Abstract
A preparation and characterization of mixtures consisting of conductive polyaniline dispersed in polystyrene (PS) matrix are described. PANI was obtained by interfacial oxidative polymerization of aniline in the presence of sulfosalicylic acid, giving the protonated form the oxidation state of emeraldine (PANI-SSA). PS was synthesized by emulsion polymerization in the presence of sodium dodecyl sulfonic (SDS) as an emulsifier. Three methods were used for the preparation of PANI-SSA/PS mixtures: (1) green in-situ polymerization of aniline in the presence of SSA as a protonating agent and ammonium persulfate ((NH4)2S2O8 as an oxidant (Method I); (2) mixing PS with PANI-SSA dissolved in sulphuric acid at the temperature of 150 °C (Method II); and (3) mixing PS dissolved in dichloromethane (DCM) with PANI-SSA at room temperature (Method III). The prepared mixtures of different PANI-SSA content were characterized by FTIR, TGA, XRD and by electrical conductivity measurements. Mixing at high temperature (Method II) led to the formation of sulfonated polystyrene (PSS) or even three dimensional resins (through cross-linking of PSS) in which highly crystalline PANI particles were dispersed.
Graphic abstract
The nanocomposites PS/PANI were elaborated by green in situ chemical polymerization (Method I) and by mixing with heating in the presence of sulphuric acid (Method II) at room temperature in the presence of dichloromethane at various percentages of PANI (Method III). These realized samples reported a high degradation temperature of PS which confirm that the PANI may even improve an inflammation property of polystyrene. The obtained composites proved a semi-crystalline character with a high level of crystallinity and the electrical properties range between 10–3 and 10–4 S/cm. These concepts are promising for the antistatic domain and charge dissipation domain.

Similar content being viewed by others
Introduction
Polymers have long been considered as insulating materials and used as main components of building materials, clothing, and packaging materials. In electrical engineering and electron technology they have been applied only as insulators, dielectrics and photoresists. Although low molecular weight of organic conductors has been known (Ferraris et al. 1973) strong acceleration of the research in this domain started in 1977 after the discovery of the conductive form of polyacetylene—the simplest polymer (Chiang et al. 1977). For this discovery Shirakawa, Heeger and MacDiarmid were awarded the Nobel Prize in chemistry in 2000. Polyacetylene is the first example of a new class of materials, namely “Intrinsic conductive organic polymers” (ICPs). Polyaniline is another example of this class of materials which can be conductive by an appropriate doping process, which can be redox or acido-base in nature. Polyaniline is one of the most studied conductor polymers due to its environmental stability and the simplicity of its preparation since it can be obtained by direct oxidation of aniline [see for example ref. (Wu et al. (2007)]. However, significant drawbacks of ICPs, in general, and polyaniline, in particular, are their difficult processability, fragility and poor mechanical properties. For these reasons ICPs, are frequently mixed with conventional thermoplastic polymers (PP, PMMA, PVC …) to obtain polymeric materials combining high electrical conductivity with good mechanical properties (Sun et al. 2017).
5-Sulfosalicylic acid has a particular interest as a doped agent of polyaniline. As proven by several studies, this dopant demonstrates the ability to induce the formation of nanoscale polyaniline structures in interfacial (Wang et al. 2010a) and free oxidative polymerizations (Chatterjee et al. 2013b; Janošević et al. 2008) as well as in electrochemical polymerizations (Chatterjee et al. 2013a). Moreover, the presence of 5-sulfosalicylate as anion dopant improves the capacity of processability PANI in solution (Trivedi and Dhawan 1993).
In the majority of articles on PANI mixtures with conventional insulating polymers, the mixture of protonated PANI with polystyrene was described (Chiang et al. 1977; Chatterjee et al. 2013a; Barthet et al. 1998; Kohut-Svelko et al. 2005; Abu and Aoki 2005; Kazimierska et al. 2009; Yang and Liau 2009; Li et al. 2011; Blinova et al. 2010; Zhang et al. 2012; Bae et al. 2003; Chen et al. 2009; Zhao et al. 2011; Yan et al. 2011; Ghasemi and Sundararaj 2012; Liao 2018). The blends were obtained by in-situ polymerization of aniline in the presence of appropriate protonating agents such as hydrochloride acid (HCl), dodecyl benzene sulfonic acid (DBSA) and lauryl sulfuric acid (LSA) dissolved in chloroform, ethanol, toluene, tetrahydrofuran *and other suitable solvents. In the majority of cases core–shell forms were obtained with different conductivity values due to variation in the level of polyaniline protonation and the morphologies of the conductive (PANI) and matrix (PS) phases.
