Abstract
Introduction
Bariatric surgery alleviates certain aspects of pelvic floor disorder, but the effect on pelvic organ prolapse (POP) is unclear. To assess the effect of bariatric surgery on POP we conducted the present meta-analysis and firstly performed a subgroup analysis based on the duration of follow-up.
Methods
Four databases including PubMed, The Cochrane Library, Web of Science, and Embase were searched to identify relevant studies published before February 24, 2023. The main outcome was the prevalence and severity of POP symptoms before and after bariatric surgery. Then we assessed the heterogeneity, publication bias and performed subgroup analyses based on follow-up time, study quality and region.
Results
Eleven studies with a total of 696 participants met the inclusion criteria. The results showed that the prevalence of POP decreased after bariatric surgery (odds ratio[OR] = 2.29, 95% confidence interval[CI]: 1.05, 5.01; P = 0.04, I2 = 78%), with significant differences observed both at 3–6 months (OR = 2.24, 95% CI: 1.25, 4.01; P = 0.007, I2 = 59%) and 12 months (OR = 4.64, 95% CI: 2.83, 7.58; P < 0.0001, I2 = 0%) of follow-up compared with pre-surgery. Pelvic Organ Prolapse Distress Inventory scores 6-item also decreased after bariatric surgery (mean difference [MD] = 2.11, 95% CI: 0.32, 3.89; P = 0.02, I2 = 55%) with significant differences observed both at 3–6 months (MD = 3.72; 95% CI: [0.10, 7.34], P = 0.04, I2 = 70%) and ≥ 12 months (MD = 3.24; 95% CI: [0.56, 5.91], P = 0.02, I2 = 56%) of follow-up.
Conclusion
Bariatric surgery alleviated POP symptoms in women with obesity both during short-term (3–6 months) and long-term (≥ 12 months) follow-up.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the improvement of living standards and changes in dietary habits, the number of people with obesity is increasing worldwide. Obesity is an epidemic disease that can adversely affect overall health and is associated with metabolic disorders such as diabetes, cardiovascular disease, polycystic ovary syndrome and pelvic floor disorders (PFDs) [1,2,3].
Pelvic organ prolapse (POP), one of the most common PFDs, is caused by the weakening of supporting structures of the pelvic floor [4]. POP is a common gynecologic disorder especially in postmenopausal women, with a prevalence of approximately 50%; although not fatal, it can seriously undermine the quality of life (QoL) of individuals [5, 6]. Obesity is a risk factor for PFD; [7, 8] increased intra-abdominal and intravesical pressure are known to be positively correlated with BMI in individuals with obesity [9]. Additionally, obesity is associated with the occurrence of diabetes mellitus and hypertension, which are also risk factors for PFD [10, 11].
Recent studies have described the effect of weight loss on PFDs symptoms in patients with obesity. Bariatric surgery (e.g., Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic adjustable gastric band) is an effective intervention for the treatment of obesity and related comorbidities [12, 13]. Several recent studies have demonstrated the positive effect of bariatric surgery on certain aspects of PFD such as urinary incontinence (UI) [14, 15], fecal incontinence (FI) [16], and sexual dysfunction[17]; and two recent meta-analyses also evaluated the effect of bariatric surgery on POP symptoms, while the conclusions were contradictory [18, 19].
With the rising rates of obesity among women, the demand for services to treat associated health risks is projected to increase. The prevalence of POP is also increasing continually with the aging of the human population which is a major public health concern that warrant more extensive investigation. In order to determine whether bariatric surgeries have the effect on alleviating POP symptoms, we performed a meta-analysis of published studies on POP symptoms in patients with surgically induced weight loss.
Materials and Methods
The study protocol of this meta-analysis was registered with PROSPERO (registration no. CRD42023407714; http://www.crd.york.ac.uk/PROSPERO) and obeyed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [20].
