Abstract
Introduction
Type 2 diabetes mellitus (T2DM) and obesity are both related to increased risk of cardiovascular disease and mortality. Early atherosclerotic vascular changes can be detected by non-invasive tests like carotid artery intima-media thickness (cIMT) and pulse wave velocity (PWV). Both cIMT and PWV are significantly impaired in T2DM patients and in obese patients, but the additional effect of T2DM on these vascular measurements in obese subjects has not been evaluated.
Methods
Two hundred morbidly obese patients with or without T2DM were enrolled in a prospective cohort study and underwent extensive laboratory testing, including cIMT and PWV measurements. The cohort was divided into a group with and a group without T2DM.
Results
Within this cohort, 43 patients (21.5%) were diagnosed with T2DM. These patients were older and had more often (a history of) hypertension as compared to patients without T2DM. HbA1c levels were significantly increased, while LDL cholesterol was significantly lower and the use of statins higher than in non-diabetic participants. cIMT and PWV were significantly increased in subjects suffering from T2DM. The variability in cIMT and PWV was related to differences in age and systolic blood pressure, but not to the presence of T2DM.
Conclusion
While T2DM negatively affects the vasculature in morbid obesity, hypertension and age seem to be the major risk factors, independent from the presence of T2DM.
Clinical Trial Registration
Dutch Trial Register NTR5172.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a growing chronic health problem worldwide and its microvascular and macrovascular complications cause significant morbidity and mortality [1]. T2DM is associated with atherosclerosis [2] and a major risk factor for cardiovascular disease (CVD) [3, 4]. Atherogenic dyslipidemia is a key factor linking T2DM to CVD and includes reduced levels of high-density lipoprotein cholesterol (HDL-C) and increased levels of triglycerides (TG) and small dense low-density lipoprotein cholesterol (LDL-C) [3]. LDL-C-lowering therapy can modify the cardiovascular risk in adults suffering from T2DM, although the risk of CVD remains higher than in non-diabetic adults [3]. Both the onset and the progression of atherosclerosis are more rapid in T2DM patients [1]. In addition, the disease may cause premature aging of the cardiovascular system [5].
Vascular health can be easily monitored by measuring carotid artery intima-media thickness (cIMT) using carotid ultrasound. cIMT is an easy and non-invasive method for the detection of structural signs of carotid atherosclerosis, which is a significant indicator of subclinical atherosclerosis and of future cardiovascular disease [1, 3, 6,7,8]. Close associations have been seen between cIMT and conventional cardiovascular risk factors, such as smoking status, hypertension, and dyslipidemia [8, 9]. Patients suffering from T2DM are also known to have an increased cIMT in comparison to non-diabetic controls [8] and the presence of T2DM increases the likelihood of early carotid atherosclerosis [9]. Both hyperglycemia and hyperinsulinemia are associated with an increased risk of atherosclerotic cardiovascular disease, and changes in cIMT are correlated with changes in fasting plasma glucose, HbA1c, and insulin levels [1, 9].
Another measure for vascular health is arterial stiffness, which is an independent risk factor for cardiovascular disease and mortality [3, 4, 10, 11]. The “gold standard” measurement for arterial stiffness is the measurement of the pulse wave velocity (PWV) [4, 5]. Subjects suffering from T2DM are known to have a higher PWV than non-diabetic subjects [4].
Both, IMT and PWV are also known to be increased in patients suffering from obesity [4, 9, 12] and obesity is also strongly associated with cardiovascular risk and the occurrence of coronary heart disease [4]. Furthermore, obese subjects are more likely to develop T2DM. It is unknown whether suffering from T2DM worsens vascular conditions in morbidly obese subjects. As IMT and PWV may be used as prospective markers for vascular disease, we selected these markers to establish the effect of T2DM on the vascular status in morbidly obese subjects.
Methods
Study Population
Data of this study are derived from the ASSISI study, a prospective cohort study established between April 2015 and April 2016 and including 200 patients scheduled for bariatric surgery within our bariatric clinic. All met the international IFSO criteria for bariatric surgery [13], i.e., patients with a BMI of ≥ 40 kg/m2 or patients with a BMI of ≥ 35 kg/m2 and obesity-related comorbidity, aged 18 to 65 years. Patients with a previous cholecystectomy, with a previous bariatric procedure, with an acute inflammatory disease within 6 weeks prior to inclusion, or using immune-modulating medication were excluded from this study. All data presented here were collected at study entry, before bariatric surgery.
The study was approved by the independent Regional Medical Research Ethics Committee Rotterdam (Maasstad Hospital, Rotterdam, The Netherlands, ABR No. NL47891.101.14) and all patients gave written informed consent. The ASSISI study is registered in the Dutch Trial Register (NTR5172).
Definitions
T2DM was defined as a HbA1c ≥ 48 mmol/mol (6.5%) [14] and/or previously diagnosed T2DM using glucose lowering medication. Hypertension was defined by a systolic blood pressure > 140 mmHg and/or the use of antihypertensive medication [15]. Hypercholesterolemia was defined as LDL-C > 2.5 mmol/l and/or the use of lipid-lowering drugs [15].