In this work mixtures of 5-sulfosalicylic acid protonated PANI with polystyrene were prepared and studied. In particular, PANI-SSA was synthesized by interfacial chemical oxidative polymerization of aniline in the presence of ammonium persulfate (APS) as an oxidizing/polymerizing agent and sulfosalicylic acid (SSA) as a protonating agent. The resulting PANI-SSA of ordered morphology showed promising properties for industrial applications (Li et al. 2016). Polystyrene was synthesized by emulsion polymerization. PS/PANI-SSA mixtures were prepared by three different methods: (1) green in situ preparation of PANI-SSA in the presence of polystyrene; (2) by mixing PS and PANI-SSA at high temperatures and (3) by mixing PS and PANI-SSA at room temperature. The obtained mixtures were characterized by different techniques, including: FTIR, TGA, XRD and four-probe conductivity measurements to determine their structure, crystallinity, electrical properties and thermal stability. These researches were vital for their intended application as packaging materials and for the dissipation of electrical charge (Pud et al. 2017).
Methodologies
Materials
Sstyrene monomer (≥ 99.5%) was purified before being used to remove the inhibitor; aniline (≥ 99.5%), ethanol (≥ 99.8%), acetone (≥ 99.9%), ammonium persulfate (APS) (≥ 98%), sodium dodecyl sulfate (SDS) (≥ 99%), sulfosalicylic acid (SSA) (95%), dichloromethane (CH2Cl2) (≥ 99.8%), sulfuric acid H2SO4 (99.99%) and dimethyl sulfoxide (DMSO) (≥ 99.7%) were used as received. All chemicals were purchased from Sigma-Aldrich Co., Ltd., Germany.
Methods
Synthesis of polyaniline by interfacial chemical oxidation of aniline
PANI was synthesized using a molar ratio of sulfosalicylic acid/aniline = 1.25 and ammonium persulfate/aniline molar ratio = 1.0. In a typical synthesis, a three-necked flask was loaded with 3 ml of aniline and 30 ml of chloroform; 10.18 g of the 5-sulfosalicylic acid in 46 ml of distilled water. The flask was then placed in an ice bath and held at one of two different temperatures: − 2 °C and 17 °C, and with rigorous agitation. 9.1 g of ammonium persulfate dissolved in 11 ml of distilled water was added drop-wise; green-coloured colloidal PANI-SSA solution appeared after 30 min. The product was allowed to stay in solution overnight. PANI-SSA was then washed once with a mixture of distilled water/ethanol and successively with distilled water until the total elimination of all traces of free acid. It was then recovered by simple filtration and finally dried in an oven at 50 °C for 48 h. To choose the most appropriate sample of synthesized PANI-SSA, solubility tests were carried out for PANI-SSA prepared at − 2 °C and that prepared at 17 °C. For this purpose, they were added to DMSO and agitated for 2 days. The sample synthesized at 17 °C exhibited rather poor solubility, whereas PANI-SSA synthesized at − 2 °C was fully dissolved in DMSO.
Synthesis of polystyrene by emulsion polymerization
The polymerization method applied was a modification of the procedures previously described in the literature (Chang et al. 1998; Chu et al. 2007; Lin et al. 2011). In particular, the following polymerization conditions were changed: the critical micellar concentration (CMC), the SDS value, the amount of the initiator and the reaction temperature. The polymerization was carried out under constant agitation in a three-necked flask immersed in a water bath. The flask was equipped with a back condenser and a thermometer. For the polymerization 10 ml of styrene, 0.23 g of SDS (CMC = 8.10–3 M) dissolved in 90 ml of distilled water and 0.1 g of APS dissolved in 10 ml of distilled water were added to the flask. The polymerization was carried out at 70 °C for 5 h under nitrogen (N2) flow. At the end of the reaction, 150 ml of ethanol was added to the recovered solution and the polystyrene/ethanol milky suspension was put in 300 ml of n-heptane with agitation to precipitate the synthesized product. The purification of PS was carried out by subsequent washing with distilled water (removal SDS used). Thereafter, polystyrene was dried in an oven at 50 °C for 2 days. The final product was recovered as a white powder.
Three methods of PS/PANI-SSA blending
Green in-situ polymerization (Method I)
In this method PS/PANI-SSA mixture was prepared by in-situ green polymerization of aniline in the presence of polystyrene (see Fig. 1). In a typical polymerization ammonium persulfate (NH4)2S2O8 was used as an oxidizing agent (Li et al. 2016), with a mole ratio of SSA/aniline = 1.25 and ammonium persulfate/aniline molar ratio of the order of 1. In a three-necked flask immersed in an ice bath and equipped with an agitator 0.01 ml of aniline with 1 g of polystyrene were dispersed in 25 ml of distilled water. The mixture was then agitated for 1 h. Thereafter, 0.34 g of SSA dissolved in 5 ml of H2O and 0.025 g of APS dissolved in 3 ml of H2O were added. The reaction was continued with agitating for 5 h at − 0.3 °C. At the end, the nanocomposite was recovered after stirring for 18 h at room temperature.
