Abstract
The oleaginous and red yeast Rhodotorula glutinis CCY 20-2-26 is known for its high-storage lipids accumulation in cells as well as for β-carotene biosynthesis. The work is focused on the study of lipogenesis and carotenoid pigments formation by the yeast grown in media with four different C/N ratios (20:1, 50:1, 70:1 and 100:1). Total fatty acids accumulation in cells reached the maximal value of 48% and yielded up to 8.9 g fatty acid/L media under C/N 70:1. On contrary, while the highest pigment accumulation in the yeast (1268 μg/g of cells) was found at the C/N ratio of 20:1, the maximal carotenoid yield (mainly β-carotene) of 12.7 mg/L was obtained under 50:1 ratio. Calculation of kinetic parameters of metabolites production revealed the metabolic correlations among glucose consumption, lipogenesis and carotenoid biosynthesis. It was demonstrated that glucose exhaustion resulted in reduction of growth and lipid accumulation in cells. Then, storage fatty acids, especially palmitic and oleic acids, begun to be degraded by β-oxidations and formed acetyl-CoA which was especially used for biosynthesis of β-carotene. This is the first study providing the new hypothesis about the metabolic connection between fatty acids and β-carotene metabolism in the red yeasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoid pigments are an impressive group of biological active compounds, exposing yellow, orange or red colour. A long polyene chain of carotenoids is responsible for their specific colour. These tetraterpenoids, synthesized by plants and several microbial producers (yeasts, fungi and bacteria) (Mata-Gómez et al. 2014) are generally located in cell membranes in several microbes or plant thylakoids (Buzzini et al. 2010). A conjugated system of double bonds and terminal cyclic structures of a carbon chain are effective tools for protection against free oxygen radical attacks and correlate to various benefits for the human health (Stahl and Sies 2012; Harrison and Bugg 2014). Therefore, carotenoids are used in various industrial fields as food, feed and cosmetic additives. β-carotene represents a precursor of vitamin A and is the most outstanding and oversupplied carotenoid pigment (Jaswir et al. 2011). Due to the positive carotenoid impact and the significant commercial application, the global market demand for natural carotenoid pigments enhanced in recent years. The global trade for carotenoid pigments achieved 1.5 billion dollars in 2014. The 2019 market value is estimated to be 1.8 billion dollars with the considerable 3.9% annual carotenoid enhancement (www.bccresearch.com). The increasing demand for these biologically active compounds has drawn a commercial attention on the provision of a suitable biosynthetic platform for their natural formation. From this point of view, several promising carotenoids synthesizing microorganisms including bacteria, algae, yeasts and fungi have been described so far (Čertík et al. 2009). Hence, the biotechnological production of carotenoid pigments in high yields requires the efficient stimulation of the carotenoid pathway with the accompanying enhanced pigment accumulation in microbial cells (Salar et al. 2013). Regulation of carotenoid metabolic pathways could be achieved by the optimisation of fermentation conditions involving modifications of physiological and nutritional factors (different types and amounts of low cost substrates, temperature, aeration, pH, light, minerals) and stress factors (osmotic stress, heavy metals, hydrogen peroxide, etc.) (Hernández-Almanza et al. 2014). These efforts could increase activities of key “pigment-forming” enzymes finally leading to the subsequent maximizing carotenoid overproduction and minimizing costs of the fermentation process.
To enhance the conversion of carbon into carotenoids, participation and mutual link of various metabolic pathways of pigment accumulation in microbial cells must be determined. Very recently, we have described the functional correlation between lipogenesis and biosynthesis of torularhodin in Rhodotorula glutinis JMT 21978 (Tkáčová et al. 2017). We found that torularhodin accumulation in the yeast was triggered by glucose consumption and subsequent utilization of specific intracellular fatty acids. However, various Rhodotorula strains differ from each other and form several other pigments than torularhodin, such as β-carotene, γ-carotene, and torulene. Because the red yeast Rhodotorula glutinis CCY 20-2-26 is known as a producer of mainly β-carotene, this paper is focused on the metabolic consecutiveness of biochemical pathways leading to the β-carotene overproduction in this yeast. It should be underlined that such comprehensive studies on the mechanism of β-carotene formation based on the calculation of kinetic parameters of the yeast growth and selected yeast metabolites under distinct cultivation conditions have not been described yet. Therefore, this strategy could be applied for aimed monitoring and regulation of β-carotene biosynthesis in fermentation processes.
