Abstract
The relationship between postoperative dietary intake and weight loss after bariatric surgery remains unclear. We performed a systematic review and meta-analysis of studies published between January 2000 and May 2023, reporting weight loss outcomes, and dietary intake before and after Roux-en-Y gastric bypass and sleeve gastrectomy. A total of 42 studies were included. There was no detectable difference in dietary intake between the two procedures. Roux-en-Y gastric bypass induced an average decrease in energy intake of 886 kcal/day at 12-month post-surgery; however, there was no correlation between daily energy intake and weight loss. These findings show a substantial reduction of energy intake in the first year after bariatric surgery but do not support a link between lower energy intake and greater weight loss.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for severe obesity [1,2,3]. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the most commonly performed procedures [4]. Both types of these bariatric procedures exert considerable effects on energy and nutrient intake, especially in the first 6–12 months, owing to their ability to influence mechanical and physiological mechanisms involved in the regulation of hunger and satiety [5]. However, the relative role of changes in energy and nutrient intake in determining weight loss and particularly long-term weight loss maintenance remains unclear.
A systematic review by Janmohammadi et al. (2019) evaluated the impact of bariatric surgery on energy and nutrient intake, specifically total energy and macronutrients. They reported that bariatric surgery significantly reduced total energy intake and increased fat and protein intake but observed no effect on carbohydrates. These findings offer some understanding of the impact of surgery on energy and macronutrient intake; however, the conclusions were based on observations made over a very broad range of time, from 3 months to 8 years postoperatively. Therefore, it is not possible to discriminate between changes that occur at short- and long-term time points after surgery and their relative role in the mechanisms of surgical weight loss. Additionally, postoperative macronutrient intakes that fall within the acceptable macronutrient distribution ranges (AMDRs; 45% carbohydrate, 20% fat, 35% protein, as percent of total energy) have been shown to optimize surgical outcomes and reduce complications [6,7,8,9], yet no study to date has compared postoperative intakes to the AMDRs. The impact of bariatric surgery on micronutrient intake in the short- and long-term has also not been systematically evaluated, despite the known risk of micronutrient deficiency after bariatric surgery [10, 11].
Understanding whether changes in energy and nutrient intake that occur early after surgery persist long-term and whether anatomically distinct procedures differentially affect nutrient intake has both clinical and mechanistic interest. This knowledge could help optimize surgical outcomes and identify elusive mechanisms of gastrointestinal (GI) physiology that could serve as a target for novel anti-obesity interventions. This systematic review and meta-analysis investigated the impact of bariatric surgery, specifically RYGB and SG, on nutrient intake in the short and long term and evaluated intakes against the recommended AMDRs. A specific objective of this review was also to assess evidence of a role of daily energy intake as a major determinant of weight loss after bariatric surgery.
Methods
This systematic review has been conducted in accordance with the PRISMA guidelines for reporting systematic reviews. Studies reporting on the effects of RYGB or SG on energy intake and/or macro/micronutrient intake were included.
Database Searches
Embase, Clinical Trials, Google Scholar, and PubMed databases were searched by two independent reviewers (DQ, B.WM). The search included all articles published between January 2000 and May 2023. The search strategy included medical subject headings (MeSH) terms and the following key search terms: ((((Roux-en-Y Gastric Bypass) OR (Bypass, Gastric) OR (Bypass, Roux-en-Y Gastric) OR (RYGB), OR (Sleeve gastrectomy) OR (SG) (Bariatric surgery) OR (weight-loss surgery) OR (metabolic surgery)) AND ((diet) OR (dietary intake) OR (diet intake) OR (nutrition) OR (nutrient) OR (nutritional intake) OR (food intake) OR (energy intake)) NOT ((gastric band surgery) OR gastric banding)))). Filters applied were full text, humans, adult: 19 + years, English, multi- and single-center study, clinical study, clinical trial, observational study, comparative studies, controlled clinical trial, and randomized control trial. The identified literature was stored in Mendeley reference manager.