Preparation by mixing PS and PANI-SSA at elevated temperature (Method II)
PS/PANI-SSA mixture (2% by weight of PANI-SSA) was prepared by mixing in sulphuric acid (Andreatta et al. 1988; Abd-Razak et al. 2015). Figure 2 shows the details of the blend preparation and the synthesis mechanism. The mixing temperature chosen in the first experiment was 170 °C. After consecutive cooling, a phase separation was observed in the resulting film. To avoid this phenomenon, we chose to work in a temperature range between 150 and 160 °C. In a typical preparation, 0.3 g polystyrene was placed into a beaker immersed in an oil bath equipped with an agitator and a thermometer. The whole bath was gradually heated to 150 °C. In parallel, PANI-SSA (2% by weight with respect to the mass of PS) was dissolved in 2 ml of sulphuric acid (H2SO4) and then added to the beaker with PS at 157 °C under rigorous agitation (1 h). The resulting blend formulation was subsequently spread on a glass plate. After first drying, the obtained film was washed several times with distilled water to remove the sulphuric acid traces and dried again.
Preparation by mixing PS and PANI-SSA at room temperature (Method III)
Mixtures of varying PANI-SSA contents in PS were prepared, namely containing 2%, 4%, 6%, 8% and 10% by weight of PANI-SSA compared to PS matrix. Figure 3 illustrates the mixing procedure. Blends were formed by mixing of 0.16 g polystyrene dissolved in 2 ml of dichloromethane with appropriate amounts of PANI-SSA also dissolved in dichloromethane. The mixtures were processed at room temperature for 2 h with constant agitation. The resulting blends were spread over glass plates, dried in the open air and then washed repeatedly with distilled water.
Characterizations techniques
FTIR-ATR infrared spectroscopic investigations were performed using a Perkin Elmer Spectrometer in the range of 400 to 4000 cm−1 with a resolution of 2 cm−1; some samples were analysed in the ATR mode and others using the KBr pellets technique.
TGA scans were performed in an inert medium (inert gas: nitrogen) at temperatures up to 600 °C with a heating speed of 10 °C min−1. The tests were performed on a METELER TGA apparatus (TGA2, Mettler Toledo Star system).
XRD investigations were carried out at room temperature, using a XPERT-PRO diffractometer, in the reflection geometry (Bragg–Brentano configuration) with the scans range from 2θ = 0° to 50° (copper cathode, Kα1, λ = 1.5406 Å). The lattice spacings were calculated using the Bragg relationship:
where θ is the half-angle of the scattering peak, n the diffraction order and λ the wavelength of the X-rays (1.5406 Å).
The crystallites sizes (L) were estimated using the Scherrer’s formula which yields the average sizes of the crystallites in a given direction, normal to the corresponding lattice plane.
FWHM is the total width at half the maximum of the diffraction peak, 0.89 is the Scherrer’s constant, λ is the wavelength of the X-rays, L is the crystallite size (Å) and θ is the diffraction angle (rad). The crystallinity degrees (Xcr) for the samples were calculated from the intensities corresponding to the crystalline and amorphous halo, according to the following formula:
where Xcr is the crystallinity degree, Icr is the total intensity of the crystalline peaks and Ia the intensity of the amorphous scattering peak.
The electrical conductivities of the samples were measured at room temperature by the standard four-probe method using a Jandel four-probe head with a constant current source and a digital voltmeter (model RM3000 test unit, Jandel Engineering Ltd., UK). This equipment allows for the measurements of resistances ranging from of 1 milliohm to 5 × 108 ohms with a precision of 0.3%. The conductivity, δ (S/cm), was calculated by applying the following relationship:
where δ is the electrical conductivity (S/cm), Φ the resistivity (Ω/cm), s is the spacing between the probes (cm) and Rsq is the square resistance (Ω). The conductivities were measured for samples in a form of films.
Results and discussion
FTIR spectroscopic studies
Polystyrene
A representative spectrum is shown in Fig. 4. The characteristic peaks of the C–H aromatic stretching vibration at 3024 cm−1 and the C–H methylene groups stretching vibrations at 2919 and 2844 cm−1 can easily be distinguished. Four bands originating from the aromatic C=C stretching vibrations are observed at 1602, 1580, 1494 and 1453 cm−1. Other bands, at 752 and 695 cm−1 are attributed to the out of plane vibrations of the C–H group. These bands confirm the presence of the di-substituted aromatic rings in the macromolecule of PS (Lu et al. 2002).
Sulfosalicylic acid
Figure 5 shows a representative spectrum of free SSA. The absorption bands at 1640 cm−1, 1546 cm−1, at 1395 cm−1 and 1317 cm−1 are characteristic of the deformations in the carbozylic group-aromatic ring moiety attached to an aromatic ring. Clear bands at 1052 cm−1, 946 cm−1 and 901 cm−1 are diagnostic of the presence of the sulfonic group. The band at ~ 865 cm−1 corresponds to C–H deformations in meta-substituted aromatic rings. Finally, a band located at 815 cm−1 corresponds to C–H deformations in para-substituted aromatic ring (Rehman et al. 2013).