Data Source and Search Strategy
Four electronic databases (PubMed, Embase, Web of Science, and Cochrane) were searched for relevant studies. The final search was carried out on February 24, 2023 using the key words “bariatric surgery” and “pelvic organ prolapse”. The full search string in PubMed was as follows: (“bariatric surgery”[MeSH] OR bariatric surgery OR bariatric OR gastric bypass OR duodenal switch OR gastric balloon OR lap-band OR AspireAssist OR vBloc therapy OR metabolic surgery OR weight loss surgery) AND (“Pelvic floor disorders”[MeSH] OR “pelvic organ prolapse”[MeSH] OR “cystocele”[MeSH] OR “rectocele”[MeSH] OR “uterine prolapse”[MeSH] OR “rectal prolapse”[MeSH] OR cystocele OR rectocele OR pelvic floor disorder OR pelvic floor dysfunction OR pelvic organ prolapse OR uterine prolapse OR rectal prolapse OR POP).
Study Selection
Inclusion criteria were as follows: i) studies evaluating the effects of bariatric surgery on POP with both pre- and postoperative data; ii) peer-reviewed study in English; and iii) no restrictions on the type of operation and follow-up time. Exclusion criteria were as follows: i) studies lacking of experimental study design (e.g., reviews, conference abstracts, case reports, etc.); ii) experiments performed on cells and animals; and iii) non-relevant or lacking data required for analysis.
Data Screening and Extraction
Selected articles were exported to Endnote and duplicates were discarded. Two reviewers independently cataloged and organized the studies. Disagreements were resolved by consulting a third investigator. Two primary reviewers independently performed data extraction according to a predesigned form. The extracted data were first author; publication year; country; study type; age range; sample size; follow-up time; type of surgery and pre- and post-operation body mass index (BMI) and POP symptoms of participants.
Measurement of Outcomes
In most of the studies, POP symptoms were evaluated with the Pelvic Organ Prolapse Distress Inventory 6-item (POPDI-6) and Pelvic Organ Prolapse Impact Questionnaire 7-item (POPIQ-7); these are subscales of Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire, respectively, which are two validated scales to assess the adverse impact of PFD on QoL [21]. The POPDI-6 has 6 questions and the POPIQ-7 has 7, each pertaining to whether a symptom exists and the degree to which it negatively affects the respondent’s QoL. Both the POPDI-6 and POPIQ-7 are scored from 0 (least distress) to 100 (greatest distress), with a higher score indicating greater symptom severity and more negative effects on QoL (Table 1).
Risk of Bias Assessment
All included studies were cohort studies. The Newcastle–Ottawa Scale (NOS) [22] was used to assess study quality; all of the included studies had a score ≥ 5, and those with a score ≥ 7 were classified as being of high quality (Table 2).
Statistical Analysis
Statistical analysis was performed using Review Manager v5.3 (https://training.cochrane.org/online-learning/core-software/revman) and Stata v12.0 (https://www.stata.com/). We calculated mean difference (MD) with 95% confidence interval (CI) for continuous variables and odds ratio (OR) with 95% CI for dichotomous variables. The chi-squared statistic and I2 tests were used to calculate heterogeneity and P < 0.05 or I2 > 50% was assessed as high heterogeneity [23]. When heterogeneity was high, a random-effects model was used and sensitivity analysis was conducted to evaluate the robustness of the results (Supplementary Fig. 1). Begg’s test[24] was used to evaluate potential publication bias.
Results
Literature Search
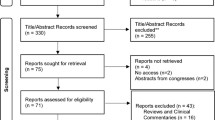
The literature search strategy is illustrated in Fig. 1 and 11 studies [25,26,27,28,29,30,31,32,33,34,35] were ultimately included in the meta-analysis.