Inclusion and Exclusion Criteria
Inclusion criteria included participants > 18 years of age undergoing RYGB or SG, outcome data that reported on dietary or nutrient intake, including total energy, macro- and micro-nutrient intake. Only studies published between January 2000 and May 2023 were included. This period was chosen to reflect the changes in the contemporary food environment, industries, products, and surgical procedures. All observational study designs were included. Non-English language articles (n = 64) were excluded during the initial search. The decision to exclude these articles was based on considerations of language proficiency and resource constraints. Animal studies, studies investigating other bariatric procedures, studies limited to the pre-operative period, and those assessing taste preferences and/or nutritional status (biomarkers) but not dietary or nutrient intake were excluded. Interventional studies that altered the dietary intake of the participants or supplemented protein, lipid, or carbohydrates were excluded. Review articles, meta-analyses, and editorials were also excluded.
Data Extraction
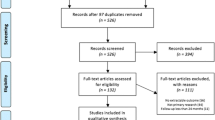
Following the removal of duplicates, titles, and abstracts were screened in triplicate against the inclusion and exclusion criteria; reasons for exclusion are outlined in Fig. 1. Data extraction from full-text articles was undertaken independently by two reviewers and included study characteristics and the pre-specified outcomes. Hand-searching of reference lists of eligible papers was also undertaken. The extracted data were synthesized using qualitative techniques, including a narrative summary approach to facilitate the interpretation of results. Short-term follow-up was considered as ≤ 12 months while studies reporting data > 12 months were classified as long term (Table 1).
PRISMA 2020 flow diagram for systematic reviews which included searches of databases and registers only. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71. https://doi.org/10.1136/bmj.n71
Key Dietary Measures
The dietary outcomes included total energy intake (kcal/day), macronutrient intake as a percentage of total energy or grams, micronutrient composition as a percentage of energy or amount (mg or µg/day), or amount of the nutrient as a proportion of dietary reference intake (DRI).
Methods of Meta-analysis
A meta-analysis was performed to investigate the impact of RYGB on total energy intake and macronutrients (%En). The meta-analysis was completed using the “metacont” command from the meta package of R software, version 4.2.3 [12].
Additionally, the meta-analysis was conducted using the “metamean” command from the “meta” package in R software, version 4.3.1 [12], and it assessed total energy intake (kcal/day), macronutrients composition as a percentage of energy (%En), before bariatric surgery and 6- and 12-month post-surgery. Quantitative variables were expressed as mean ± standard deviation (SD). The random-effects model was applied because observational studies are inherently heterogeneous. The statistical method used to weight the measures of association among the included studies was the inverse of the variance [raw means (MRAW)].
The primary criterion for including studies in the meta-analysis was the presence of complete data (mean and SD) at the specified time points (0, 6, 12, and 24 months). The second criterion was to refrain from analyzing two different types of bariatric surgery (RYGB and SG) together. Since there are limited studies at the 24-month time point, RYGB at the specified time points including 0, 6, and 12 months were chosen for the analysis (n = 16).
Energy Intake and Weight Loss
A secondary objective of this review was to determine if daily energy intake is a major determinant of weight loss. Hence, we conducted a meta-analysis to investigate the relationship between average daily energy intake and excess weight loss (EWL) at 12-month post-surgery. To classify the results and establish a comparison parameter, a modification of the Reinhold classification was applied. In this modified classification, an excellent outcome is defined as a reduction in excess weight greater than 75%, a good result falls within a range of 50 to 75% reduction in excess weight, and a failure occurs if the weight loss is less than 50%.
Results
The initial search identified 7322 articles. Following the removal of duplicates and studies that did not meet the eligibility criteria, 540 studies were screened for relevance by title and abstract review, and an additional 18 records were identified through a manual search of the reference lists of eligible articles. Further details are presented in the PRISMA diagram (Fig. 1). A total of 42 studies were eligible for full-text data extraction and analysis.
Study Design
Of the 42 studies included, 27 were longitudinal prospective studies, 13 were cross-sectional, and two were case–control studies.
Demographic and Clinical Characteristics
The age of participants ranged between 30 and 50 years. Participants were predominantly female; specifically, 59% (n = 24) of the studies had more females than males, 29% (n = 12) had female-only participants, and 12% (n = 5) had a similar number of males and females. The mean sample size across studies was 74 ± 63 participants and ranged from 10 [13] to 355 [14] participants. The follow-up period ranged from 6 months [15,16,17] to 8 years of post-surgery [18]. Twenty-nine (70%) studies utilized RYGB, five (12%) SG and seven (17%) studies compared RYGB and SG.