Experimental
Microorganism and cultivation conditions
Red yeast Rhodotorula glutinis CCY 20-2-26, isolated from water of Little Danube was chosen in this research as an effective carotenoid producer (Marova et al. 2004). R. glutinis was maintained on Petri dishes with 7% malt agar at 4 °C and regularly re-inoculated every 2 months. The 4-day culture obtained from malt agar (28 °C, yellow light) was used for cultivation experiments. Cultures were inoculated in the 30 mL of YPG media in 100-mL Erlenmeyer flasks containing 2% glucose, 1% peptone and 1% yeast extract for 72 h. These cultures were used to inoculate the production media (1 × 105 cells/mL). Production media (20 mL in 100-mL Erlenmeyer flasks) with different four C/N ratios were used. Each media contained yeast extract (5 g/L) and glucose in various concentration to reach the ratio 20:1 (20 g/L), 50:1 (50 g/L), 70:1 (70 g/L) and 100:1 (100 g/L). Resulting C/N ratios were calculated to amount of carbon and nitrogen present in the production medium. Cultivation time was 4 (for C/N ratio 20:1) or 8 days. All cultivation experiments were performed in triplicates and carried out using IKA® KS 4000 ic Control Incubator Shaker with Cooling Coil (180 rpm) at 28 °C under constant yellow light. The external light source represented a duralumin plate with 15 LED rows (30 LED DIOD in a one row) that was located on the upper cover of rotary incubator. The system was cooled by two external ventilators. The wavelength was set up by the external infra-red remote control and signal was received by the infra-red RGB receiver. Altering voltage 220 V passed through the system. The intensity of the exposure was 1225–1230 lx (Digital Luxmeter PU 550, Metra).
Carotenoid pigments isolation and analysis
Cells of R. glutinis were harvested by centrifugation and washed by 0.9% NaCl solution and distilled water. Biomass was lyophilized and subsequently homogenized by mortar with the sea sand. Carotenoids together with lipids were double extracted by mixture chloroform/methanol (2:1) for 2 h at laboratory temperature with constant stirring (Folch et al. 1957). After extraction, the mixture was filtrated to remove cells and extracts were mixed with distilled water (1.2-fold of total extract volume). The mixture was stirred vigorously for 1 min and centrifuged to effect phase separation. The chloroform layer containing lipids and pigments was filtered through anhydrous Na2SO4 and evaporated under vacuum. Lipid extract with carotenoid pigments was re-suspended in the 1 mL of mixture of hexane/chloroform (9:1, v/v) and afterwards analysed by high-performance liquid chromatography (HP 1100, Agilent) according to Klempova et al. (2013). Carotenoid pigments were identified using known standards (Sigma, Germany) and evaluated by ChemStation B 01 03 (Agilent Technologies).
Fatty acid isolation and analysis
Biomass (10–15 mg) was mixed and incubated (50 °C, 3 h) with 1 mL of dichloromethane containing 0.1 mg of internal standard (C17:0) and 2 mL of methanolic HCl solution to form fatty acid methyl esters (FAMEs) (Certik and Shimizu 2000). After incubation, 1 mL of distilled water and 1 mL of hexane were added simultaneously and stirred vigorously. Mixture was centrifuged (3000×g, 5 min) and hexane phase with pre-extracted FAMEs was analysed by gas chromatography (GC-6890 N, Agilent Technologies) according to Gajdoš et al. (2015). FAMEs were evaluated by retention times of internal standard (Sigma, USA) and quantified by ChemStation B0103 (Agilent Technologies).
Measurement of residual glucose
Residual contents of glucose in production media were measured spectrophotometrically by a method using 3,5-dinitrosalicylic acid (DNS) at 540 nm (Miller 1959).