Characteristics and Quality of Included Studies
The characteristics of the included studies are summarized in Table 1. In total, there were 696 participants in the 11 included studies, which all had a prospective cohort design. Four studies were conducted in the United States [25, 28, 29, 33], 3 in Israel [27, 30, 31, 35], 2 in France [32, 34], and 1 in Italy [26]. Five studies focused on the prevalence of POP [25,26,27,28,29], of which 3 also reported changes in POPDI-6 [26,27,28] and 2 also reported POPIQ-7 (n = 2)[26, 28] scores; the remaining 6 studies reported changes in POPDI-6 (n = 5)[31,32,33,34,35] or POPIQ-7 (n = 1)[30] scores. Of the 5 studies that examined the prevalence of POP, symptomatic POP was self-reported in 4 [26,27,28,29]; in 1 study, POP was also objectively analyzed with the Pelvic Organ Prolapse Quantification System[25] in addition to being self-reported. Regarding postoperative follow-up time, 4 studies reported outcomes at 6 months [26, 28, 29, 35], 3 at 12 months [26, 28, 29], 2 at 3–6 months [27, 31], 1 at 6–12 months [25], and 1 at 12–24 months [31]; and 3 studies had a median follow-up time of 11.3 months [34], 18.6 months [33], and 3.152 years [30]. To facilitate the subgroup analysis, we divided the follow-up time into 3–6 months [26,27,28,29, 31, 35], 6–12 months (excluding 6 and 12 months) [25, 34], and ≥ 12 months [26, 28,29,30, 33] (Table 3). Study quality according to the NOS was high for 9 studies [25,26,27,28, 30, 32,33,34,35,36,37] and moderate for 2 studies [29, 31] (Table 2).
Primary Results
As shown in Fig. 2, the prevalence of POP symptoms decreased significantly after bariatric surgery. And POPDI-6 scores also decreased after the surgery which indicates an improvement in POP symptoms (Fig. 3).
Secondary Results
Subgroup analyses according to follow-up time, study region, and study quality failed to detect the source of heterogeneity (Table 3). The prevalence of POP was decreased both at 3–6 months and 12 months of follow-up compared to before the surgery (Fig. 4) and the POPDI-6 scores also decreased after bariatric surgery both at 3–6 months and ≥ 12 months of follow-up (Fig. 5). Only 2 studies reported the change in POP prevalence and POPDI-6 scores at 6–12 months after surgery, and no significant difference was found compared with pre surgery (Figs. 4 and 5).
Publication Bias
Publication bias was assessed with Begg’s funnel plots and no publication bias was found (Supplementary Fig. 2).
Discussion
There is accumulating evidence of a link between obesity and PFDs, which significantly impact the QoL of patients and thereby impose a substantial social and economic burden. Many previous studies have demonstrated an improvement in PFDs after bariatric surgery, but the effects of this intervention on POP—a common type of PFD—remain unclear. In this meta-analysis, we demonstrated that the prevalence of POP and POPDI-6 scores were decreased after bariatric surgery, providing evidence for the clinical benefits of this intervention for POP.
Bariatric surgery has been shown to positively impact weight loss and some aspects of PFDs such as UI [14, 15], FI [16], and sexual dysfunction [17]. However, the effects of bariatric surgery on POP are controversial. Consistent with our results (Fig. 2), one meta-analysis of 4 relevant studies reported that POPDI-6 scores were significantly decreased after bariatric surgery [19]. However, Montenegro et al. [18] compared the prevalence of POP before versus after bariatric surgery based on the same 5 studies in their meta-analysis and reached conclusions that differed from ours (Fig. 1). A possible reason for the discrepancy is the selection of data from one of the studies [29]; Montenegro et al. [18] used 6 months post-surgery data from this study although the total follow-up time was up to 12 months [29]. We pooled the 12-month data for our meta-analysis, which is more reasonable. Moreover, the results of the subgroup analysis by follow-up time showed that the prevalence of POP was decreased both at 3–6 and 12 months after bariatric surgery, supporting the robustness of our results (Fig. 4).
As the variable follow-up time across studies are bound to have an impact on the accuracy of the results, we performed a subgroup analysis based on the duration of follow-up to increase statistical power for the first time. We found that both POP prevalence and POPDI-6 scores decreased at 3–6 and ≥ 12 months of follow-up (Figs. 4 and 5). A caveat is that the follow-up time of the included studies was relatively short, with just 3 studies having a follow-up time more than 1 year (1–2 years [31] and a mean follow-up of 18.6 months [33] and 3.152 years [30]). Additionally, we performed subgroup analyses by study quality and region (Table 3). We found that POPDI-7 scores in high-quality studies were consistent with those in the overall analysis whereas POP prevalence showed no significance. The discrepancy may be related to the smaller number of included studies and higher heterogeneity, but may also indicate the instability of the current results. In the subgroup analysis by region, we found that 3 studies with data for POP prevalence in the US and 4 studies with data for POPDI scores from Israel showed different results from the overall analysis, which needs to be validated in future work. In addition, one of the included studies found that in obese patients with symptoms of UI, these symptoms along with POP were significantly improved after bariatric surgery, whereas no improvement in POP symptoms was observed after bariatric surgery in patients without UI symptoms, indicating that the improvement in PFD symptoms after this intervention is more obvious in obese women with multiple PFD symptoms.