PANI-SSA
Figure 6 shows an FTIR spectrum of PANI doped with SSA. The peaks at 1569 cm−1, 1490 cm−1, 1640 cm−1 and 1296 cm−1 are affected by the stretching vibrations of the C=C, C=N and C–N of the quinoïd and the benzoïd rings, respectively. The peak at 1132 cm−1 is attributed to C–H stretching in quinoïd rings, whereas the peak at 809 cm−1 is attributed to C–H stretching vibrations in the benzoïd rings. A distinct band at 885 cm−1, attributed to the C–H vibrations in the SSA ring, confirms the doping of PANI-SSA by the sulfosalicylic acid. The peaks at 1094 cm−1 and 1111 cm−1 originate from the presence of the Ar–SO3− moiety, confirming again the presence of the sulfosalicylate anion in doped PANI. The band observed at 1378 cm−1 is associated with the vibrations of the RCOO− group of SSA (Chatterjee et al. 2013a, b; Wang et al. 2010b), indicating the doping of PANI by the two acid functions of the protonating agent. Finally, the peak at about 1243 cm−1 confirms the protonated state of the PANI chains (C–N+) (Chatterjee et al. 2013a; Dong et al. 2016; Ertit Taştan et al. 2016; Marjanović et al. 2010).
PS/PANI-SSA blend prepared by green in-situ polymerization
In Fig. 7, the FTIR spectra of pure PS and PS/PANI-SSA (1 wt%), synthesized by the green in-situ polymerization, are compared. Although the PS/PANI-SSA spectrum is dominated by peaks originating from polystyrene, bands diagnostic of protonated PANI and are distinguished at 1225 cm−1 and 1027 cm−1 as well as peaks originating from the sulfosalicylate anion at 1714 cm−1 (Naar et al. 2012; Neetika et al. 2012; Arasi et al. 2009).
PS/PANI-SSA blends prepared by mixing at elevated temperature
Similarly, as in the previous case, in the FTIR spectrum of PS/PANI-SSA (2 wt%) presented in Fig. 8, bands from all components of the mixture can be distinguished. In particular, peaks at 2939 cm−1 and 670 cm−1 can be attributed to PS, peaks at 1599 cm−1, 1492 cm−1, 1601 cm−1 and 650 cm−1 to the protonated chain of polyaniline while peak at 878 cm−1 to the sulfosalicylate anion (Chatterjee et al. 2013a, b; Wang et al. 2010b; Martins et al. 2003) (Marjanović et al. 2010). It should be noted that the presence of peaks at 1030 cm−1, 1122 cm−1 and 3355 cm−1 seems to suggest partial sulfonation of polystyrene. This is not unexpected taking into account the presence of sulfuric acid in the blending medium as well as high blending temperature.
PS/PANI-SSA blends prepared by mixing at room temperature
Figure 9 shows FTIR spectra of PS and PS/PANI-SSA blends with increasing contents of PANI-SSA: 2%, 4%, 6%, 8% and 10%. Although peaks characteristic of the polystyrene matrix dominates the spectra, bands originating from the protonated PANI chains and those characteristic of the sulfosalicylate anion appear at 1560 cm−1, 1470 cm−1, 1150 cm−1, 842 cm−1 and 907 cm−1, and grow in intensity with increasing content of PANI-SSA.
Thermogravimetric analysis
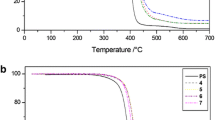
A representative thermogram of PS is shown in Fig. 10a. A single mass loss in the temperature range from 317 to 437 °C can be observed. The degradation temperature of PS may vary from 160 to 330 °C depending on its molar mass and the polymerization method applied (Lin et al. 2011). The experimentally measured degradation temperature for polystyrene synthesized in the frame of this research via emulsion polymerization indicates its relatively high molecular mass. PANI-SSA loses its mass at three steps: the first step, associated with the removal of adsorbed water, starts at 38 °C is practically ended at 109 °C with the total mass loss of 21%. The second mass loss occurs in the temperature range from 205 to 350 °C can be attributed to dopants’ loss (21% of the total mass loss). The third step starts at 430 °C and ends at 580 °C (ca. 15% mass loss), corresponding to the degradation of the polymer backbone (Trivedi and Dhawan 1993; Dong et al. 2016). The TG curve of PS/PANI-SSA is very similar to that of pure PS which is not unexpected taking into account small amount of PANI-SSA in this blend (1 wt%). It should be, however, noted that the value of the degradation temperature is shifted to higher temperatures as compared to the pure PS.
A representative thermogram of PS/PANI-SSA (2 wt%) blend prepared by mixing at elevated temperature is shown in Fig. 10b. Significant deterioration of the thermal stability can be noticed in this case, which can be provisionally attributed to partial sulfonation of the polystyrene matrix.
Martins et al. (2003) demonstrated that the weight loss in sulfonated polystyrene starts at ca. 200 °C. Thus, the shape of the obtained TG profile strongly indicates sulfonation of the matrix. Note also 42% of residual mass at 600 °C.