Determination of kinetic parameters
Kinetic analysis describes formation of biomass (total dry cell weight—DCW), total fatty acids content (TFAs) and carotenoids content (CARs) in yeast cells during each day of cultivation. Fatty acids free cell weight (ffCW) was applied for other calculations. Several kinetic parameters were determined according to Certik and Shimizu (2000). Basic parameter productivity (P) represents final production of DCW, TFAs and CARs during cultivation process (mg L−1, g L−1). The relative productivity (P r) expresses accumulation of TFAs and CARs in DCW (%, mg g−1). Rate of product synthesis (ν) illustrates average speed of total production of DCW, TFAs and CARs (g L−1 day−1; mg L−1 day−1). Specific product synthesis (q) performs total accumulation of CARs and TFAs in DCW all along R. glutinis was cultivated (% day−1; mg g−1 day−1). Finally, the yield of product (Y) shows the entire amount of DCW, TFAs and CARs formation from total consumed substrate (g 100 g−1 Sc; %).
Results and discussion
Rhodotorula glutinis growth and substrate consumption
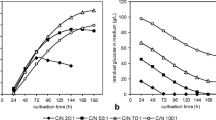
Kinetic parameters of Rhodotorula glutinis growth and glucose consumption were determined during the yeast cultivation in media with various C/N ratios. Low glucose concentration (CN20) led to early C-source depletion that caused absence of the stationary phase of cell growth (Fig. 1a, b). The maximal dry cell weight production (8.8 g/L) was reached after 48 h and DCW yield was the lowest compared to other used C/N ratios (Fig. 1a). Glucose exhaustion was also observed during cultivation on media with CN50 (Fig. 1b). Like CN20, glucose exhaustion after 144 h caused the drop in DCW yield. Further increase of glucose concentration had a positive effect on DCW production with the highest yield 18.6 g/L using media with CN70. However, CN100 (100 g/L of glucose) did not display any significant increase in DCW formation. On contrary, the maximal yield of DCW employing CN100 media (16.1 g/L) was lower than the maximal CN50 media DCW yield (16.7 g/L). This fact could be a result of the longer lag phase with a negative impact on the cell growth. The possible reason is that high glucose concentration could lead to high osmotic pressure. Similar data were observed with strains Sporobolomyces pararoseus (Han et al. 2016), Lipomyces starkeyi (Lin et al. 2011), Rhodosporidium toruloides (Li et al. 2007) and Trichosporon fermentans (Zhu et al. 2008).
Growth of Rhodotorula glutinis CCY 20-2-26, expressed as a dry cell weight (DCW) formation (a), residual glucose content in media (b), total fatty acid accumulation in cells (c) and total fatty acids yield (d) during cultivation on media with various C/N ratios: 20:1 (filled diamond), 50:1 (open diamond), 70:1 (filled triangle) and 100:1 (open triangle). DCW dry cell weight, TFAs total fatty acids content
Total fatty acid formation
Total fatty acids productivity—P(TFAs) of R. glutinis CCY 20-2-26 was also influenced by the presence of carbon source in the medium. Total fatty acids were accumulated in cells until glucose depletion. After glucose was consumed from media (48th hour for CN20, 144th hour for CN50), the yields of fatty acids began to decrease. The maximal P(TFAs) was obtained using CN70 (8.9 g/L) where glucose was not completely consumed (Fig. 1c). This fact was also illustrated by the relative productivity P r(TFAs) since it reached 47.6% (Fig. 1d). Enhancement of C/N ratio to CN100 did not display such an efficiency of TFAs formation (6.2 g/L) and the relative productivity P r(TFAs) (reached only 38.3%). Moreover, TFA formation using CN100 was almost equal to the formation obtained by the CN50.
The further kinetic parameters ν, q and Y provided another view on total fatty acids production during the cultivation process. The parameter ν(TFAs) confirmed the maximal lipid formation using ratio CN70 as it was referred previously by P and P R parameters (Fig. 2a). The rate of TFAs synthesis increased gradually up to value 1.1 g/L/day obtained in the stationary phase. Similar value of v(TFAs) was reached during cultivation of R. glutinis on media with CN50 (1.0 g/L/day at 144th hour). However, it decreased 1.6-times (0.6 g/L/day) when the substrate was completely utilized from media. Moreover, this parameter showed that high ratio of CN100 had no significant effect on oleaginicity of R. glutinis and the maximal rate of TFAs synthesis was obtained during the stationary phase with value 0.7 g/L/day. Specific product synthesis (q) represents the rate of accumulation of TFAs in biomass. The highest q(TFAs) was observed employing CN70 with average rate 6.7%/day (Fig. 2b). Cells cultivated on media with CN50 showed similar average rate (6.6%/day) until the glucose was exhausted form media (144th hour). The depletion of carbon source caused the cessation of TFAs accumulation. Interesting results were achieved by calculation of glucose conversion to TFAs (Y) (Fig. 2c). The maximal yield of TFAs was achieved on media with ratio CN50 (15.6 g TFAs/100 g of glucose after 144th hour); however, the cultivation on CN70 media showed also good effectiveness with the obtained yield (14.9 g TFAs/100 g glucose). Y(TFAs) data calculated from media with CN100 were lower compared to CN50 and CN70, thus confirming that such high CN ratio (glucose concentration) was not favourable for lipogenesis of R. glutinis.