This meta-analysis was subject to several limitations. First, the number of relevant studies is limited and some variables showed heterogeneity and instability across studies. Second, the diagnosis of POP was mainly made based on self-reported symptoms, and there was no definitive diagnosis that was independently confirmed. Third, there were variations in the surgical techniques used and phenotypic characterization of obesity across studies, with relatively short follow-up periods in some cases. Fourth, all of the included studies had a nonrandomized cohort design; as such, the risk of selection bias was unavoidable. Finally, the analysis examined the effect of bariatric surgery on POP without taking into account the potential complications associated with this procedure.
Conclusion
This meta-analysis provides evidence that bariatric surgery alleviated POP symptoms in women with obesity both during short-term (3–6 months) and long-term (≥ 12 months) follow-up. However, due to the limitations of the number and quality of the current studies, further randomized controlled trials on a larger scale and of longer duration are needed to validate these findings.
Data Availability
Requests for reprints should be directed to the corresponding authors.
References
Katsareli EA, Dedoussis GV. Biomarkers in the field of obesity and its related comorbidities. Expert Opin Ther Targets. 2014;18(4):385–401.
Young N, Atan IK, Rojas RG, et al. Obesity: how much does it matter for female pelvic organ prolapse? Int Urogynecol J. 2018;29(8):1129–34.
Friedman T, Dietz HP. Does obesity change the perception of pelvic organ prolapse? Arch Gynecol Obstet. 2022;305(6):1491–5.
Lim VF, Khoo JK, Wong V, et al. Recent studies of genetic dysfunction in pelvic organ prolapse: the role of collagen defects. Aust N Z J Obstet Gynaecol. 2014;54(3):198–205.
Vrijens D, Berghmans B, Nieman F, et al. Prevalence of anxiety and depressive symptoms and their association with pelvic floor dysfunctions-A cross sectional cohort study at a Pelvic Care Centre. Neurourol Urodyn. 2017;36(7):1816–23.
Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–90.
Vergeldt TF, Weemhoff M, IntHout J, et al. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J. 2015;26(11):1559–73.
Giri A, Hartmann KE, Hellwege JN, et al. Obesity and pelvic organ prolapse: a systematic review and meta-analysis of observational studies. Am J Obstet Gynecol. 2017;217(1):11-26.e3.
Lee UJ, Kerkhof MH, van Leijsen SA, et al. Obesity and pelvic organ prolapse. Curr Opin Urol. 2017;27(5):428–34.
Chang KM, Hsieh CH, Chiang HS, et al. Risk factors for urinary incontinence among women aged 60 or over with hypertension in Taiwan. Taiwan J Obstet Gynecol. 2014;53(2):183–6.
Aniuliene R, Aniulis P, Steibliene V. Risk Factors and Types of Urinary Incontinence among Middle-Aged and Older Male and Female Primary Care Patients in Kaunas Region of Lithuania: Cross Sectional Study. Urol J. 2016;13(1):2552–61.
Adams TD, Davidson LE, Litwin SE, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377(12):1143–55.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–36.
Sheridan W, Da Silva AS, Leca BM, et al. Weight loss with bariatric surgery or behaviour modification and the impact on female obesity-related urine incontinence: A comprehensive systematic review and meta-analysis. Clin Obes. 2021;11(4):e12450.
Gabriel I, Tavakkoli A, Minassian VA. Pelvic Organ Prolapse and Urinary Incontinence in Women After Bariatric Surgery: 5-Year Follow-up. Female Pelvic Med Reconstr Surg. 2018;24(2):120–5.
Mohamed F, Jeram M, Coomarasamy C, et al. Does Bariatric Surgery Improve Faecal Incontinence? A Systematic Review and Meta-analysis. Obes Surg. 2021;31(7):2942–53.
