Abstract
Purpose
A suitable option for severe obesity treatment is a surgical approach. After surgery, metabolic markers and weight frequently return to adequate values; however, concerning systemic inflammatory mediators, the results are inconsistent. Furthermore, it has been suggested that leucocyte function may be affected even after weight normalization.
This study aimed to determine if the surgical treatment of obesity influences the production of cytokines by LPS-stimulated as a function of leucocytes.
Materials and Methods
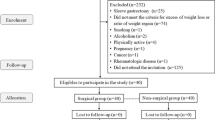
We performed a cross-sectional study that investigated the production of cytokines in response to lipopolysaccharide (LPS) along a kinetic of simulation by leucocytes recovered from individuals with normal weight (NW, n = 8), persons living with obesity (Ob, n = 7), persons living with obesity and diabetes mellitus (Ob-DM, n = 17), and persons that used to live with obesity who underwent bypass surgery (fOb + bypass, n = 8) and recover normal weigh.
Results
IL-6 levels were significantly higher in the Ob and fOb + bypass groups than in NW (p = 0.043). IL-10 secretion without LPS was significantly higher in the NW group than in the other groups explored (p < 0.05). When exposed to LPS, the IL-10 levels increased in all groups except the NW group. As also observed for IL-18 and IL-33, the secretion curve of the fOb + bypass group was more similar to the Ob group, even when they had reached normal weight, as opposed to the NW group.
Conclusion
Our results show that in patients with fOb + bypass, inflammatory and anti-inflammatory cytokine production dynamics remain disrupted even with improved metabolic control and normal weight recovery.
Graphical Abstract



Similar content being viewed by others
Data Availability
All data are available from the corresponding author upon request.
References
Organization WH. Obesity and overweight https://www.who.int/news/item/11-10-2017-tenfold-increase-in-childhood-and-adolescent-obesity-in-four-decadesnew-study-by-imperial-college-london-and-who.
Perdomo CM, Cohen RV, Sumithran P, Clement K, Fruhbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023;401(10382):1116–30. https://doi.org/10.1016/S0140-6736(22)02403-5.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. https://doi.org/10.1038/nature05485.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. https://doi.org/10.3390/ijms15046184.
Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochem Biophys Acta. 2014;1842(3):446–62. https://doi.org/10.1016/j.bbadis.2013.05.017.
Moreno-Navarrete JM, Escote X, Ortega F, Serino M, Campbell M, Michalski MC, et al. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56(11):2524–37. https://doi.org/10.1007/s00125-013-3015-9.
Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes Targets Ther. 2014;7:587–91. https://doi.org/10.2147/DMSO.S67400.
Fruhbeck G, Gomez-Ambrosi J. Control of body weight: a physiologic and transgenic perspective. Diabetologia. 2003;46(2):143–72. https://doi.org/10.1007/s00125-003-1053-4.
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–85. https://doi.org/10.1038/nature21363.
Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(10):2322–30. https://doi.org/10.1161/ATVBAHA.111.230979.
Park JY. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr. 2018;27(4):213–22. https://doi.org/10.7570/jomes.2018.27.4.213.
Choban PS, Jackson B, Poplawski S, Bistolarides P. Bariatric surgery for morbid obesity: why, who, when, how, where, and then what? Cleve Clin J Med. 2002;69(11):897–903. https://doi.org/10.3949/ccjm.69.11.897.
Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;(8):CD003641. https://doi.org/10.1002/14651858.CD003641.pub4.
Illan-Gomez F, Gonzalvez-Ortega M, Orea-Soler I, Alcaraz-Tafalla MS, Aragon-Alonso A, Pascual-Diaz M, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5. https://doi.org/10.1007/s11695-012-0643-y.
Poitou C, Perret C, Mathieu F, Truong V, Blum Y, Durand H, et al. Bariatric surgery induces disruption in inflammatory signaling pathways mediated by immune cells in adipose tissue: a RNA-Seq study. PLoS One. 2015;10(5):e0125718. https://doi.org/10.1371/journal.pone.0125718.
Sams VG, Blackledge C, Wijayatunga N, Barlow P, Mancini M, Mancini G, et al. Effect of bariatric surgery on systemic and adipose tissue inflammation. Surg Endosc. 2016;30(8):3499–504. https://doi.org/10.1007/s00464-015-4638-3.
Freitas WR Jr, Oliveira LVF, Perez EA, Ilias EJ, Lottenberg CP, Silva AS, et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg. 2018;28(7):1931–42. https://doi.org/10.1007/s11695-017-3104-9.
Moulin CM, Marguti I, Peron JP, Halpern A, Rizzo LV. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes Surg. 2011;21(1):112–8. https://doi.org/10.1007/s11695-010-0250-8.
Cuellar-Tamez RX, Villarreal-Calderon JR, Rubio-Infante N, Castillo EC, Garcia-Garza M, Elizondo-Montemayor L, et al. Bariatric surgery-induced weight loss reduces B cell activating cytokines and IgG immunoglobulins related to autoimmunity. Surg Endosc. 2020. https://doi.org/10.1007/s00464-020-08004-6.
Viana EC, Araujo-Dasilio KL, Miguel GP, Bressan J, Lemos EM, Moyses MR, et al. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-alpha. Prospect Clin Trial Obes Surg. 2013;23(8):1252–61. https://doi.org/10.1007/s11695-013-0894-2.
Jurets A, Itariu BK, Keindl M, Prager G, Langer F, Grablowitz V, et al. Upregulated TNF expression 1 year after bariatric surgery reflects a cachexia-like state in subcutaneous adipose tissue. Obes Surg. 2017;27(6):1514–23. https://doi.org/10.1007/s11695-016-2477-5.
