the total amount of metal ions, including only 4.0% of the total amount of Pb2+ in the aqueous source phase. Based on this consideration, after the extraction approximately 45% of the metal ions in the organic phase was coordinated by crown ether molecules, while more than a half of them were in solvated state. As a result, the selectivity of the extraction was lost. Due to the intensive phase contact, some ions with a higher organophilicity were able to get into the organic phase even without host molecules, or metal ions might also be able to pass from the aqueous phase to the organic phase in the form of hydrate-enveloped microdomains or with small waterdrops. Based on these results, the reported crown ether cannot be used for selective liquid-liquid extraction separation under competitive conditions.
Transport through a BLM-system
For studying the transport process, we investigated the factors which have a decisive influence on either the selective molecular recognition or on the process itself at a macroscopic level. As critical factors the following ones were chosen: stirring speed, quality of apolar organic membranes, counterions of Pb2+, pH of the aqueous phases, concentration of the source phase, concentration of the carrier in the BLM and temperature.
Effect of stirring speed on transport kinetics
The time-dependence of the transport and the effect of stirring speed on the kinetics were studied over a time interval of 0–48 h (Fig. 4). A passive transport mechanism governed by the concentration gradient between the aqueous phases can typically be characterized by a saturation curve. In this case a nearly linear (0.91 < R2 < 0.96) relationship was observed since the studied time-interval belongs to the initial stage of this equilibrium process.
Higher stirring speed resulted in a more efficient flow of carriers inside the BLM, which increased the transport efficiency. If transport molecules move faster, the speed of the transport also increases. A stirring speed of 250 rpm was chosen as optimum, as it was the highest value at which the applied device can still operate stably. Further studies were performed uniformly at this stirring speed. Applying higher stirring speed, the LM-phase can get unstable and mixing of the phases might take place. This deviance in operation appears as a large increase in the slope of the curve illustrating the kinetics of the process at a stirring speed of 300 rpm (see curve e) in Fig. 4). In the case of this high stirring speed, the transported quantities were no longer entirely from the carrier facilitated transport, thus the results are not exact for the analysis of the carrier-mediated BLM-technique. In order to make the results comparable, the measurements were performed uniformly after a transport time of 20 h . Previous crown ether-based Pb2+-selective phase transport studies using comparable BLM-systems report similar mass transfer values without differences of orders of magnitude [9, 10].
Effect of using different solvents as BLM
Since solvents can affect the stability of supramolecular complexes formed inside the membrane by their interactions with ions or molecules, it is important to study the effects of several apolar, high-density organic solvents with different dielectric constants, which could be used as BLMs. In order to prevent change in volumes of the liquid phases during the transport, they were saturated with each other before the measurements. The solvents used as BLMs and the obtained transport results are summarized in Table 3.
Based on the results, dichloromethane was used as a BLM for further studies. In general, the use of halogenated aromatic solvents significantly reduced the efficiency of the transport. Tetrachloroethylene can be a good alternative to dichloromethane if the separation process requires a longer time, as almost the same efficiency could be achieved with this solvent as a BLM and due to its reduced tension, no change in the volume of the membrane phase is expected even over a longer period of time.
Membrane saturation
The Pb2+-content of the membrane is the sum of the free Pb2+ dissolved in the membrane and the Pb2+ bound by the carrier molecules. The total amount of Pb2+ inside the membrane was determined at each 30-min-interval according to the described procedure (see Subsection 2.1.). The results are presented in Fig. 5.
Time dependence of Pb2+-content in the membrane; the membrane saturation percentage value refers to the amount of Pb2+ inside the membrane regarded to the molar amount of dissolved carrier molecules (T = 20 °C, pH = 7, c (Pb2+ in source phase) = 1 mol/L, c (carrier in BLM) : c (Pb2+ in source phase) = 1 : 25, acetate counterion)
The membrane reached saturation within a relatively short period of time, thus the Pb2+-flux is mainly influenced by the complex dissociation at the receiving phase-membrane interface. The first step of the transport can be equivalent to an extraction-type separation, which is followed by a facilitated transport through the membrane, and finally an interfacial complex dissociation takes place, which is also the rate-determining step of the whole process. Additionally, in accordance with the extraction experiments in the case of single-component aqueous solutions, it is also seen, that the Pb2+-content of the membrane is determined by the amount of the dissolved carrier and the presence of the metal ions within the BLM is only possible by binding to transport molecules. Membrane saturation values close to 100% confirm the previously determined 1 : 1 complex-stoichiometry of the crown ether analogues for Pb2+ [19].