Twenty-four percent of studies (n = 10) were conducted between 2000 and 2010, 42% (n = 17) between 2011 and 2015, and 34% (n = 14) were between 2016 and 2020. There were 15% (n = 6) from North America, 39% (n = 16) from Europe, 34% (n = 14) from South America, 7% (n = 3) from the Middle East, and 5% (n = 2) from East Asia.
Methods of Dietary Assessment
Various dietary assessment methods were employed across different studies. Nineteen (46%) studies utilized a food recall [13, 16, 18,19,20,21,22,23,24,25,26,27,28,29,30], and seventeen (41%) used dietary records [14, 28, 31,32,33,34,35,36,37,38,39,40], while the remaining studies used food frequency questionnaires (n = 8; 20%) [17, 41,42,43,44,45] or employed mixed methods (n = 5; 12%) [15, 17, 41, 45, 46]. The best method regarding flexibility, accuracy, and reflection of the actual diet is a dietary diary. Food diaries do not rely on memory and thus are considered more accurate than 24-h food recall and food frequency questionnaires (FFQs) [47]. In addition, FFQs are restricted to specific food items of the questionnaire and may fail to fully capture habitual dietary intake. Dietary assessment is crucial to accurately assess the impact of bariatric surgery on dietary intake and the impact of dietary intake on surgical outcomes.
Daily energy (kcals) was reported in 90% of studies (n = 37) [8, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46, 48,49,50,51,52]; all studies reported macronutrient intake whereas only ten studies (24%) [14, 19, 21, 26, 30, 33, 35, 38, 43, 45] reported micronutrient intake.
Studies Comparing RYGB and SG
Only seven (17%) studies investigated the difference in energy and nutrient intake between RYGB and SG [8, 14,15,16, 24, 25, 48]. Six of these comparative studies were conducted over a short period of postoperative observation (6–12-month post-surgery) and found no significant differences in dietary intake, including energy and macronutrient intake, between RYGB and SG [8, 15, 16, 24, 25, 48]. Additionally, no differences in weight loss between RYGB and SG were reported. Only one of the seven studies investigated the long-term (> 12-month post-surgery) difference in dietary intake [14]. This was a 5-year prospective study, and its results also show no difference in dietary intake or weight loss outcomes.
Energy and Macronutrient Intakes
Energy and macronutrient intakes were assessed using a dietary diary in 54% of the included studies, whereas 15% used FFQs and 39% used 24-h dietary recalls.
The weighted mean (WM) of total energy intake before surgery was 2049 kcal/day (95% CI: 1845; 2252). There was a decrease in energy intake at 6-month post-surgery; WM, 1038 kcal/day (95% CI, 935; 1141), followed by a slight increase at 12-month post-surgery WM, 1284 kcal/day (95% CI, 1134; 1433) [8, 18, 27,28,29, 38,39,40, 42,43,44,45,46, 52, 53].
Carbohydrate intakes remained relatively unchanged by surgery; WM of intake before surgery was 49%En (95% CI: 43; 54), 44%En (95% CI: 40; 49) at 6 months and 46%En (95% CI: 42; 49) at 12-month post-surgery [19, 21, 22, 24,25,26, 31,32,33,34,35,36,37, 40, 48, 49, 51, 52, 54]. Carbohydrate intake pre-surgery and at all time points, post-surgery was within the AMDRs (45–65%En).
Protein intake before surgery had a WM of 16%En (95% CI: 13; 19), and intake increased to 19%En (95% CI, 16; 22) at 6 months, which was maintained at 12 months 18%En (95% CI, 16; 21) [8, 18, 27,28,29, 38,39,40, 42,43,44,45,46, 52, 53]. Protein intake pre-surgery and at all time points, post-surgery was within the AMDRs (10–35%).
Fat intake WM was 36%En (95% CI: 32; 41) before surgery and remained unchanged at 6- and 12-month post-surgery (6 months 36%En (95% CI: 31; 41); 12 months 36% En (95% CI: 33; 39)) [8, 18, 27,28,29, 38,39,40, 42,43,44,45,46, 52, 53]. Fat intake was slightly higher than AMDRs (25–35%) pre-surgery and at all time points after surgery.
The change in dietary intake following RYGB surgery in short term (< 12 months) was more commonly reported than long term (> 12 months), therefore the meta-analysis was only performed on short-term data, < 12 months. There was a significant reduction in energy intake by 1003 kcal/d (MD = − 1003, 95% CI − 1145 to − 862; p < 0.001) at 6 months compared to before RYGB (Fig. 2). A significant reduction in energy intake from baseline was also observed at 12-month post-RYGB by a mean of 886 kcal/d (MD = − 886, 95% CI − 1039 to − 732; p < 0.001) (Fig. 3).