Figure 10c shows the TGA curves of PS/PANI-SSA blends of increasing PANI-SSA content (2 wt%, 4 wt%, 6 wt% 8 wt% and 10 wt %), prepared by mixing in dichloromethane solutions. Similarly, as in the case of the blend prepared by in situ polymerization (see Fig. 10a) the TG curve is dominated by the effect of PS decomposition with the contribution of PANI-SSA increasing with the rising of its content in the mixture. Again, the temperature of the decomposition onset is shifted to higher values as compared to the case of pure PS, indicating intermolecular interactions between the blend components slightly interfering the decomposition process.
X-ray diffraction results
X-ray scattering patterns of PS, PANI-SSA (− 2 °C) and three types of the prepared blends are presented in Fig. 11. As showed the Fig. 11 a pure PS is amorphous of large peak of halo at 2θ = 19°.By contrast, PANI-SSA (− 2 °C) is partially crystalline showing distinct bragg reflections at 2θ = 7.02°, 8.07°, 16.39°, 20.39° and 24.62° (see Fig. 11b and Table 1).
As it can be seen, the peak centred at 7.02°, attributed to the frequency along the polymer chain, is observed for PANI-SSA and also for PS/PANI-SSA (8 wt%) at 6.37° (Fig. 11c). It can be assigned as the periodic distance between the dopant and N atom on the main chains of the PANI. The broad band at 16.39° is related to the repeat units of the polyemeraldine chains and the periodicity parallel to the polymer chains of PANI, while the band at 24.62° is ascribed to the periodicity in the direction perpendicular to the polymer chain (Wu et al. 2015; Sekerak et al. 2018). The two strong and narrow peaks at 8.07° and 20.39° are also ascribed to periodicity parallel (Wu et al. 2015; Sekerak et al. 2018; Zhang et al. 2002). These results clearly demonstrate high crystallinity index of PANI-SSA. The X-ray pattern PS/PANI-SSA (1 wt%, in situ) shown in Fig. 11c. Presents a broad peak at 2θ = 7.21° accompanied by narrow peaks at 2θ = 14.07°, 16.93°, 18.54°, 22.15°, 24.86°, 25.63° and 27.79° indicating the presence of crystalline phase. X-ray scattering pattern of PS/PANI-SSA 8wt% shown in Fig. 11d shows two broad peaks at 12.89° and 30.35° ascribed to the presence of an amorphous phase. The narrow peaks centred at 5.09°, 6.37°, 16.56°, 18.25° and 19.72° are attributed to the crystalline zones.
Thus, it can be noticed that this blend is semi-crystalline with high degree of order due to the dopant structure. Figure 11e shows the diffraction pattern of PS/PANI-SSA 2 wt% prepared by mixing at high temperature in the presence of sulfuric acid. Several narrow peaks can be noticed at 2θ = 6.55°, 16.61°, 19.84°, 23.72° and 26.23° ascribed to periodicity in crystalline PANI (Zhang et al. 2002). Other narrow peaks appearing at 2θ = 38.27° and 44.54° can probably be assigned to a 3D network formed from the motifs of crystalline PANI surrounded by sulfonated polystyrene (Du et al. 2014). This result can rationalize the presence of 42% residual mass of PS/PANI-SSA 2 wt% at 600 °C, as evidenced by TGA (see Fig. 10b).
The crystallinity degree, Xcr, of PANI-SSA (− 2 °C) is about 24%, for PS/PANI-SSA 1 wt% in situ is equal to 98%, for the PS/PANI-SSA 8–69% and for PS/PANI-SSA 2 wt% is over 98%. It is worth noting noted that the blend prepared by Method I exhibits higher crystallinity than pure PANI-SSA. This may be due to different crystallites morphologies and sizes and due to several mechanisms of the supramolecular structure formation.
The average crystallite size of PANI-SSA (− 2 °C) is about 201 Å, for PS/PANI-SSA 1 wt% in situ is equal to 166 Å, for PS/PANI-SSA 8 wt% is equal to 177.951 Å and for PS/PANI-SSA 2 wt% thermal blend is about 173 Å.
The theoretical inter chain distance obtained using simulation Gauss View (Naar et al. 2013) is about 13.75 Å for PS/PANI-SSA 1% in situ nanocomposite (see Fig. 12a). Returning to the results included in Table 1, we find the experimental value of 12.57 Å at 2θ = 7.02°, which is confirmed by the results of FTIR indicating that the dopant is located between two tetra-aniline segments. For the PS/PANI-SSA 8% nanocomposite prepared at room temperature a distance is found of 17.32 Å. This is the exact experimental distance found at 2θ = 5.09° (Table.1). It is calculated for PANI doped from one side by SSA, as seen in Fig. 12b.