Effect of the presence of polar organic solvents
We thought it is worth investigating the effect of the presence of polar organic solvents, since they are often present as contaminants in liquid phase applications. These solvents are able to participate as cosolvents in the BLMs and can improve membrane permeability with maintaining selectivity. Fig. 6 shows the kinetics of the transport using an aqueous source phase containing 5 or 10% ethanol as a polar cosolvent.
The results show that even 5% of a polar cosolvent increased the permeability of the membrane significantly, thereby suppressed carrier facilitated transport. The altered transport mechanism of the ions is also supported by the changing of the kinetics from a linear to an exponential (R2 = 0.99) relationship. In experiments with alkali metal ions, a similar increase in membrane permeability was observed, thus the presence of polar cosolvents leads to a loss of selectivity. As the process is highly sensitive to solvent contaminants, this fact should be taken into consideration when designing BLM-based separation methods.
Effect of counterions
The effect of different counterions on the efficiency of the transport was also studied. If the source phase contained Pb(NO3)2 instead of Pb(CH3COO)2, no significant change was observed. This fact suggests that the differences in lipophilicity of various salts do not have a significant effect on the transport. On the other hand, it is consistent with the statement that the rate-determining step of the process is the dissociation of the Pb2+ - carrier molecule complex instead of the interfacial extraction or of the membrane transition.
This property may also be triggered by the tendency of crown ethers to ligand aggregation, especially on interphases [32, 33]. The aggregation of the carrier molecules within the membrane at the receiving phase interface may contribute to the inhibition of material transfer.
From this observation it also follows that during the membrane transition, the movement of ions is mainly mediated by transport molecules. In this case, the counterions travel as companions with the positively charged complexes. Due to the strong agitation, the complexes dissociate several times inside the membrane and then new associations are formed. If the motion of the ions was mainly governed by passive diffusion—as in many stationary LM- containing applications, such as ion-selective electrochemical or optical membranes—differences in lipophilicity caused by different counterions would presumably influence the transport.
It is known, that the membrane-receiving phase interface plays an important role in the membrane transport, as the metal ion - carrier complex is likely to accumulate in the membrane, consequently causes a decrease in the ion-flux [15]. Since the rate-determining step is the membrane-receiving phase transition, the efficiency can be improved in this step. Selectivity is provided by both the selective extraction at the source phase-membrane interface and the selective membrane transport by the carrier molecules, thus precipitation offers an alternative to improve the efficiency by inducing complex dissociation at the receiving phase-membrane interface without losing selectivity.
The ionic balance was successfully shifted to the direction of the receiving phase by replacing the it with 1 mol/L solutions of various acids (HNO3, H2SO4, HCl) or base (NaOH) (Table 4). The results for acetate salt (see the first line of Table 4) was obtained by using distilled water as a receiving phase in accordance with the previous measurements.
Improved transport efficiency could be achieved by precipitation (in the case of NaOH, HCl or H2SO4) or by acidifying the receiving phase (replacing it with HNO3, HCl or H2SO4). Carrier molecules become protonated under acidic conditions which leads to a faster dissociation of the Pb2+ - crown ether complexes at the membrane interface. Furthermore, in the case of H2SO4 and HCl precipitation also takes place, which facilitates the transport of metal ions and increases the concentration gradient between the aqueous phases by reducing the interfacial ion concentration in the receiving phase. The highest transport rate could be achieved by using these acids, since both of the mentioned transport enhancing effects take place.
It is noteworthy to mention that theoretically, the precipitation at the interface of the receiving phase—if the precipitate is sufficiently insoluble in water—could provide the maximum achievable concentration gradient during the transport. Thus, the total content of the source phase could be transported to the receiving phase with shifting the ionic balance.
Effect of temperature, pH and concentration of the carrier in the membrane
The optimization of temperature, pH and concentration parameters was performed together in an experimental design matrix by varying the 3 variables according to a full-factorial design. The factors and their investigated levels are constructed in Table 5. The effect of the concentration of the carrier in the membrane and the metal salt concentration of the source phase were examined together by expressing them as the ratio of these two concentrations (cr), respectively as reported earlier. Changes in the concentration ratio were adjusted by varying only the content of the carrier in the membrane.