Compared to pre-surgery intakes there was no significant difference in any of the macronutrients (%En) at 6 months (carbohydrates MD = − 4.3, 95% CI − 9.0 to 0.5, p < 0.08; protein MD = 3.4, 95% CI − 2.0 to 8.8, p = 0.22; fat MD = − 0.23, 95% CI − 1.8 to 1.4; p = 0.77) or 12 months after RYGB (carbohydrates MD = − 2.7, 95% CI − 6.5 to 1.1, p = 0.17; protein MD = 1.8, 95% CI − 2.1 to 5.7, p = 0.36); fat MD = − 0.01, 95% CI − 1.6953 to 1.6731, p = 0.99) Table 2.
Micronutrients Intake
Only ten studies investigated the micronutrient intake following RYGB and/or SG [14, 19, 21, 26, 30, 33, 35, 38, 43, 45]. As summarized in Table 2, in the short term (≤ 12 months), vitamin B12 intakes met the UK RDA of 2.4 µg/day in all the studies while zinc intake was < 8 mg/day in two studies [33, 35] at 3- and 12- month post-surgery. Dias et al. reported iron intake < 8 mg/day at 3- and 12-month post-surgery [33]. In the long term (> 12-month post-surgery), Silveria et al. reported vitamin B12 and zinc intakes in line with the UK RDAs, but iron remained suboptimal at 20-month post-surgery (Table 3; [43]). Overall, both surgical procedures resulted in lower intakes of folic acid, vitamins D, E, C, and calcium, in the long term [14, 26, 30, 38, 39, 45].
Relationship Between Weight Loss and Dietary Intake
A specific objective of this review was to test if daily energy intake is a major determinant of weight loss in the included studies. Total energy intake (Total EI, kcal/d) and percentage of excess weight loss (EWL%) reduction were assessed in a meta-analysis. Five studies were included in the subgroup ≥ 50 EWL% and < 75 EWL%, comprising a total of 211 patients. For this subgroup, at a 12-month follow-up, there was an average reduction of 861 kcal/d in a before and after analysis of RYGB (MD = − 861, 95% CI − 1324 to − 398; I2 = 98%).
In the analysis of a second subgroup, including two studies and a total of 108 patients with EWL% ≥ 75%, the average reduction in Total EI was 887 kcal/d in a before and after RYGB analysis (MD = − 887, 95% CI − 919 to − 855; I2 = 0%). Figure 4 illustrates the association between energy intake (kcal/day) before and after RYGB surgery and the EWL% 12-month post-surgery.
The association between energy intake (kcal/day) both before and after RYGB surgery and the percentage of excess weight loss (EWL%) 12-month post-surgery, including subgroup analysis (Number of studies = 7). SD = standard deviation; CI = confidence interval; pct = %. A successful outcome is defined as achieving ≥ 75% of EWL, an acceptable outcome falls within the range of 50% to 75% of EWL, and an unsuccessful outcome is indicated by < 50% of EWL
In the overall analysis, there was no difference in the reduction of total energy consumption between the two subgroups (test for subgroup differences (random effects model, p = 0.91)). These results show that there is no association between total energy intake (kcal/day) before and after RYGB surgery and the EWL% at 12-month post-surgery.
Discussion
This review reports on the impact of bariatric surgery, both RYGB and SG, on total energy, and macro- and micro-nutrient intake. The findings indicate that there are no significant differences in energy intake, including energy and macronutrient intake, between RYGB and SG. Twelve months post-bariatric surgery, the proportion of carbohydrate intakes was reported to range between 35 and 53% of total energy intake, which largely aligns with AMDRs (45–65%). Protein intake was reported to range between 10 and 52%, suggesting that some patients have higher protein intake than AMDRs (10–35%). However, protein intake after bariatric surgery is generally found to fall below the recommended intake [20]. The American Society for Metabolic and Bariatric Surgery guidelines suggest that the daily protein intake should be between 60 and 120 g (1–1.5 per kilogram of desirable body weight) after surgery [55]. At 6-month post-surgery, we found that the reported average daily protein intake ranged widely from a minimum of 19.5 g/day (below recommended intake) to a maximum of 101.5 g/day (within recommended levels of intake).