Lo T, Rudge EJM, Chase RP, Subramaniam R, Heshmati K, Lucey EM et al. Early changes in immune cell metabolism and function are a hallmark of sleeve gastrectomy: a prospective human study. medRxiv. 2020;. https://doi.org/10.1101/2020.07.31.20161687.
Min T, Prior SL, Dunseath G, Churm R, Barry JD, Stephens JW. Temporal effects of bariatric surgery on adipokines, inflammation and oxidative stress in subjects with impaired glucose homeostasis at 4 years of follow-up. Obes Surg. 2020;30(5):1712–8. https://doi.org/10.1007/s11695-019-04377-3.
Lylloff L, Bathum L, Madsbad S, Grundtvig JLG, Nordgaard-Lassen I, Fenger M. S100A8/A9 (calprotectin), interleukin-6, and C-reactive protein in obesity and diabetes before and after Roux-en-Y gastric bypass surgery. Obes Facts. 2017;10(4):386–95. https://doi.org/10.1159/000478097.
Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999;99(3):294–9. https://doi.org/10.1016/S0002-8223(99)00077-2.
Devevre EF, Renovato-Martins M, Clement K, Sautes-Fridman C, Cremer I, Poitou C. Profiling of the three circulating monocyte subpopulations in human obesity. J Immunol. 2015;194(8):3917–23. https://doi.org/10.4049/jimmunol.1402655.
Kopp A, Bala M, Weigert J, Buchler C, Neumeier M, Aslanidis C, et al. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine. 2010;49(1):51–7. https://doi.org/10.1016/j.cyto.2009.10.001.
Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, et al. Increased levels of calprotectin in obesity are related to macrophage content: impact on inflammation and effect of weight loss. Mol Med. 2011;17(11–12):1157–67. https://doi.org/10.2119/molmed.2011.00144.
Lowry SF VZK, Rock CS, Thompson WA, Oldenburg HSA, Rogy MA, Moldawer LL. Tumor necrosis factor as a mediator of sepsis. In: Schlag GA RH, Berlin, Heidelberg, Springer, editor. Shock, Sepsis and Organ Failure 1993;13–4.
Newton RC, Uhl J, Covington M, Back O. The distribution and clearance of radiolabeled human interleukin-1 beta in mice. Lymphokine Res. 1988;7(3):207–16.
Castell J, Klapproth J, Gross V, Walter E, Andus T, Snyers L, et al. Fate of interleukin-6 in the rat. Involvement of skin in its catabolism. Eur J Biochem. 1990;189(1):113–8. https://doi.org/10.1111/j.1432-1033.1990.tb15466.x.
Wang X, Wong K, Ouyang W, Rutz S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol. 2019;11(2). https://doi.org/10.1101/cshperspect.a028548.
Gotoh K, Fujiwara K, Anai M, Okamoto M, Masaki T, Kakuma T, et al. Role of spleen-derived IL-10 in prevention of systemic low-grade inflammation by obesity [Review]. Endocr J. 2017;64(4):375–8. https://doi.org/10.1507/endocrj.EJ17-0060.
Sabater M, Moreno-Navarrete JM, Ortega FJ, Pardo G, Salvador J, Ricart W, et al. Circulating pigment epithelium-derived factor levels are associated with insulin resistance and decrease after weight loss. J Clin Endocrinol Metab. 2010;95(10):4720–8. https://doi.org/10.1210/jc.2010-0630.
Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88(3):1055–8. https://doi.org/10.1210/jc.2002-021437.
Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in health and disease. Int J Mol Sci. 2019;20(3). https://doi.org/10.3390/ijms20030649.
Acknowledgements
All the authors acknowledge and appreciate the hard work of the M.Sc. Esteban Domínguez-Cerezo for helping with data collection. We recognize and thank the bariatric surgeons: Dr. Enrique Luque de León and Dr. Arturo Rodríguez González, from the Gastrointestinal Surgery Department, UMAE Hospital de Especialidades-IMSS, for referencing the included patients. We also thank Dr. Constantino López-Macías for carefully reviewing the different versions of the manuscript. We appreciate the technical support of Jessica L. Prieto-Chávez at the Cytometry Laboratory of the Health Research Coordination (CIS), located at CMN Siglo XXI of the IMSS.
Funding
The study was supported by the FIS/IMSS/PROT/G16/1579 grant from IMSS granted to EFO.
Author information
Authors and Affiliations
Contributions
LAAP, EFO, and FJSG proposed and discussed the seed idea. EFO and LCR recollected the samples and filled the database. LCR treated the samples and performed stimulation assays. ACV, LCR, IMH, and LAAP performed flow cytometry analysis for leucocyte immunophenotyping and cytokine/chemokine immunoassays. ACV, IMH, and LAAP performed statistical analysis. ACV and DCE organized data and wrote the first draft of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Disrupted leucocyte response to LPS remains after bariatric surgery.
• Bariatric surgery induces metabolic control but does not recover leucocyte function.
• Deregulated cytokine production even after weight normalization/bypass surgery.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cérbulo-Vázquez, A., Cabrera-Rivera, L., Mancilla-Herrera, I. et al. Metabolic Recovery with the Persistence of Proinflammatory Leucocyte Dysfunction After Bariatric Intervention for Obesity. OBES SURG 34, 1575–1583 (2024). https://doi.org/10.1007/s11695-024-07135-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-024-07135-2