The kinetic analysis of fatty acid production in Rhodotorula glutinis CCY 20-2-26 using different C/N ratios: 20:1 (filled diamond), 50:1 (open diamond), 70:1 (filled triangle) and 100:1 (open triangle). The analysis of average speed of total fatty acids (TFAs) synthesis (ν) (a), the analysis of specific TFAs synthesis (q) (b) during cultivation and the study of glucose conversion (Y) to TFAs (c) content related to 100 g of glucose (Glc)
The mechanism of fatty acid biosynthesis by oleaginous yeasts is very well known (Ratledge 2004). The essential point of fatty acids overproduction is a nitrogen source exhaustion and presence of a sufficient amount of carbon source. In nitrogen-limited conditions, a decreased mitochondrial AMP concentration was observed due to higher activity of AMP-deaminase. Change in AMP concentration causes the accumulation of citric acid from citric acid cycle and its transport to cytosol. Citric acid in cytosol is cleaved by ATP-citrate lyase which supplies acetyl-CoA for fatty acids overproduction (Tang and Chen 2014). Using CN20 ratio in this work is clearly not suitable for lipid overproduction due to limited concentration of carbon source. CN50 and CN70 provided a similar yield (glucose conversion ratio around 15%) until the carbon source was spent from media. Braunwald et al. (2013) studied also the effect of the C/N ratio on metabolism of R. glutinis; however, authors were not able to achieve the nitrogen source depletion and obtained yields of fatty acids which were less than 1 g/L. The maximal conversion ratios of glucose to lipid reported by R. toruloides (Li et al. 2007) and L. starkeyi (Lin et al. 2011) were 9.6 and 6%, respectively. This suggests that R. glutinis has a significant potential for the single-cell oil production employing media with the limited nitrogen source.
Individual fatty acids production
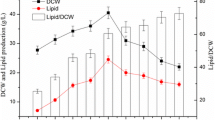
Red yeast R. glutinis predominantly synthesizes palmitic (PA, C16:0), stearic (SA, C18:0), oleic (OA, C18:1), linoleic (LA, C18:2) and α-linolenic (ALA, C18:3) acids (Braunwald et al. 2013). ALA is mainly synthesized in the early stages of cell life cycle since it is important part of newly created cell membranes. After the exponential phase of growth, the content of ALA in fatty acids dropped below 5%. Relative productivities (P r) of other main fatty acids are displayed on Fig. 3. Biomass obtained from media with higher C/N ratio (CN70 and CN100) continually accumulated each fatty acid during whole cultivation process. On the other hand, biomass obtained from media with lower C/N ratio (CN20 and CN50) changed the rate of accumulation of individual fatty acids especially after glucose exhaustion. The content of two of the most abundant fatty acids—PA and OA—dropped dramatically after glucose disappeared from cultivation media. The P r of PA decreased for 34% (CN20) and 15% (CN50) and P r of OA decreased for 15% (both CN20 and CN50). After glucose depletion, the SA accumulation fell slightly (less than 10%). The content of fatty acids with higher number of double bonds (LA and α-ALA) has not been changed during the starvation phase, since the β-oxidation of highly unsaturated fatty acids requires other enzymes and even NADPH (van Roermund et al. 1998). Thus, degradation of these fatty acids is not favourable from the energetic point of view. This result differs from our previous study (Tkáčová et al. 2017), where torularhodin-producing R. glutinis strain utilized preferentially just the oleic acid after glucose depletion.