Similarly, the daily fat intake ranged from 28 to 40%, which is slightly higher than the recommended AMDRs (25–35%). Micronutrient intake of zinc and iron appears lower than the recommended daily intake levels after surgery. Remarkably, and contrary to widespread assumptions, we found no relationship between average total daily energy intake and the degree of excess weight loss, or BMI achieved 12-month post-surgery.
More studies compared RYGB with gastric banding than with SG [55,56,57,58]; reflecting the relatively more recent introduction of SG in clinical practice [59]. Moreover, of the studies that compared RYGB with SG, the majority investigated eating behaviors rather than dietary intake [60,61,62,63]. Only seven studies compared dietary intake between RYGB and SG [8, 14,15,16, 24, 25, 48]. The most robust among these studies was conducted by Moizé and colleagues and investigated the changes in dietary intake over 5 years of post-surgery in a Mediterranean population. The authors found no difference between RYGB and SG in dietary intake. The daily dietary intake of micronutrients, including calcium, magnesium, phosphorus, and iron, was lower than the RDA for both types of surgery. However, a significant limitation of this study was its uneven distribution of participants between the two groups (RYGB, n = 294 versus SG, n = 61) [14].
Common limitations of studies comparing dietary intake between RYGB and SG were the short-term follow-up period and the paucity/absence of micronutrient intake data. Furthermore, energy intake in relation to weight loss may be influenced by physical activity [56], and this was not addressed in any of these studies.
There were 35 out of 42 studies reviewed here that examined the impact of RYGB and SG independently on dietary intake. The number of independent descriptive studies is higher than comparative studies on dietary intake after RYGB and SG. In addition, there are more descriptive studies on RYGB than on SG, especially in relation to the long-term outcomes.
In this systematic review, only 11 out of 42 studies investigated the micronutrient intake following RYGB and/or SG. In the short-term (≤ 12 months), vitamin B12 intakes met the RDA while zinc intake was less than 8 mg/day. In the longer term (> 12 months), vitamin B12 and zinc intakes were in line with the RDAs, but iron intake appeared to remain suboptimal at 20-month post-surgery. There are many studies on micronutrient status, however, far fewer studies are available on the micronutrient intake following RYGB and/or SG. Therefore, more studies are needed to further investigate the impact of bariatric surgery on micronutrient intake.
Much of the available evidence from studies that investigated dietary intake in relation to weight loss maintenance suggests that energy intake is not associated with long-term maintenance of weight loss following bariatric surgery [18, 31, 42, 45, 57]. In this systematic review, we have found no relationship between energy intake and weight loss after bariatric surgery. The finding that energy intake is not related to the weight loss outcomes of bariatric surgery is in contrast with the widespread belief that overeating is the cause of inadequate weight loss after bariatric surgery [46, 58,59,60].
Our review cannot make a firm conclusion on whether energy intake may contribute instead to long-term weight regain after surgery. Obesity is a multifactorial condition, and it is therefore plausible that resilient and/or recurrent pathophysiologic mechanisms may predispose individuals to disease recurrence. Bariatric surgery imposes substantial anatomical alterations to the GI tract [61]. Given the multiple metabolic and endocrine functions of the GI tract, changing the anatomy of the stomach and small intestine may affect energy homeostasis through several physiological mechanisms rather than merely due to mechanical restriction of energy intake [62]. Nutrient passage through the GI tract elicits secretion of several GI hormones (glucagon-like peptide-1, peptide YY, oxyntomodulin, GLP-2, glucose-dependent insulinotropic polypeptide, ghrelin) that play important roles in the regulation of hunger, satiety, and other metabolic functions [63,64,65,66]. In this context, the outcome of the interplay between the physiologic effects of GI surgery and pathophysiologic mechanisms of obesity is more likely to determine weight loss outcomes than either mechanical or voluntary restrictions of food intake.
Obesity is a complex, multifactorial medical condition caused by numerous factors, such as behavioral, psychological, biological, and social factors. Additionally, dietary intake is highly influenced by cultural background, socioeconomic setting, geographic environment, and product availability [67, 68]. These influencing factors were not considered in this systematic review, thus future systematic reviews should consider such an investigation. Additionally, methods used to assess dietary and nutrient intake are associated with known biases that can affect reporting of intakes, but this is a known limitation of dietary and nutrient assessment.
