Abstract
Background and Aims
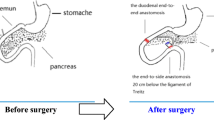
Duodenal–jejunal bypass (DJB) shows great effects on weight loss and diabetes improvement. Previously, we reported that the bilio-pancreatic (BP) limb plays an important role in glycemic improvement and in serum bile acid (BA) level increase as reported by Miyachi et al. (Surgery 159(5):1360–71, 2016). This study aimed to investigate the mechanism of BA elevation after DJB and the relationship between these effects and BP-limb length.
Methods
Otsuka Long-Evans Tokushima Fatty rats with diabetes were randomly assigned into four groups: one sham group and three DJB groups. Three DJB groups were defined according to the BP-limb length: 0 cm, 15 cm, and 30 cm. The lengths of the alimentary limb and common channel were set equally in each DJB groups. Body weight, glucose tolerance, and BA levels in the liver, bile juice, portal vein, and intestinal contents were assessed postoperatively. Changes in enterohepatic circulation of BAs were assessed using labeled BA.
Results
BA elevation after DJB was higher with longer BP-limb. In the 30-cm group, the serum total BA level and BA levels in the portal vein, liver, and bile juice were greater than those in other groups. The enterohepatic circulation was shortened in the 15-cm and 30-cm groups.
Conclusions
Shortening of the “enterohepatic circulation” by early reabsorption of BAs in the BP-limb, not by the early influx of bile juice into the ileum, was the main cause of BA elevation after DJB. Thus, glycemic improvement and elevation of BA concentration after DJB depend on the BP-limb length.






Similar content being viewed by others
References
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–36.
Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5.
Lee WJ, Almulaifi AM, Tsou JJ, et al. Duodenal-jejunal bypass with sleeve gastrectomy versus the sleeve gastrectomy procedure alone: the role of duodenal exclusion. Surg Obes Relat Dis. 2015;11(4):765–70.
Seki Y, Kasama K, Umezawa A, et al. Laparoscopic sleeve gastrectomy with duodenojejunal bypass for type 2 diabetes mellitus. Obes Surg. 2016;26(9):2035–44.
Naitoh T, Kasama K, Seki Y, et al. Efficacy of sleeve gastrectomy with duodenal-jejunal bypass for the treatment of obese severe diabetes patients in Japan: a retrospective multicenter study. Obes Surg. 2018;28(2):497–505.
Ionut V, Bergman RN. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5(5):1263–82.
Meryn S, Stein D, Straus EW. Pancreatic polypeptide, pancreatic glucagon and enteroglucagon in morbid obesity and following gastric bypass operation. Int J Obes. 1986;10(1):37–42.
Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893–901.
Gerhard GS, Styer AM, Wood GC, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–64.
Liou AP, Paziuk M, Luevano Jr JM, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8.
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53.
Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298(5):714–9.
Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278(11):9435–40.
Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419–25.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113(10):1408–18.
Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26.
Lu TT, Makishima M, Repa JJ, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–15.
Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24(10):1803–23.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77.
Thomas C, Auwerx J, Schoonjans K. Bile acids and the membrane bile acid receptor TGR5—connecting nutrition and metabolism. Thyroid. 2008;18(2):167–74.
Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10(3):e0122273.
Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015 Jul 21;6:7715.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51.
Miyachi T, Nagao M, Shibata C, et al. Biliopancreatic limb plays an important role in metabolic improvement after duodenal-jejunal bypass in a rat model of diabetes. Surgery. 2016;159(5):1360–71.
Funakoshi A, Miyasaka K, Jimi A, et al. Little or no expression of the cholecystokinin—a receptor gene in the pancreas of diabetic rats (Otsuka Long-Evans Tokushima Fatty = OLETF rats). Biochem Biophys Res Commun. 1994;199(2):482–8.
Kawano K, Hirashima T, Mori S, et al. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract. 1994;24(Suppl):S317–20.