Total carotenoid production
R. glutinis is known as a significant producer of carotenoid pigments. Several approaches for pigment overproduction have been applied, e.g. physiological regulation via C/N ratio (Hernández-Almanza et al. 2014). The study determined that lower C/N ratios were more applicable for the maximizing of carotenoid formation as glucose exhaustion directly contributed to the significant carotenoid pigments (CARs) production. That is in consistence with our result since the highest accumulation—P r(CARs)—was reached on media with CN20 and the obtained level of accumulation was 1267.8 µg CARs/g of biomass after 96 h. (Figure 4a). After glucose deprivation, the relative productivity of carotenoids accrued from 534.5 to 1020.9 µg/g (48–96 h). The same positive effect was also observed employing CN50 media and carotenoid accumulation rose from 574.9 µg CARs/g of biomass (144th hour) to 947.6 µg CARs/g of biomass (192nd hour). Although the maximal accumulation of carotenoids was achieved on media with CN20, the yeast cultivation on media with ratio CN50 leads to maximal yield 12.7 mg CARs/L due to higher DCW production (Fig. 4b). On the other hand, cells obtained from media with higher ratios (CN70 and CN100) accumulated carotenoids mostly during the exponential growth phase.
The accumulation of carotenoids (Pr CARs) in Rhodotorula glutinis CCY 20-2-26 cells (a) and the carotenoid yield (P CARs) (b) using different C/N ratios: 20:1 (filled diamond), 50:1 (open diamond), 70:1 (filled triangle) and 100:1 (open triangle). CARs total content of carotenoids, DCW dry cell weight
Braunwald et al. (2013) monitored the maximal carotenoid formation by high-ratio CN60. Lower C/N ratios led to decreased formation of both biomass and pigments. Somashekar and Joseph (2000) determined that carotenoids were produced by R. gracilis in high level by application of low C/N ratio CN10 with total carotenoid content 26 mg/g DCW after 94 h of cultivation. Schmidt et al. (2011) determined that low C/N ratio led to rapid cell growth of Phaffia rhodozyma, but repressed astaxanthin formation. It was discussed that Crabtree effect positively influenced the carotenoid production when the strain was cultivated in media with high initial substrate concentration. However, this effect of the aerobic alcohol fermentation in the substrate excess did not impact carotenoids formation in R. glutinis as it is a non-fermentative strain (Viega et al. 2000; Rodrigues et al. 2006; Deák 2008).
Calculation of other kinetic parameters confirmed the association of glucose depletion and carotenoids biosynthesis elevation. The rate of carotenoids synthesis increased to maximal values at 72nd hour (2.9 mg/L/day) using media with ratio CN20 and at 144th hour (1.6 mg/L/day) for media with ratio CN50. This represents 1.3-times resp. 1.5-times fold of v(CARs) compared to values obtained while glucose was still present in media. Due to the absence of the stationary phase of growth on media with CN20, the rate of biosynthesis decreased rapidly after reaching maximum since the cells were in the death phase. However, v(CARs) remained stable after glucose exhaustion from media with CN50 (Fig. 5a). Application of higher C/N ratios led to continual repressing of carotenoid production after the exponential phase of growth. The specific rate of carotenoids synthesis (q) was the highest while the yeast was cultivated on media with CN20 ratio. The maximal obtained rate was nearby 340.3 µg/g/day at 72nd hour after glucose was consumed from media (Fig. 5b). The efficiency of glucose conversion to carotenoids (Y) reached maximal values when lower C/N ratios were used in cultivation media (0.03% for CN20 or 0.02% for CN50) (Fig. 5c). Concerning high C/N ratio, the yield of carotenoids reached the highest value in the early stationary phase.