Conclusions and Future Directions
In general, there is a paucity of comparative studies investigating the impact of RYGB and SG on dietary intake. Furthermore, most studies, both descriptive and comparative, investigated only short-term changes in energy intake and fewer data are available about the intake of micronutrients after bariatric surgery than macronutrients. Available data, however, do not support widespread assumptions that excess energy intake may explain poorer weight loss outcomes of bariatric surgery. While the role of energy intake on longer-term weight maintenance/weight regain needs to be further investigated, the results of this review suggest caution in attributing the efficacy of surgical treatment of obesity to a mere reduction of food intake.
More studies comparing nutrition intake after the two most performed bariatric procedures, especially in the long term, are needed. Further research is also necessary to understand the exact role of gastric versus intestinal anatomic manipulations in regulating post-operative energy intake and the role of energy intake as a mechanism of weight loss or metabolic control. More studies are also required to better assess the micro- and macro-nutrient intake after surgery using robust dietary assessment methods and to improve nutrition care strategies.
References
Rubino F. From bariatric to metabolic surgery: definition of a new discipline and implications for clinical practice. Curr Atheroscler Rep. 2013. https://doi.org/10.1007/s11883-013-0369-x.
Pareek M, Schauer PR, Kaplan LM, et al. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol. 2018;71:670–87.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. J Am Med Assoc. 2004;292:1724–37.
Osland E, Yunus RM, Khan S, et al. Weight loss outcomes in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech. 2017;27:8–18.
Tadross JA, Le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes. 2009;33:S28–32.
Cambi MPC, Baretta GAP. Bariatric diet guide: plate model template for bariatric surgery patients. ABCD Arquivos Brasileiros de Cirurgia Digestiva (São Paulo). 2018;31:4–7.
Lee E, Choi J, Ahn A, et al. Acceptable macronutrient distribution ranges and hypertension. Clin Exp Hypertens. 2015;37:463–7.
Moizé V, Andreu A, Rodríguez L, et al. Protein intake and lean tissue mass retention following bariatric surgery. Clin Nutr. 2013;32:550–5.
Dagan SS, Goldenshluger A, Globus I, et al. Nutritional Recommendations for adult bariatric surgery patients: clinical practice. Adv Nutr. 2017;8:382–94.
Parrott J, Frank L, Rabena R, et al. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 update: micronutrients. Surgery for Obesity and Related Diseases. 2017;13:727–41.
Roust LR, Dibaise JK. Nutrient deficiencies prior to bariatric surgery. Curr Opin Clin Nutr Metab Care. 2017;20:138–44.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2023. https://www.R-project.org/
Da Silva MM, Sala PC, Cardinelli CS, et al. Comparison of virtual nutri plus® and dietpro 5i® software systems for the assessment of nutrient intake before and after roux-en-y gastric bypass. Clinics. 2014;69:714–22.
Moizé V, Andreu A, Flores L, et. al (2013) Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or roux-en-y gastric bypass in a mediterranean population. J Acad Nutr Diet. https://doi.org/10.1016/j.jand.2012.11.013
El Labban S, Safadi B, Olabi A. The effect of Roux-en-Y gastric bypass and sleeve gastrectomy surgery on dietary intake, food preferences, and gastrointestinal symptoms in post-surgical morbidly obese Lebanese subjects: a cross-sectional pilot study. Obes Surg. 2015;25:2393–9.
Golzarand M, Toolabi K, Djafarian K. Changes in body composition, dietary intake, and substrate oxidation in patients underwent laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a comparative prospective study. Obes Surg. 2018;29:406–13.
Molin Netto BD, Earthman CP, Farias G, et al. Eating patterns and food choice as determinant of weight loss and improvement of metabolic profile after RYGB. Nutrition. 2017;33:125–31.
Kruseman M, Leimgruber A, Zumbach F, et al. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc. 2010;110:527–34.
Gesquiere I, Foulon V, Augustijns P, et al. Micronutrient intake, from diet and supplements, and association with status markers in pre- and post-RYGB patients. Clin Nutr. 2017;36:1175–81.
Faria SL, Faria OP, Buffington C, et al. Dietary protein intake and bariatric surgery patients: a review. Obes Surg. 2011;21:1798–805.