Baseline Characteristics
Baseline characteristics were obtained during standard preoperative screening by the internist prior to bariatric surgery and included the medical history and current medication [16]. Anthropometric characteristics included height, weight, waist circumference, and blood pressure.
Laboratory Measurements
Preoperative laboratory testing was carried out in all participants using non-fasting samples. C-reactive protein (CRP), glucose, total cholesterol, HDL-C, and triglycerides (TG) were determined using the DxC analyzer (Beckman Coulter, Crea, CA, USA). LDL-C values were calculated using the Friedewald formula. HbA1c was measured using an HPLC G8 analyzer (Tosoh Bioscience, King of Prussia, PA, USA). Apo B was determined by rate nephelometry using IMAGE analyzer (Beckman Coulter).
Carotid Intima-Media Thickness
Carotid intima-media thickness (cIMT) measurements were performed according to the consensus guidelines for carotid ultrasound for CVD risk assessment as described previously [17]. The measurement was carried out using the ART-LAB (Esaote, Italy) by a trained and experienced sonographer, who was unaware of the patient’s medical history. Ultrasound scans were performed with the patients lying in a supine position with the head resting comfortably and the neck slightly hyperextended and rotated in the opposite direction of the probe. The ultrasound images were obtained from the distal 1 cm of the far wall of each common carotid artery (CCA) using B-mode ultrasound producing two echogenic lines. These lines represent the combined thickness of the intimal and medial layers of the arterial wall. Each CCA was imaged in three different projections: CCA right side 90-120-150 and CCA left side 210-240-270 degrees. The segments were measured semi-automated in triplicate.
Pulse Wave Velocity
Pulse wave velocity (PWV) measurements were carried out using the Mobil-O-Graph (I.E.M, Germany) as previously described [18]. The Mobil-O-Graph uses an inflatable cuff to measure the PWV. The cuff was placed on the patient’s bare left upper arm. Triplicate manual measurements were performed. PWV was calculated by the provided software and was expressed in meter per second.
Statistical Analysis
All analyses were performed using SPSS (PASW) 18.0 software (SPSS Inc., Chicago, IL, USA).
Data are given as mean ± standard deviations. Skewed variables are given in median and interquartile range (IQR). Categorical data were described in absolute numbers and percentages. Differences between subjects with and without T2DM were analyzed using independent t tests, chi-square tests, Fisher’s exact tests, and independent sample Mann-Whitney U tests. For statistical analysis, cIMT was defined as the mean of the six individual measurements, as described above, and PWV was defined as the mean of three individual measurements. Systolic and diastolic blood pressures were integrated in the mean arterial pressure (MAP), which is the sum of one-third systolic blood pressure and two-third diastolic blood pressure.
The association between IMT and PWV, both intermediate measures of subclinical atherosclerosis, with T2DM was evaluated with univariate linear regression analysis. To evaluate the effects of both the occurrence of T2DM and the severity of dysregulation of glucose homeostasis, linear regression analyses were performed using both the previously described definition of T2DM and the HbA1c level. In further analyses, covariables included gender, age, smoking status, BMI, waist circumference, TG, HDL-C, LDL-C, CRP, and MAP. Correlations between covariables (multicollinearity) were checked for confounding and interaction effects using stratified analysis. Within the regression models of T2DM and HbA1c on both IMT and PWV, a significant interaction effect was observed between HDL-C and TG, while gender was a significant confounder. Other confounding factors were ignored in the analyses for having a low correlation (< 0.30) or small impact on the outcome measures. After stratification for gender, the associations of both T2DM and HbA1c level on cIMT and PWV were evaluated with multivariable linear regression analysis (model: backward stepwise). The following variables were entered into the models: gender, age, smoking status, waist circumference, TG, HDL-C, LDL-C, CRP, HDL-C × TG, and MAP. Results of these models were reported as B coefficients with 95% confidence intervals. Goodness of fit was evaluated with the adjusted R2. A p value < 0.05 (two-sided) was considered a statistically significant difference.
Results
The total cohort included 200 patients, 52 men and 148 women, with a mean age of 41 (± 11.8) years and a mean BMI of 42.7 (± 5.2) kg/m2. Within this cohort, 43 patients (21.5%) were diagnosed with T2DM. T2DM patients were older and suffered more frequently from hypertension. In addition, these patients had more frequently a history of myocardial infarction. While mean HbA1c values were significantly increased in diabetes patients, their LDL-C levels were significantly lower than those in non-diabetics. Diabetic patients were more frequently treated with lipid-lowering drugs and antihypertensive drugs. Of all diabetic patients, 35 patients (81.4%) were treated with glucose-lowering drugs; 13 patients received monotherapy with metformin, 8 received a combination of insulin and metformin, 10 received a combination of metformin with another oral glucose lowering drug, and 4 received another treatment. Additional characteristics are displayed in Table 1.