The kinetic analysis of the carotenoid production in Rhodotorula glutinis CCY 20-2-26 using different C/N ratios: 20:1 (filled diamond), 50:1 (open diamond), 70:1 (filled triangle) and 100:1 (open triangle). The analysis of average speed of total carotenoids (CARs) synthesis (ν) (a), the analysis of specific CARs synthesis (q) during cultivation (b) and the study of glucose conversion (Y) to CARs (c) content related to 100 g of glucose (Glc)
Production of individual carotenoids
This study revealed that the strain R. glutinis CCY 20-2-26 is able to synthesize four major carotenoid pigments: β-carotene, γ-carotene, torularhodin and torulene. The ratio of produced pigments was strictly dependent on C/N ratio of cultivation media. Major pigment produced during cultivation on media with lower C/N ratio (CN20, CN50) was β-carotene (BCAR). Figure 6 shows that highest productivity of β-carotene was achieved on media with CN50 and the yield was 7.2 mg/L (56% of all produced pigments). Increased yield of β-carotene was accompanied by reduced yield of γ-carotene, since it is a direct precursor of β-carotene. The productivities of individual pigments employing media with higher C/N ratio (CN70 and CN100) were very similar to each other with prevalence of torulene and γ-carotene. These results suggest that β-carotene production is triggered by glucose exhaustion from media. Similar data were obtained using torularhodin-producing strain R. glutinis, where lower C/N ratios favoured torularhodin production (Tkáčová et al. 2017). El-Banna et al. (2012) reported that β-carotene was overproduced in R. glutinis var. glutinis using C/N ratios 10:1, 15:1 and 20:1 while torularhodin amount was maximized by lower C/N ratios 2.5:1 and 5:1, respectively. Torulene, a precursor of torularhodin, was also produced in significant levels when the strain was cultivated in media with lower C/N ratios (2.5:1, 5:1 and 10:1). On the other hand, Braunwald et al. (2013) detected enhanced torulene and torularhodin biosynthesis by R. glutinis in all tested C/N ratios.
Yield of individual carotenoids from Rhodotorula glutinis CCY 20-2-26 by the application of different C/N ratios: 20:1 (a), 50:1 (b), 70:1 (c) and 100:1 (d). CAR carotenoid pigment, THR torularhodin (filled circle), TOR torulene (open circle), GCAR γ-carotene (open square), BCAR β-carotene (filled square)
Further kinetic parameters also proved the findings described above. The rate of β-carotene synthesis achieved the maximal level (1.4 mg/L/day and 1.0 mg/L/day) during cultivation on media with CN20 and CN50 ratios, respectively (Fig. 7a). The yield of β-carotene with regard to consumed glucose Y(BCAR) showed that rapid biosynthesis of β-carotene was triggered when the small amount of glucose (less than 5 g/L) was present in media (0.008% for both CN20 and CN50) (Fig. 7b). It should be emphasized that all kinetic parameters clearly indicated that β-carotene biosynthesis significantly increased even glucose was completely consumed. These results suggest that the cells were able to efficiently utilize another source of carbon for β-carotene formation.
The kinetic analysis of the β-carotene (BCAR) production in Rhodotorula glutinis CCY 20-2-26 using different C/N ratios: 20:1 (filled diamond), 50:1 (open diamond), 70:1 (filled triangle) and 100:1 (open triangle). The analysis of average speed of BCAR synthesis (ν) (a), and the study of glucose conversion (Y) to BCAR content related to 100 g of glucose (Glc) (b)
Connection between fatty acids and carotenoid metabolism
This work deals with a possible connection between fatty acids and carotenoid metabolism. The association between carbon utilization and metabolites (pigments, fatty acids) production was described using various kinetic parameters such as productivity, the rate of biosynthesis, and yield per glucose. During the early stage of a life cycle, glucose was mainly used for cell mass biosynthesis and the conversion of glucose into free-fat biomass was almost 70.8 and 64.5% under CN20 and CN50, respectively, after 24 h of cultivation. After the exponential phase of biomass growth on nitrogen-limited media (while glucose was still present), the carbon exhaustion from media resulted in reduction of fatty acids. Carotenoid pigments were continually synthesized, however, the glucose conversion into pigments was low and the carotenoid profile was stable (30% of β-carotene, 30% of γ-carotene, 25% of torulene and 15% of torularhodin).
After glucose depletion, the cells began to use energy and carbon from storage lipids (fatty acids bounded to triacylglycerols). By a set of β-oxidation reactions, fatty acids were metabolized to acetyl-CoA molecules and reducing equivalents in peroxisomes. Results of our work clearly demonstrated that primarily the palmitic and oleic acids underwent degradation process and accumulation of carotenoid pigments (mainly β-carotene, its content rose to 60% of total pigments) elevated rapidly. Our results suggested that acetyl-CoA obtained from degradation of palmitic and oleic acids after glucose consumption was shifted towards carotenoid biosynthesis pathway (Fig. 8).