Miller GD, Norris A, Fernandez A. Changes in nutrients and food groups intakes following laparoscopic Roux-en-Y gastric bypass (RYGB). Obes Surg. 2014;71:1926–32.
Carrasco F, Rojas P, Csendes A, et al. Changes in ghrelin concentrations one year after resective and non-resective gastric bypass: associations with weight loss and energy and macronutrient intakes. Nutrition. 2012;28:757–61.
Dagan SS, Ben TT, Keidar A, et al. Inadequate protein intake after laparoscopic sleeve gastrectomy surgery is associated with a greater fat free mass loss. Surg Obes Relat Dis. 2017;13:101–9.
Verger EO, Aron-Wisnewsky J, Dao MC, et al. Micronutrient and protein deficiencies after gastric bypass and sleeve gastrectomy: a 1-year follow-up. Obes Surg. 2015;26:785–96.
Lim H-SS, Kim YJ, Lee J, et al. Establishment of adequate nutrient intake criteria to achieve target weight loss in patients undergoing bariatric surgery. Nutrients. 2020;12:1–12.
Ruz M, Carrasco F, Rojas P, et al. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–32.
Reid RER, Oparina E, Plourde H, et al. Energy intake and food habits between weight maintainers and regainers, five years after Roux-en-Y gastric bypass. Can J Diet Pract Res. 2016;77:195.
Ortega J, Ortega-Evangelio G, Cassinello N, et al. What are obese patients able to eat after Roux-en-Y gastric bypass? Obes Facts. 2012;5:339–48.
Benson-Davies L, Davies L, Kattelmann L, et al. Food preferences in patients after Roux-en Y gastric bypass surgery: a pilot study examining eating behaviors and weight maintenance. Top Clin Nutr. 2013;28:8–14.
de Torres Rossi RG, dos Santos MTA, Souza FIS, et al. Nutrient intake of women 3 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2012;22:1548–53.
Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13:23–8.
Ferreira Nicoletti C, Morandi Junqueira-Franco MV, Dos Santos JE. Protein and amino acid status before and after bariatric surgery: a 12-month follow-up study. Surg Obes Relat Dis. 2013;9:1008–12.
Dias MCG, Ribeiro AG, Scabim VM, et al. Dietary intake of female bariatric patients after anti-obesity gastroplasty. Clinics. 2006;61:93–8.
Bavaresco M, Paganini S, Lima TP, et al. Nutritional course of patients submitted to bariatric surgery. Obes Surg. 2010;20:716–21.
Schieferdecker MEM, CAMPOS ACL, Taconeli CA, et al food intake evaluation during the first year of postoperative of patients with type 2 diabetes mellitus or glycemic alteration submitted to Roux-en-Y gastric bypass. Arquivos brasileiros de cirugía digestiva 31 2018:4–7
Pinto SL, Juvanhol LL, Bressan J. Increase in protein intake after 3 months of RYGB is an independent predictor for the remission of obesity in the first year of surgery. Obes Surg. 2019;29:3780–5.
Gjessing HR, Nielsen HJ, Mellgren G, et al. Energy intake, nutritional status and weight reduction in patients one year after laparoscopic sleeve gastrectomy. Springerplus. 2013. https://doi.org/10.1186/2193-1801-2-352.
Mercachita T, Santos Z, Limão J, et al. Anthropometric evaluation and micronutrients intake in patients submitted to laparoscopic Roux-en-Y gastric bypass with a postoperative period of ≥1 year. Obes Surg. 2014;24:102–8.
Wardé-Kamar J, Rogers M, Flancbaum L, et al. Calorie intake and meal patterns up to 4 years after Roux-en-Y gastric bypass surgery. Obes Surg. 2004;14:1070–9.
Coluzzi I, Raparelli L, Guarnacci L, et al. Food intake and changes in eating behavior after laparoscopic sleeve gastrectomy. Obes Surg. 2016;26:2059–67.
Sarwer DB, Moore RH, Gibbons AW, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4:640–6.
Laurenius A, Larsson I, Melanson KJ, et al. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur J Clin Nutr. 2013;67:168–73.
Leiro LS, Melendez-Araújo MS. Diet micronutrient adequacy of women after 1 year of gastric bypass. Arq Bras Cir Dig. 2014;27(Suppl 1):21–5. https://doi.org/10.1590/s0102-6720201400s100006.
Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7.
Chou JJ, Lee WJ, Almalki O, et al. Dietary intake and weight changes 5 years after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27:3240–6.