Another similar distance is found (17.37 Å) in Fig. 12c, attributed also to the same angle. It is assigned to the electrostatic interactions between the PS and the non-doped emeraldine form. This observation confirms that dichloromethane plays a role of a compatibilizer between polystyrene and polyaniline by electrostatic interactions ensured by hydrogen bonding as shown in Fig. 12b, c. The distance 6.86 Å derived from the XDR pattern (Table 1) of PS/PANI-SSA 8% (Table 1) is also confirmed by Gauss View simulations yielding at a distance of 6.84 Å (Fig. 12d). It is related to the distance between polyemeraldine base and polystyrene. Thus, the simulations are highly consistent with the experiment.
DC conductivity by four-probe measurements
The results of the conductivity measurements are summarized in Table2. First, it is important to mention that the conductivity of PANI-SSA synthesized at − 2 °C is higher than that of PANI-SSA synthesized at 17 °C. Thereafter, it is found that the blend prepared by is situ polymerization (Method I) exhibits a reasonable value of conductivity despite the fact that the conducting phase constitutes in this case only 1%. This result confirms that this preparation method results in a rather low percolation threshold favouring the electrical transport. Low percolation threshold is also found for blends prepared by solution mixing at room temperature (Method III). Finally, a high-temperature blending is observed for the blend prepared by the method II and gives interesting conductivity values.
Conclusion
Three methods of preparation conductive mixtures of PS with polyaniline protonated with 5-sulfosalicylic acid were developed, and all of them produce low threshold mixtures of electrical percolation and exhibit conductivities in the range of 5-sulfosalicylic acid. The process of mixing of PS and PANI-SSA at high temperature and in the presence of sulfuric acid resulted in partial sulfonation of polystyrene matrix which lowered the thermal stability of the blend, but did not affect, however, its electrical properties to a large extent. X-ray diffraction investigations indicated that the presence of PANI-SSA improves the crystallinity of the mixture. The experimental X-ray results turned out to be perfectly consistent with molecular simulations.
References
Abd-Razak SI, Wahab IF, Fadil F, Dahli FN, Md Khudzari AZ, Adeli H (2015) A review of electrospun conductive polyaniline based nanofiber composites and blends: processing features, applications, and future directions. Adv Mater Sci Eng 2015:1–19. https://doi.org/10.1155/2015/356286
Abu YM, Aoki K (2005) Corrosion protection by polyaniline-coated latex microspheres. J Electroanal Chem 583(1):133–139. https://doi.org/10.1016/j.jelechem.2005.05.014
Andreatta A, Cao Y, Chiang JC, Heeger AJ, Smith P (1988) Electrically-conductive fibers of polyaniline spun from solutions in concentrated sulfuric acid. Synth Metal 26(4):383–389. https://doi.org/10.1016/0379-6779(88)90233-0
Arasi AY, Jeyakumari JL, Sundaresan B, Dhanalakshmi V, Anbarasan R (2009) The structural properties of Poly (aniline)—analysis via FTIR spectroscopy. Acta Mol Biomol Spectrosc 74(5):1229–1234. https://doi.org/10.1016/j.saa.2009.09.042
Bae WJ, Jo WH, Park YH (2003) Preparation of polystyrene/polyaniline blends by in situ polymerization technique and their morphology and electrical property. Synth Metal 132(3):239–244. https://doi.org/10.1016/s0379-6779(02)00451-4
Barthet C, Armes SP, Lascelles SF, Luk SY, Stanley HME (1998) Synthesis and characterization of micrometer-sized, polyaniline-coated polystyrene latexes. Langmuir 14(8):2032–2041. https://doi.org/10.1021/la971064z
Blinova NV, Reynaud S, Roby F, Trchová M, Stejskal J (2010) The polymerization of aniline in polystyrene latex particles. Synth Metal 160(15–16):1598–1602. https://doi.org/10.1016/j.synthmet.2010.05.014
Chang H-C, Lin Y-Y, Chern C-S, Lin S-Y (1998) Determination of critical micelle concentration of macroemulsions and miniemulsions. Langmuir 14(23):6632–6638. https://doi.org/10.1021/la971109w
Chatterjee K, Mitra M, Ganguly S, Kargupta K, Banerjee D (2013) Thermoelectric performance of electrodeposited nanostructured polyaniline doped with sulfo-salicylic acid. J Appl Polym 131(4):39920–39926. https://doi.org/10.1002/app.39920