Our recent study (Tkáčová et al. 2017) revealed the correlation between carotenoid and lipid metabolism in torularhodin-synthesizing strain Rhodotorula glutinis JMT21978. We have applied the similar approach using various kinetic parameters to perform the detailed analysis of regulation of β-carotene biosynthesis in strain Rhodotorula glutinis CCY 20-2-26. Obtained data confirm the connection between catabolism of fatty acids and anabolism of carotenoid pigments after exhaustion of extracellular carbon source. However, the strain R. glutinis CCY 20-2-26 degraded both palmitic and oleic acid for β-carotene production, while strain R. glutinis JMT21978 used oleic acid exclusively.
Conclusions
The kinetic analysis was applied to study growth, lipogenesis and carotenoid pigment biosynthesis by R. glutinis CCY 20-2-26 cultivated under various C/N ratios. It was proved that carotenoid overproduction was triggered by carbon (glucose) depletion from the medium with lower C/N ratios 20:1 and 50:1. Glucose consumption was followed by a substantial decrease in the both growth rate and lipid accumulation and storage palmitic and oleic acids began to be cleaved from triacylglycerols and degraded by the β-oxidation. Formed acetyl-CoA entered mevalonate/isoprenoid/carotenoid pathways to biosynthesize especially β-carotene. Thus, for the first time it was described and established the metabolic association among the glucose exhaustion, storage fatty acids utilization and carotenoid overproduction in the β-carotene-forming R. glutinis.
References
Braunwald T, Schwemmlein L, Graeff-Hönninger S, French WT, Hernandez R, Holmes WE, Claupein W (2013) Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl Microbiol Biotechnol 97:6581–6588. https://doi.org/10.1007/s00253-013-5005-8
Buzzini P, Goretti M, Branda E, Turchetti B (2010) Basidiomycetous yeast for production of carotenoids. In: Flickinger M (ed) Encyclopedia of industrial biotechnology: bioprocess, bioseparation, and cell technology. Wiley, New York, pp 469–481
Certik M, Shimizu S (2000) kinetic analysis of oil biosynthesis by an arachidonic acid-producing fungus, Mortierella alpina 1S-4. Appl Microbiol Biotechnol 54:224–230. https://doi.org/10.1007/s002530000361
Čertík M, Hanusová V, Breierová E, Márová I, Rapta P (2009) Biotechnological production and properties of carotenoid pigments. In: Hou CT, Shaw J-F (eds) Biocatalysis and agricultural biotechnology. Tailor and Francis-CRC Press, Boca Raton, pp 355–375
Deák T (2008) Handbook of food spoilage yeasts, 2nd edn. CRC Press, New York, pp 62–64
El-Banna AA, El-Razek AMA, El-Mahdy AR (2012) Some factors affecting the production of carotenoids by Rhodotorula glutinis var. glutinis. Food Nutr Sci 3:64–71. https://doi.org/10.4236/fns.2012.31011
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gajdoš P, Nicaud JM, Rossignol T, Čertík M (2015) Single cell oil production on molasses by Yarrowia lipolytica strains overexpressing DGA2 in multicopy. Appl Microbiol Biotechnol 99:8065–8074. https://doi.org/10.1007/s00253-015-6733-8
Han M, Xu Z, Du Ch, Qian H, Zhang W (2016) Effects of nitrogen on the lipid and carotenoid accumulation of oleaginous yeast Sporidiobolus pararoseus. Bioprocess Biosyst Eng 39:1425–1433. https://doi.org/10.1007/s00449-016-1620-y
Harrison PJ, Bugg TDH (2014) Enzymology of the carotenoid cleavage dioxygenases: reaction mechanisms, inhibition and biochemical roles. Arch Biochem Biophys 544:105–111. https://doi.org/10.1016/j.abb.2013.10.005
Hernández-Almanza A, Montanez JC, Aguilar-González MA, Martínez-Ávila C, Rodríguez-Herrera R, Aguilar CN (2014) Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci 5:64–72. https://doi.org/10.1016/j.fbio.2013.11.007