Freire RH, Borges MC, Alvarez-Leite JI, et al. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition. 2012;28:53–8.
Prentice RL, Mossavar-Rahmani Y, Huang Y, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174:591–603.
Coupaye M, Msika S, Dupré T, et al. Comparison of nutritional status during the first year after sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Surg. 2013;24:276–83.
Taylor MA, Szczerbinski L, Citko A, Niemira M, Gorska M, Hady HR, Kretowski A. Sex-specific glucose homeostasis and anthropometric responses to sleeve gastrectomy in obese patients. Nutrients. 2019;11(10):2408. https://doi.org/10.3390/nu11102408.
da Silva FBLBL, Gomes DLL, de Carvalho KMBMB, et al. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition. 2016;32:1250–3.
Bobbioni-Harsch E, Huber O, Morel P, et al. Factors influencing energy intake and body weight loss after gastric bypass. Eur J Clin Nutr. 2002;56:551–6.
Giusti V, Fanny T, Vetta D, et al. Energy and macronutrient intake after gastric bypass for morbid obesity: a 3-y observational study focused on protein consumption. Am J Clin Nutr. 2016;103:18–24.
Sarwer DB, Wadden TA, Moore RH, Eisenberg MH, Raper SE, Williams NN. Changes in quality of life and body image after gastric bypass surgery. Surg Obes Relat Dis. 2010;6(6):608–14. https://doi.org/10.1016/j.soard.2010.07.015.
Dagan SS, Keidar A, Raziel A, et al. Do bariatric patients follow dietary and lifestyle recommendations during the first postoperative year? Obes Surg. 2017. https://doi.org/10.1007/s11695-017-2633-6.
Mechanick JI, Kushner RF, Sugerman HJ, et al. American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariat. Obesity. 2009;17:S3–72.
Myers A, Dalton M, Gibbons C, et al. Structured, aerobic exercise reduces fat mass and is partially compensated through energy intake but not energy expenditure in women. Physiol Behav. 2018. https://doi.org/10.1016/j.physbeh.2018.11.005.
Sioka E, Tzovaras G, Oikonomou K, et al. Influence of eating profile on the outcome of laparoscopic sleeve gastrectomy. Obes Surg. 2012;23:501–8.
Brolin RE, Robertson LB, Kenler HA, et al. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg. 1994;220:782–90.
Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Monaco-Ferreira DV, Leandro-Merhi VA. Weight regain 10 years after Roux-en-Y gastric bypass. Obes Surg. 2017;27:1137–44.
Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–89.
Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care. 2013;36:287–91.
Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis. 2018;14:708–14.
Suzuki K, Jayasena CN, Bloom SR. The gut hormones in appetite regulation. J Obes. 2011;2011:528401.
Pedersen SD. The role of hormonal factors in weight loss and recidivism after bariatric surgery. Gastroenterol Res Pract. 2013;2013:528450. https://doi.org/10.1155/2013/528450.
Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–22.
Brug J. Determinants of healthy eating: motivation, abilities and environmental opportunities. Fam Pract. 2009. https://doi.org/10.1093/fampra/cmn063
Story M, Kaphingst KM, Robinson-O’Brien R, Glanz K. Creating healthy food and eating environments: policy and environmental approaches. Annu Rev Public Health. 2008;29:253–72. https://doi.org/10.1146/annurev.publhealth.29.020907.090926.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The principal investigator, Professor Francesco Rubino, reports receiving research funding from Medtronic, Johnson & Johnson, and Novo Nordisk, consulting fees from GI Dynamics and lecturing fees from Johnson & Johnson, Medtronic, and Novo Nordisk. Prof. Rubino also serves on the data safety advisory board of GT Metabolic Solutions and is President of the Metabolic Health Institute (non-profit). All other authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
There was no difference in dietary intake between Roux-en-Y gastric bypass and sleeve gastrectomy.
Daily energy intake after surgery does not correlate with postoperative weight loss outcomes.
Postoperative micronutrient intake of iron, calcium, and zinc is lower than recommended.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qanaq, D., O’Keeffe, M., Cremona, S. et al. The Role of Dietary Intake in the Weight Loss Outcomes of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy: A Systematic Review and Meta-analysis. OBES SURG (2024). https://doi.org/10.1007/s11695-024-07183-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11695-024-07183-8