Loh HH, Shahar MA, Loh HS, et al. Female sexual dysfunction after bariatric surgery in women with obesity: A systematic review and meta-analysis. Scand J Surg. 2022;111(1):14574969211072396.
Montenegro M, Slongo H, Juliato CRT, et al. The Impact of Bariatric Surgery on Pelvic Floor Dysfunction: A Systematic Review. J Minim Invasive Gynecol. 2019;26(5):816–25.
Lian W, Zheng Y, Huang H, et al. Effects of bariatric surgery on pelvic floor disorders in obese women: a meta-analysis. Arch Gynecol Obstet. 2017;296(2):181–9.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283(15):2008–12.
Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005;193(1):103–13.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Romero-Talamás H, Unger CA, Aminian A, et al. Comprehensive evaluation of the effect of bariatric surgery on pelvic floor disorders. Surg Obes Relat Dis. 2016;12(1):138–43.
Cuicchi D, Lombardi R, Cariani S, et al. Clinical and instrumental evaluation of pelvic floor disorders before and after bariatric surgery in obese women. Surg Obes Relat Dis. 2013;9(1):69–75.
Leshem A, Shimonov M, Amir H, et al. Effects of Bariatric Surgery on Female Pelvic Floor Disorders. Urology. 2017;105:42–7.
McDermott CD, Terry CL, Mattar SG, et al. Female pelvic floor symptoms before and after bariatric surgery. Obes Surg. 2012;22(8):1244–50.
Whitcomb EL, Horgan S, Donohue MC, et al. Impact of surgically induced weight loss on pelvic floor disorders. Int Urogynecol J. 2012;23(8):1111–6.
Olivera CK, Herron DM, Kini SU, et al. Long-term quality of life and pelvic floor dysfunction after bariatric surgery. Am J Obstet Gynecol. 2012;207(5):431.e1-4.
Leshem A, Groutz A, Amir H, et al. Surgically induced weight loss results in a rapid and consistent improvement of female pelvic floor symptoms. Scand J Urol. 2018;52(3):219–24.
Mazoyer C, Treacy P, Turchi L, et al. Laparoscopic Roux-En-Y Gastric Bypass Versus Sleeve Gastrectomy on Pelvic Floor Disorders in Morbidly Obese Women: a Prospective Monocentric Pilot Study. Obes Surg. 2019;29(2):609–16.
Wasserberg N, Haney M, Petrone P, et al. Morbid obesity adversely impacts pelvic floor function in females seeking attention for weight loss surgery. Dis Colon Rectum. 2007;50(12):2096–103.
Knepfler T, Valero E, Triki E, et al. Bariatric surgery improves female pelvic floor disorders. J Visc Surg. 2016;153(2):95–9.
Shimonov M, Groutz A, Schachter P, et al. Is bariatric surgery the answer to urinary incontinence in obese women? Neurourol Urodyn. 2017;36(1):184–7.
Myers DL, Sung VW, Richter HE, et al. Prolapse symptoms in overweight and obese women before and after weight loss. Female Pelvic Med Reconstr Surg. 2012;18(1):55–9.
Gozukara YM, Akalan G, Tok EC, et al. The improvement in pelvic floor symptoms with weight loss in obese women does not correlate with the changes in pelvic anatomy. Int Urogynecol J. 2014;25(9):1219–25.
Funding
This study was supported by grants from the Beijing Natural Science Foundation (No. Z190021), the National Natural Science Foundation of China (No. 81971366) and the CAMS Innovation Fund for Medical Sciences (CIFMS 2020-I2M-C&T-B-043).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
Bariatric surgery alleviated POP symptoms in women with obesity.
The prevalence of POP decreased both at 3–6 months and 12 months of follow-up.
POPDI-6 scores decreased both at 3–6 months and ≥ 12 months of follow-up.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, Z., Wang, X., Hu, X. et al. Effect of Surgically Induced Weight Loss on Pelvic Organ Prolapse: A Meta-analysis. OBES SURG 33, 3402–3410 (2023). https://doi.org/10.1007/s11695-023-06867-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06867-x