Jaswir I, Noviendri D, Hasrini RF, Octavianti F (2011) Carotenoids: sources, medical properties and their application in food and nutraceutical industry. J Med Plants Res 5:7119–7131. https://doi.org/10.5897/JMPRx11.011
Klempova T, Basil E, Kubatova A, Certik M (2013) Biosynthesis of gamma-linolenic acid and beta-carotene by Zygomycetes fungi. Biotechnol J 8:794–800. https://doi.org/10.1002/biot.201200099
Li Y, Zhao ZK, Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides y4 in fed-batch culture. Enzyme Microb Technol 41:312–317. https://doi.org/10.1016/j.enzmictec.2007.02.008
Lin J, Shen H, Tan H, Zhao X, Wu S, Hu C, Zhao ZK (2011) Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. J Biotechnol 152:184–188. https://doi.org/10.1016/j.jbiotec.2011.02.010
Marova I, Breierova E, Koci R, Friedl Z, Slovak B, Pokorna J (2004) Influence of exogenous stress factors on production of carotenoids by some strains of carotenogenic yeasts. Ann Microbiol 54:73–85
Mata-Gómez LC, Montañez JC, Méndez-Zavala A, Aguilar CN (2014) Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact 13:1–11. https://doi.org/10.1186/1475-2859-13-12
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Ratledge C (2004) Fatty acids biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815. https://doi.org/10.1016/j.biochi.2004.09.017
Rodrigues F, Ludovico P, Lea͂o C (2006) Sugar metabolism in yeasts: an overview of aerobic and anaerobic glucose catabolism. In: Gábor P, Carlos R (eds) Biodiversity and ecology of yeasts. The yeast handbook. Springer, Berlin, pp 101–121
Salar RK, Certik M, Brezova V, Brlejova M, Hanusova V, Breierová E (2013) Stress influenced increase in phenolic content and radical scavenging capacity of Rhodotorula glutinis CCY 20-2-26. 3. Biotech 3:53–60. https://doi.org/10.1007/s13205-012-0069-1
Schmidt I, Schewe H, Gassel S, Jin Ch, Buckingham J, Hümbelin M, Sandmann G, Schrader J (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89:555–571. https://doi.org/10.1007/s00253-010-2976-6
Somashekar D, Joseph R (2000) Inverse relationship between carotenoid and lipid formation in Rhodotorula gracilis according to the C/N ratio of the growth medium. World J Microbiol Biotechnol 16:491–493. https://doi.org/10.1023/A:1008917612616
Stahl W, Sies H (2012) Photoprotection by dietary carotenoids: concept, mechanisms, evidence and future development. Mol Nutr Food Res 56:287–295. https://doi.org/10.1002/mnfr.201100232
Tang X, Chen WN (2014) Investigation of fatty acid accumulation in the engineered Saccharomyces cerevisiae under nitrogen limited culture condition. Bioresour Technol 162:200–206. https://doi.org/10.1016/j.biortech.2014.03.061
Tkáčová J, Čaplová J, Klempová T, Čertík M (2017) Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann Microbiol 67:541–551. https://doi.org/10.1007/s13213-017-1284-0
Van Roermund CWT, Hettema EH, Kal AJ, van den Berg M, Tabak HF, Wanders RJA (1998) Peroxisomal β-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even position. EMBO J 17:677–687. https://doi.org/10.1093/emboj/17.3.677
Viega A, Arrabaça DJ, Loureiro-Dias MC (2000) Cyanide-resistant respiration is frequent, but confined to yeasts incapable of aerobic fermentation. FEMS Microbiol Lett 190:93–97. https://doi.org/10.1111/j.1574-6968.2000.tb09268.x
Zhu LY, Zong MH, Wu H (2008) Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour Technol 99:7881–7885. https://doi.org/10.1016/j.biortech.2008.02.033
Acknowledgements
Authors acknowledge to Grant VEGA 1/0574/15 from Ministry of Education, Science, Research and Sport of the Slovak Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tkáčová, J., Klempová, T. & Čertík, M. Kinetic study of growth, lipid and carotenoid formation in β-carotene producing Rhodotorula glutinis . Chem. Pap. 72, 1193–1203 (2018). https://doi.org/10.1007/s11696-017-0368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0368-4