Chatterjee K, Dhara P, Ganguly S, Kargupta K, Banerjee D (2013) Morphology dependent ammonia sensing with 5-sulfosalicylic acid doped nanostructured polyaniline synthesized by several routes. Sens Actuator B Chem 181:544–550. https://doi.org/10.1016/j.snb.2013.02.042
Chen E-C, Lin Y-W, Wu T-M (2009) Fabrication, morphology and thermal degradation behaviors of conductive polyaniline coated monodispersed polystyrene particles. Polym Degrad Stab 94(4):550–557. https://doi.org/10.1016/j.polymdegradstab.2009.01
Chiang CK, Fincher CR, Park YW, Heeger AJ, Shirakawa H, Louis EJ, MacDiarmid AG (1977) Electrical conductivity in doped polyacetylene. Phys Rev Lett 39(17):1098–1101. https://doi.org/10.1103/physrevlett.39.1098
Chu H-H, Yeo Y-S, Chuang KS (2007) Entry in emulsion polymerization using a mixture of sodium polystyrene sulfonate and sodium dodecyl sulfate as the surfactant. Polymer 48(8):2298–2305. https://doi.org/10.1016/j.polymer.2007.02.057
Dong X, Zhang X, Wu X, Cui H, Chen D (2016) Investigation of gas-sensing property of acid-deposited polyaniline thin-film sensors for detecting H2S and SO2. J Sens 16(11):1889. https://doi.org/10.3390/s16111889
Du X, Xu Y, Xiong L, Bai Y, Zhu J, Mao S (2014) Polyaniline with high crystallinity degree: synthesis, structure, and electrochemical properties. J Appl Polym. https://doi.org/10.1002/app.40827
Ertit Taştan B, Duygu E, İlbaş M, Dönmez G (2016) Enhancement of microalgal biomass production and dissolved inorganic C fixation from actual coal flue gas by exogenous salicylic acid and 1-triacontanol growth promoters. Nrg 103:598–604. https://doi.org/10.1016/j.energy.2016.03.020
Ferraris J, Cowan DO, Walatka V, Perlstein JH (1973) Electron transfer in new highly conducting donor-acceptor complex. J Am Chem Soc 95(3):948–949
Ghasemi H, Sundararaj U (2012) Electrical properties of in situ polymerized polystyrene/polyaniline composites: the effect of feeding ratio. Synth Metal 162(13–14):1177–1183. https://doi.org/10.1016/j.synthmet.2012.04.037
Janošević A, Ćirić-Marjanović G, Marjanović B, Holler P, Trchová M, Stejskal J (2008) Synthesis and characterization of conducting polyaniline 5-sulfosalicylate nanotubes. Nanotechnology 19(13):135606–135614. https://doi.org/10.1088/0957-4484/19/13/135606
Kazimierska E, Smyth MR, Killard AJ (2009) Size-dependent electrocatalytic reduction of nitrite at nanostructured films of hollow polyaniline spheres and polyaniline–polystyrene core–shells. Electrochim Acta 54(28):7260–7267. https://doi.org/10.1016/j.electacta.2009.07.039
Kohut-Svelko N, Reynaud S, Dedryvère R, Martinez H, Gonbeau D, François J (2005) Study of a nanocomposite based on a conducting polymer: polyaniline. Langmuir 21(4):1575–1583. https://doi.org/10.1021/la0481243
Liao G (2018) Green preparation of sulfonated polystyrene/polyaniline/silver composites with enhanced anticorrosive properties. Int J Chem 10(1):81. https://doi.org/10.5539/ijc.v10n1p81
Lin Y, Ng KM, Chan C-M, Sun G, Wu J (2011) High-impact polystyrene/halloysite nanocomposites prepared by emulsion polymerization using sodium dodecyl sulfate as surfactant. J Colloid Interface Sci 358(2):423–429. https://doi.org/10.1016/j.jcis.2011.03.009
Li Y, Wang Z, Wang C, Zhao Z, Xue G (2011) Controlling the morphology of micrometre-size polystyrene/polyaniline composite particles by swelling–diffusion–interfacial-polymerization method. Polymer 52(2):409–414. https://doi.org/10.1016/j.polymer.2010.11.025
Li Y, Hu Y, Ye S, Wu Y, Yang C, Wang L (2016) Functional polyaniline-assisted decoration of polystyrene microspheres with noble metal nanoparticles and their enhanced catalytic properties. New J Chem 40(12):10398–10405. https://doi.org/10.1039/c6nj02200f
Lu X, Ng HY, Xu J, He C (2002) Electrical conductivity of polyaniline–dodecylbenzene sulphonic acid complex: thermal degradation and its mechanism. Synth Metal 128(2):167–178. https://doi.org/10.1016/s0379-6779(01)00668-3
Marjanović B, Juranić I, Mentus S, Ćirić-Marjanović G, Holler P (2010) Oxidative polymerization of anilinium 5-sulfosalicylate with peroxydisulfate in water. Chem Pap 64(6):783–790. https://doi.org/10.2478/s11696-010-0064-0
Martins CR, Ruggeri G, De Paoli M-A (2003) Synthesis in pilot plant scale and physical properties of sulfonated polystyrene. J Braz 14(5):797–802. https://doi.org/10.1590/s0103-50532003000500015
Naar N, Lamouri S, Jeacomine I, Pron A, Rinaudo M (2012) A comprehensive study and characterization of colloidal emeraldine-base. J Macromol Sci. https://doi.org/10.1080/10601325.2012.714688