The results were plotted on surface diagrams as shown in Fig. 7. Based on the calculated statistical characteristics (ANOVA), all of the 3 examined parameters had a significant effect on transport. The fitted nonlinear model proved to be adequate. Additional information related to the experimental design, such as measurement results, the statistical analyses of the data as well as the investigation of the normality, constant variance and independence of the fitted nonlinear model can be found in the Supplementary Material.
Surface diagrams as results of parameter optimization by a full-factorial experimental design, A: \({J}_{{\mathrm{Pb}}^{2+}}\) with cr adjusted to its ‘medium’ level, B: \({J}_{{\mathrm{Pb}}^{2+}}\) with pH adjusted to its ‘medium’ level, C: \({J}_{{\mathrm{Pb}}^{2+}}\) with T adjusted to its ‘medium’ level
Increasing the concentration ratio of carrier molecules had a positive effect on the transport. This is not surprising, as it allows the membrane transition of water-soluble ions to take place faster.
The optimum of the temperature was within the studied range and was found to be 15 °C. In our opinion, this fact indicates that the transport can also take place in the opposite direction between the two aqueous phases. Thus, the ion flux toward the aimed transport direction has an optimum temperature, above which the reverse transport becomes more favourable.
The effect of pH was significant only in the case of changing the pH of the receiving phase. In the case of using an acidic source phase the obtained values were equal to those of neutral pH. This observation also supports that the rate-determining step of the process is the material transfer through the receiving phase-membrane interface. Since if the pH of one aqueous phase was changed, the pH of the other aqueous phase remained unchanged, so proton transport did not take place.
Changes in pH in both directions from the neutral value accelerate the transport. In the case of pH values in the alkaline range, the increased efficiency is caused by precipitation with hydroxide ions, as suggested previously. The positive effect of the acidic medium on transport is presumably due to the protonation of the basic heteroaromatic nitrogen of the carrier in the presence of the increased proton concentration in the receiving phase. Carrier molecules become protonated upon reaching the membrane surface. Protonation makes the nitrogen lose its electron donor ability, leading to the loss of the most significant attractive intermolecular interaction within the complex. Finally, the complex dissociates and the carrier molecule releases the Pb2+, which as a result enters the receiving phase. Fig. 8 shows the suggested mechanism of the phase transport.
Based on the results, the use of an acidic receiving phase may also be a useful alternative to shifting the ion-balance in ionizable carrier-based separation.
Pb2+-selectivity of the BLM-technique under competitive conditions
The BLM-based separation studies were also performed under competitive conditions using a source phase with the composition shown in Table 1, with a molar ratio of 1:25 = crown ether : Pb2+-salt and at 15 °C (according to the parameter optimization), using neutral pH. Although the lowest efficiency of transport was achieved at pH = 7, acidifying or alkalizing the receiving phase influenced only the effectiveness of the complex dissociation, which does not belong to the selectivity-determining steps of the process. Therefore, the use of neutral pH was advantageous for studying the selectivity under competitive conditions. The measured values were corrected in the same manner as described in the case of the extraction experiments (see Subsection 3.1.). The ionic composition in the receiving phase was characterized using flux and selectivity factors after a transport time of 20 h. The results obtained by using an aqueous source phase with a total ion concentration of 860 mmol/L are shown in Table 6.
Based on the determined flux values, the amount of Pb2+ in the receiving phase after the transport decreased with approximately 71% compared to the experiments using a single-component source phase containing only Pb2+ under the same conditions. Thus, the efficiency of the process was reduced to less than one-third due to the competition. (Since the carrier-mediated transport depends primarily on the cr ratio, in the case of highly selective procedures we would expect nearly the same Pb2+-flux if the same cr ratio was adjusted.) Although, unlike the extraction separation, the Pb2+-selectivity remained, but it was not significant (the initial metal ion fluxes approached the flux of Pb2+ in several cases, resulted in a relatively low selectivity toward Pb2+: 0.01 < |Δα| < 0.73). In the case of multicomponent systems with a relatively high total concentration of competing ions the transport of the less preferred ions also takes place, indicating the complexation of these ions by the carrier molecules. As the ionic strength influences the complexation, a relatively high concentration of the source phase is expected to increase the probability of the complex formation with the competing ions, thereby improving the effectiveness of their carrier facilitated transport. It also suggests that the differences in selectivities are mainly due to the differences between the kinetic stabilities of the complexes formed with different metal ions inside the membrane. The crown ether also forms complexes with the other metal ions as they can pass through the membrane and they can also inhibit Pb2+-transport by reducing the number of carrier molecules, which can interact with Pb2+, but these complexes dissociate faster than Pb2+-complexes, thus other metal salts are transported less efficiently within the membrane.