Kawano K, Hirashima T, Mori S, et al. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41(11):1422–8.
Moran TH, Katz LF, Plata-Salaman CR, et al. Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Phys. 1998;274(3 Pt 2):R618–25.
Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982.
Imoto H, Shibata C, Ikezawa F, et al. Effects of duodeno-jejunal bypass on glucose metabolism in obese rats with type 2 diabetes. Surg Today. 2014 Feb;44(2):340–8.
Tsuchiya T, Naitoh T, Nagao M, et al. Increased bile acid signals after duodenal-Jejunal bypass improve non-alcoholic steatohepatitis (NASH) in a rodent model of diet-induced NASH. Obes Surg. 2018;28(6):1643–52.
Nakatani H, Kasama K, Oshiro T, et al. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58(10):1400–7.
Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17(9):1671–7.
Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65.
Roberts MS, Magnusson BM, Burczynski FJ, et al. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41(10):751–90.
Nakahara M, Furuya N, Takagaki K, et al. Ileal bile acid-binding protein, functionally associated with the farnesoid X receptor or the ileal bile acid transporter, regulates bile acid activity in the small intestine. J Biol Chem. 2005;280(51):42283–9.
Pellicoro A, Faber KN. Review article: the function and regulation of proteins involved in bile salt biosynthesis and transport. Aliment Pharmacol Ther. 2007;26(Suppl 2):149–60.
Neimark E, Chen F, Li X, et al. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology. 2004;40(1):149–56.
Lee H, Zhang Y, Lee FY, et al. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47(1):201–14.
Byrne JA, Strautnieks SS, Mieli-Vergani G, et al. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123(5):1649–58.
Hallen S, Bjorquist A, Ostlund-Lindqvist AM, et al. Identification of a region of the ileal-type sodium/bile acid cotransporter interacting with a competitive bile acid transport inhibitor. Biochemistry. 2002;41(50):14916–24.
Balakrishnan A, Wring SA, Polli JE. Interaction of native bile acids with human apical sodium-dependent bile acid transporter (hASBT): influence of steroidal hydroxylation pattern and C-24 conjugation. Pharm Res. 2006;23(7):1451–9.
Kim RB, Leake B, Cvetkovic M, et al. Modulation by drugs of human hepatic sodium-dependent bile acid transporter (sodium taurocholate cotransporting polypeptide) activity. J Pharmacol Exp Ther. 1999;291(3):1204–9.
Malinen MM, Ali I, Bezencon J, et al. Organic solute transporter OSTalpha/beta is overexpressed in nonalcoholic steatohepatitis and modulated by drugs associated with liver injury. Am J Physiol Gastrointest Liver Physiol. 2018;314(5):G597–g609.
Acknowledgments
The authors thank Ms. Emiko Shibuya and Ms. Saori Shoji for their technical assistance, and the staff of Institute for animal Experimentation, Graduate School of Medicine, Tohoku University, for assistance with animal husbandry and care.
Funding
This study was supported by the grant-in-aid for scientific research from Japan Society for the Promotion of Science (Grant No. 17K10575).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Animal Rights
All procedures in this study were approved by ethics committee for animal research of our institute.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(PDF 15 kb)
Supplementary Table 2
(PDF 15 kb)
Supplementary Fig. 1
(PDF 52 kb)
Supplementary Fig. 2
(PDF 71 kb)
Supplementary Fig. 3
(PDF 70 kb)
Supplementary Fig. 4
(PDF 71 kb)
Supplementary Fig. 5
(PDF 13447 kb)
Rights and permissions
About this article
Cite this article
Ise, I., Tanaka, N., Imoto, H. et al. Changes in Enterohepatic Circulation after Duodenal–Jejunal Bypass and Reabsorption of Bile Acids in the Bilio-Pancreatic Limb. OBES SURG 29, 1901–1910 (2019). https://doi.org/10.1007/s11695-019-03790-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03790-y