Naar N, Djurado D, Saad L, Pron A (2013) Spectroscopic and structural properties of dopant functionalized polyaniline prepared in a one-step procedure. J Macromol Sci A 50(6):631–638. https://doi.org/10.1080/10601325.2013.784556
Neetika G, Kumar D, Tomar SK (2012) Thermal behaviour of chemically synthesized polyanilines/polystyrene sulphonic acid composites. J Mater Chem 2(2):79–85. https://doi.org/10.5923/j.ijmc.20120202.07
Pud AA, Nikolayeva OA, Vretik LO, Noskov YV, Ogurtsov NA, Kruglyak OS, Fedorenko EA (2017) New nanocomposites of polystyrene with polyaniline doped with lauryl sulfuric acid. Nanoscale Res Lett. https://doi.org/10.1186/s11671-017-2265-8
Rehman S, Islam N, Ahad S, Fatima SZ, Pandith AH (2013) Preparation and characterization of 5-sulphosalicylic acid doped tetraethoxysilane composite ion-exchange material by sol–gel method. J Hazard Mater 260:313–322. https://doi.org/10.1016/j.jhazmat.2013.05.036
Sekerak NM, Hutchins KM, Luo B, Kang JG, Braun PV, Chen Q, Moore JS (2018) Size control of cross-linked carboxy-functionalized polystyrene particles: four orders of magnitude of dimensional versatility. Eur Polym 101:202–210. https://doi.org/10.1016/j.eurpolymj.2018.01.028
Sun L, Sun X, Zheng Y, Lin Q, Su H, Wang Y, Qi C (2017) Preparation and characterization of core-shell polystyrene/polyaniline/Pd composites and their catalytic properties for the reduction of 4-nitrophenol. J Appl Polym 134(19):44812–44818. https://doi.org/10.1002/app.44812
Trivedi DC, Dhawan SK (1993) Investigations on the effect of 5-sulfosalicylic acid on the properties of polyaniline. Synth Metal 58(3):309–324. https://doi.org/10.1016/0379-6779(93)91140-w
Wang X, Wang X, Wu Y, Bao L, Wang H (2010a) Interfacial synthesis of polyaniline nanostructures induced by 5-sulfosalicylic acid. Mater Lett 64(17):1865–1867. https://doi.org/10.1016/j.matlet.2010.06.006
Wang X, Wang X, Wu Y, Bao L, Wang H (2010b) Interfacial synthesis of polyaniline nanostructures induced by 5-sulfosalicylic acid. Mater Lett 64(17):1865–1867. https://doi.org/10.1016/j.matlet.2010.06.006
Wu Q, Wang Z, Xue G (2007) Controlling the structure and morphology of monodisperse polystyrene/polyaniline composite particles. Adv Funct Mater 17(11):1784–1789. https://doi.org/10.1002/adfm.200700170
Wu W, Pan D, Li Y, Zhao G, Jing L, Chen S (2015) Facile fabrication of polyaniline nanotubes using the self-assembly behavior based on the hydrogen bonding: a mechanistic study and application in high-performance electrochemical supercapacitor electrode. Electrochim Acta 152:126–134. https://doi.org/10.1016/j.electacta.2014.11.130
Yang L-Y, Liau W-B (2009) Environmental responses of nanostructured polyaniline films based on polystyrene–polyaniline core–shell particles. Mater Chem Phys 115(1):28–32. https://doi.org/10.1016/j.matchemphys.2008.10.074
Yan J, Wang C, Gao Y, Zheng Z, Zhong S, Miao X, Wang H (2011) Anchoring conductive polyaniline on the surface of expandable polystyrene beads by swelling-based and in situ polymerization of aniline method. Chem Eng 172(1):564–571. https://doi.org/10.1016/j.cej.2011.06.009
Zhang Z, Wei Z, Wan M (2002) Nanostructures of polyaniline doped with inorganic acids. Macromolecules 35(15):5937–5942. https://doi.org/10.1021/ma020199v
Zhang L, Wang T, Liu P (2012) Superparamagnetic sandwich Fe3O4@PS@PANi microspheres and yolk/shell Fe3O4@PANi hollow microspheres with Fe3O4@PS nanoparticles as “partially sacrificial templates.” Chem Eng Trans 187:372–379. https://doi.org/10.1016/j.cej.2012.01.125
Zhao S, Wang Z, Wang J, Yang S, Wang S (2011) PSf/PANI nanocomposite membrane prepared by in situ blending of PSf and PANI/NMP. J Membr Sci 376(1–2):83–95. https://doi.org/10.1016/j.memsci.2011.04.008
Acknowledgements
The authors are grateful to the Directorate General for Scientific Research and Technological Development/Ministry of Higher Education and Scientific Research (Algeria) for its support through providing chemical products and are also grateful to thank the master student Bouchalaala Mounir to have participated to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamlaoui, F.Z., Naar, N. Characterization of polyaniline/polystyrene blends prepared by three different methods. Chem. Pap. 75, 1431–1443 (2021). https://doi.org/10.1007/s11696-020-01395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01395-9