In order to reduce the probability of complex formation with competing ions, the effects of diluting the source phase were examined. The source phase was diluted 10-fold and the transport was carried out using an unchanged ratio of 1 : 25 = carrier : Pb2+ under the same conditions as reported. The results are shown in Table 7.
In the case of the diluted source phase, the competitive inhibition on the efficiency of the transport was weaker. The presence of the competing ions reduced the initial Pb2+-flux by less than half compared to the single-component Pb2+-containing system. Selectivity was significantly improved compared to the competitive transport studies using a source phase with a total ion concentration of 860 mmol/L (0.50 < |Δα| < 1.00).
This trend was further investigated by a 100-fold dilution of the applied multicomponent source phase with a total ion concentration of 860 mmol/L (Table 8).
Using relatively low total ion concentrations of the source phases, the process proceeded with an excellent Pb2+-selectivity (0.99 < |Δα| in all cases). Moreover, the efficiency of the transport was reduced only to 2/3 of the Pb2+-flux compared to the single-component systems under the same conditions, allowing efficient and selective separation of similarly diluted multicomponent systems applying this BLM-technique.
For a better interpretation, the tendency of the initial Pb2+-flux and the Pb2+-selectivity under competitive conditions as a function of the total ion concentration of multicomponent systems are also summarized in Fig. 9.

source phase (cs total); A: Decrease of Pb2+-flux as a function of cs total (‘’ refers to the initial Pb2+-flux under competitive conditions, while ‘’ refers to the Pb2+-flux in single-component system), B: The ratio of Pb2+-flux regarded to the total ion flux of the system, a characteristic of selectivity as a function of cs total (‘’ refers to the total ion flux through the membrane under competitive conditions)
The tendency of the initial Pb2+-flux and the Pb2+-selectivity under competitive conditions by varying the total ion concentration of the multicomponent \({J}_{{Pb}^{2+} comp.}\)\({J}_{{Pb}^{2+} sing.}\)\({J}_{comp. total}\)
Both Pb2+-flux and Pb2+-selectivity decreased as a function of the total concentration of ions in the source phase, which observation also confirms the complex formation with various competing ions inside the membrane and assumes the dependence of the kinetic stabilities of the complexes on the ionic strength. Since ionic strength has an effect on complexation, a higher ionic strength results in a longer average lifetime of the complexes inside the membrane. As a result, the transport rate of the competing ions also increased. Based on the results, both the efficiency of the transport and the selectivity of the BLM-system could be significantly improved by diluting the multicomponent solutions before separation, as the membrane transport is influenced by the concentration gradient in addition to the cr ratio. The preference of the carrier toward various competing ions could be minimized by reducing the concentration gradient between the phases, due to the decreasing ionic strength, that presumably resulted in a decreasing kinetic stability of the complexes.
Conclusion
A detailed study on a liquid-liquid extraction- and a BLM-technique-based separation using a previously reported Pb2+-selective acridono-18-crown-6 ether selector molecule was performed.
The results of liquid-liquid extraction studies showed that the lipophilic macrocycle formed a reversible complex with Pb2+ with a 1:1 complex-stoichiometry, allowing a rapid separation of metal ions by making the heavy metal salts soluble in the organic phase. The metal ion content of the organic phase after the extraction was directly proportional to the amount of the selector molecules dissolved in the organic phase. As the results of the extraction experiments showed a large standard deviation and the selectivity of the process was lost under competitive conditions, the applied selector molecule proved to be unsuitable for conventional liquid-liquid extraction-based heavy metal ion separation.
LM-based separation was also studied by applying a widely used BLM-cell-type apparatus. We examined the effects of the following factors: stirring speed, quality of apolar organic membranes, counterions of Pb2+, pH of the aqueous phases, concentration of the source phase, concentration of the carrier in the BLM and temperature. The transport efficiency increased almost linearly in the initial stage of the process by increasing the stirring speed. The optimum stirring speed was chosen to be 250 rpm, since this was the highest value at which the results obtained from the transport were well-reproducible. Among the solvents used as apolar LMs, dichloromethane proved to be the most effective. The use of aromatic solvents significantly reduced the transport efficiency. Studies on membrane saturation showed that the rate-determining step of the separation process is the complex dissociation, which takes place at the LM-receiving phase interface. The BLM-technique proved to be extremely sensitive to the presence of added protic polar solvents. Pb2+-transport through the dynamically stirred BLM showed a weak dependence on counterions, however, the ionic balance of the process can be effectively shifted to increase transport efficiency with precipitating agents or by using an acidic receiving phase. These observations indicate that the membrane-receiving phase interface plays the most important role in the membrane transport. Since the accumulation of the metal ion - carrier complex in the membrane has a strong influence on the ion-flux, the selection of a suitable receiving phase is essential for an effective separation. The effects of temperature, pH and the ratio of the carrier concentration were examined together in a statistical experimental design. Increasing the ratio of the carrier to Pb2+, as well as acidifying or alkalizing the receiving phase resulted in an increased transport efficiency, while the optimum temperature was within the studied range, suggesting the reversibility of the process.
Studies on selectivity and efficiency of the transport in multicomponent systems were performed by adjusting the previously defined optimal conditions using a source phase, which contained a 9-component-metal-ion-mixture with a nearly equimolar initial ion concentration. Based on the results, the selectivity of the transport could be improved by diluting the source solutions of the multicomponent ion mixtures.
Based on the studies performed on the BLM-system, the sensor molecule can be suitable for the development of additional LM-based Pb2+-selective separation methods and this paper will hopefully provide conducive information for further macrocyclic carrier-based ion separation techniques in the future.
References
Maruyama, T., Hannah, S.A., Cohen, J.M.: Metal removal by physical and chemical treatment processes. J. Water Pollut. Control Fed. 47, 962–975 (1975). (https://www.jstor.org/stable/25038696)
Mandal, B., Ghosh, N.: Extraction chromatographic method of preconcentration and separation of lead (II) with high molecular mass liquid cation exchanger. Desalination 250, 506–514 (2010). https://doi.org/10.1016/j.desal.2009.06.050
Luo, Y.Y. Oxidation and dissolution of lead in chlorinated drinking water. Adv. Environ. Res. 1, 84–97 (1997). (https://ci.nii.ac.jp/naid/20001618058/en)
Sarı, A., Tuzen, M., Cıtak, D., Soylak, M.: Adsorption characteristics of Cu (II) and Pb (II) onto expanded perlite from aqueous solution. J. Hazard. Mat. 148, 387–394 (2007). https://doi.org/10.1016/j.jhazmat.2007.02.052
Fu, F., Wang, Q.: Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92, 407–418 (2011). https://doi.org/10.1016/j.jenvman.2010.11.011
Bahadir, T., Bakan, G., Altas, L., Buyukgungor, H.: The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzym. Microb. Tech. 41, 98–102 (2007). https://doi.org/10.1016/j.enzmictec.2006.12.007
Minhas, F.T., Solangi, I.B., Memon, S., Bhanger, M.I.: Kinetic study of an effective Pb (II) transport through a bulk liquid membrane containing calix[6]arene hexaester derivative as a carrier. Separ. Sci. Technol. 45, 1448–1455 (2010). https://doi.org/10.1080/01496391003652791
Diaconu, I., Ruse, E., Aboul-Enein, H.Y., Bunaciu, A.A.: Analytical applications of transport through bulk liquid membranes. Crit. Rev. Anal. Chem. 46, 332–341 (2016). https://doi.org/10.1080/10408347.2015.1064759
Lamb, J.D., Izatt, R.M., Garrick, D.G., Bradshaw, J.S., Christensen, J.J.: The influence of macrocyclic ligand structure on carrier-facilitated cation transport rates and selectivities through liquid membranes. J. Membrane Sci. 9, 83–107 (1981). https://doi.org/10.1016/S0376-7388(00)85119-4
Lamb, J.D., Christensen, J.J., Oscarson, J.L., Nielsen, B.L., Asay, B.W., Izatt, R.M.: The relationship between complex stability constants and rates of cation transport through liquid membranes by macrocyclic carriers. J. Am. Chem. Soc. 102, 6820–6824 (1980). https://doi.org/10.1021/ja00542a026
Mirea, C.M., Diaconu, I., Ruse, E., Serban, E.A., Clej, D.D., Popa, G.A., Popa, D.F., Nechifor, G. The removal of heavy metals using the bulk liquid membrane technique. National Research-Development Institute for Cryogenic and Isotopic Technologies – ICIT, Valcea, Romania, 19, 45–54 (2016). ISSN: 1582-2575 (http://dspace.incdecoind.ro/handle/123456789/1151)
Casanueva-Marenco, M.J., Galindo-Riano, M.D., Granado-Castro, M.D.: Coupled transport of Pb (II) ions through a bulk liquid membrane as a preconcentration method for saline natural waters. Curr. Anal. Chem. 14, 135–144 (2018). https://doi.org/10.2174/1573412913666170705095341
Raghuraman, B.J., Tirmizi, N.P., Kim, B.S., Wiencek, J.M.: Emulsion liquid membranes for wastewater treatment: equilibrium models for lead- and cadmium-di-2-ethylhexyl phosphoric acid systems. Environ. Sci. Technol. 29, 978–984 (1995). https://doi.org/10.1021/es00004a018
Yourd, E.R., Tyson, J.F.: Comparison of supported liquid membranes and solid-phase extraction for quantitative removal of lead from aqueous solutions. Can. J. Chem. 81, 1061–1069 (2003). https://doi.org/10.1139/v03-130
Gherasim, C.V.I., Bourceanu, G., Olariu, R.I., Arsene, C.: Removal of lead (II) from aqueous solutions by a polyvinyl-chloride inclusion membrane without added plasticizer. J. Membrane Sci. 377, 167–174 (2011). https://doi.org/10.1016/j.memsci.2011.04.042
Peterson, R.T., Lamb, J.D. in Rational Design of Liquid Membrane Separation Systems (Chapter 4 of ACS Symposium Series Vol. 642: Chemical Separations With Liquid Membranes ISBN: 9780841234475, doi: https://doi.org/10.1021/bk-1996-0642), American Chemical Society, 57–74 (1996). https://dopi.org/https://doi.org/10.1021/bk-1996-0642.ch004
Albaraka, Z.: Carrier-mediated liquid membrane systems for lead (II) ion separations. Chem. Pap. 74, 77–88 (2020). https://doi.org/10.1007/s11696-019-00868-w
Szalay, L., Farkas, V., Vass, E., Hollósi, M., Móczár, I., Pintér, Á., Huszthy, P. Synthesis and selective lead (II) binding of achiral and enantiomerically pure chiral acridono-18-crown-6 ether type ligands. Tetrahedron: Asymm., 15, 1487–1493 (2004). https://doi.org/https://doi.org/10.1016/j.tetasy.2004.03.024
Németh, T., Kormos, A., Tóth, T., Balogh, G.T., Huszthy, P.: Synthesis and cation binding of acridono-18-crown-6 ether type ligands. Monatsh. Chem. 146, 1291–1297 (2015). https://doi.org/10.1007/s00706-015-1541-5
Golcs, Á., Horváth, V., Huszthy, P., Tóth, T.: Fast potentiometric analysis of lead in aqueous medium under competitive conditions using an acridono-crown ether neutral ionophore. Sensors 18, 1407–1421 (2018). https://doi.org/10.3390/s18051407
Niu, Y., Han, X., Huang, L., Song, J.: Methylene blue and lead (II) removal via degradable interpenetrating network hydrogels. J. Chem. Eng. Data 65, 1954–1967 (2020). https://doi.org/10.1021/acs.jced.9b01134
Sharma, G., Kandasubramanian, B.: Molecularly imprinted polymers for selective recognition and extraction of heavy metal ions and toxic dyes. J. Chem. Eng. Data 65, 396–418 (2020). https://doi.org/10.1021/acs.jced.9b00953
Chen, Y., Zhao, H., Li, Y., Zhao, W., Yang, X., Meng, X., Wang, H.: Two-step preparation of an amidoxime-functionalized chelating resin for removal of heavy metal ions from aqueous solution. J. Chem. Eng. Data 64, 4037–4045 (2019). https://doi.org/10.1021/acs.jced.9b00402
Chu, Y., Zhu, S., Wang, F., Lei, W., Xia, M., Liao, C.: Tyrosine-immobilized montmorillonite: an efficient adsorbent for removal of Pb2+ and Cu2+ from aqueous solution. J. Chem. Eng. Data 64, 3535–3546 (2019). https://doi.org/10.1021/acs.jced.9b00304
Younes, A.A., El-Maghrabi, H.H.: Removal of lead ions from wastewater using novel Schiff-base functionalized solid-phase adsorbent. Separ. Sci. Technol 55, 1589–1602 (2020). https://doi.org/10.1080/01496395.2019.1604758
Taylor-Pashow, K.M., Pribyl, J.G.: PolyHIPEs for separations and chemical transformations: a review. Solvent Extr. Ion Exch. 37, 1–26 (2019). https://doi.org/10.1080/07366299.2019.1592924
Singh, S.K., Asfari, Z., Trébouet, D.: Recent advances in the extraction of target metal ions with liquid membrane processes incorporating macrocycle carriers. Sep. Purif. Rev. 42, 28–86 (2013). https://doi.org/10.1080/15422119.2012.681744
Ramkumar, J., Chandramouleeswaran, S. A perceptive on bulk liquid membrane: a brief review. Indian J. Adv. Chem. Sci. 3, 293–298 (2015). (http://www.ijacskros.com/)
Kazemi, S.Y., Hamidi, A.S.: Competitive removal of lead (II), copper (II), and cadmium (II) ions through a bulk liquid membrane containing macrocyclic crown ethers and oleic acid as ion carriers. J. Chem. Eng. Data 56, 222–229 (2011). https://doi.org/10.1021/je1007108
Tarahomi, S., Rounaghi, G.H., Chamsaz, M.: Study of competitive transport of some heavy metal cations across bulk liquid membranes containing phenylaza-15-crown-5 and cryptand [2.2.2.] as carriers using flame atomic absorption spectrometry. Russ. J. Appl. Chem. 88, 1219–1228 (2015). https://doi.org/10.1134/S1070427215070174
Riddick, J. A., Bunger, W. B., Sakano, T. K. in Organic solvents: Physical properties and methods of purification (in the series: Techniques of Chemistry), Vol. 4 (Ed.: A. Weissberger), Wiley-Interscience: New York, New York, 1344–1400 (1986). ISBN: 0471084670
Horwitz, E.P., Dietz, M.L., Fisher, D.E.: Extraction of strontium from nitric acid solutions using dicyclohexano-18-crown-6 and its derivatives. Solvent Extr. Ion Exch. 8, 557–572 (1990). https://doi.org/10.1080/07366299008918017
Mohapatra, P.K., Lakshmi, D.S., Bhattacharyya, A., Manchanda, V.K.: Evaluation of polymer inclusion membranes containing crown ethers for selective cesium separation from nuclear waste solution. J. Hazard. Mat. 169, 472–479 (2009). https://doi.org/10.1016/j.jhazmat.2009.03.124
Acknowledgements
Thanks to Dániel Ster for his valuable technical assistance during this work. The authors express their thanks to Panna Vezse for her help in interpreting the results. The financial supports of the National Research, Development and Innovation Office (Grant Number: K128473) and those of the New National Excellence Program (ÚNKP-19-3) of the Ministry for Innovation and Technology are gratefully acknowledged.
Funding
Open access funding provided by Budapest University of Technology and Economics. The funding institutions had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
Conceptualization: ÁG; Formal Analysis: ÁG; Funding acquisition: PH; Investigation: ÁG, LB; Methodology: ÁG, LB; Project administration: TT; Resources: LB, PH, TT; Supervision: PH, TT; Writing—original draft: ÁG; Writing—review and editing: LB, PH, TT.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10847_2020_1036_MOESM1_ESM.docx
Table S1 Results of the 33-type full-factorial experimental design for parameter optimization of the BLM-transport, Table S2 ANOVA-table of the results of the experimental design at a significance level of 95 %, Table S3 ’Effect estimates’ table of the results of the experimental design at a significance level of 95 %, Fig. S1 The ‘normal probability plot’ for checking the normality assumption, Fig. S2 Predicted against residual values, Fig. S3 Raw residuals against case numbers. (DOCX 49 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Golcs, Á., Bezúr, L., Huszthy, P. et al. Liquid-liquid extraction and facilitated membrane transport of Pb2+ using a lipophilic acridono-crown ether as carrier. J Incl Phenom Macrocycl Chem 99, 117–129 (2021). https://doi.org/10.1007/s10847-020-01036-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01036-4