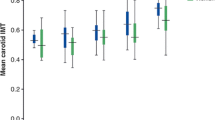
The median cIMT was significantly increased in patients suffering from T2DM, when compared to non-diabetics (0.576 mm (IQR 0.531–0.726) and 0.540 mm (IQR 0.481–0.634), respectively, p = 0.016). The median PWV was also significantly higher in diabetic patients compared to non-diabetics (7.6 m/s (IQR 6.9–8.4) and 6.8 m/s (IQR 5.9–8.0), respectively, p = 0.005) (Fig. 1).
Univariate linear regression showed that the presence of T2DM was associated with an increased IMT (crude beta 0.048, adjusted R2 0.021, p = 0.033). Additionally, higher HbA1c levels were associated with an increased cIMT (crude beta 0.001, adjusted R2 0.019, p = 0.041). In a multiple regression analysis, both T2DM and HbA1c did not contribute to the cIMT after stratification for gender and adjusted for age, LDL-C, HDL-C, and MAP. The other parameters included in the analysis did not have any significant effect on the cIMT.
Using univariate analysis, T2DM was associated with increased PWV (crude beta 0.685, adjusted R2 0.039, p = 0.007). Furthermore, HbA1c levels were also associated with an increased PWV (crude beta 0.023, adjusted R2 0.043, p = 0.005). In the multivariable analysis, stratified for gender and after adjustment for age, waist circumference, and MAP, T2DM was not associated with increased PWV. HbA1c tended to show a small contribution to PWV in men only (Table 2).
Discussion
Vascular disease as measured by cIMT and PWV is significantly worse in morbidly obese subjects suffering from T2DM, in comparison to their non-diabetic counterparts. This is in line with previously described differences in non-obese subjects [4, 8]. Our data suggest that the presence of T2DM is not a significant contributor to the levels of IMT and PWV within the morbidly obese subjects after adjustment for age, blood pressure, and lipid profile. PWV was mainly determined by differences in gender, age, waist circumference, and blood pressure.
cIMT and PWV values are increased in both T2DM [8] and obesity [4, 9, 12]. However, the impact of having T2DM in a morbidly obese population on subclinical atherosclerosis has not been described before. Our study suggests that patients with T2DM and morbid obesity have a significantly impaired vascular function, compared to their non-diabetic obese counterparts. However, it should be noted that other cardiovascular risk factors, linked to T2DM, may have a greater impact on vascular function, than T2DM itself. The consequence of our study is that in subjects with morbid obesity and T2DM, one should consider more strict targets of conventional cardiovascular risk factors.
In general, it is well known that cIMT is mainly influenced by age and gender in which men and elderly people have an increased cIMT when compared to women and young subjects [19,20,21,22,23]. In terms of classic cardiovascular risk factors, blood pressure is the main contributor to increased cIMT. Blood pressure influences cIMT, for example in young healthy subjects [20, 23, 24], in lean subjects suffering from T2DM [25], and in elderly patients with cardiovascular risk factors [21]. Additionally, a decrease in blood pressure, for example by administering antihypertensive drugs, can provide a decrease in measured cIMT [26]. The second important cardiovascular risk factor to influence cIMT is dyslipidemia [20,21,22] and intensive lipid-lowering therapy can also positively influence IMT [26,27,28], although this effect was not observed in diabetic subjects [29]. The results in these non-obese study populations are in agreement with the results in our study. Smoking habit is also thought to influence cIMT in different populations [20,21,22,23], but this association was not observed in our morbidly obese subjects.
The determinants of arterial stiffness, measured as the pulse wave velocity, have been less well studied compared to cIMT. In healthy middle-aged and elderly subjects, not suffering from T2DM or CVD, PWV is mainly determined by age, blood pressure, and waist circumference [30], which is in accordance with the results in our morbidly obese population. Fasting glucose levels are thought to have a modest impact on PWV and only when diabetic and cardiovascular patients are included in the study group [30]. Additionally, both HbA1c [25] and TG [24] levels are thought to play a role in the development of arterial stiffness, although that effect is only seen in selected subjects and cannot be confirmed in all studies or patient groups. Both parameters were no major determinant of PWV within our morbidly obese study population.
Obesity is both a major and modifiable risk factor for the development of metabolic and cardiovascular disease [12]. Weight loss can be an important pillar in the prevention strategy for cardiovascular events. For example, bariatric surgery can significantly reduce cardiovascular morbidity and mortality. One study showed that cIMT values were significantly decreased 1 year after bariatric surgery [31]. An interesting finding in the current study is the fact that the degree of obesity, in terms of both BMI and waist circumference, did not influence the value of cIMT or PWV. It can be suggested that being morbidly obese indeed increases the values of PWV and cIMT, but that the level of obesity does not really matter once a certain BMI threshold is surpassed (ceiling effect). A certain ceiling effect was previously described for markers of dyslipidemia [32, 33] and may be true for other cardiovascular risk factors or vascular outcome measures, such as cIMT and PWV. Consequently, once morbid obesity is a fact, further increment of body weight may not cause further deterioration of subclinical atherosclerosis.
When analyzing the impact of diabetes on cIMT or vascular function, differences in cardiovascular risk management between diabetics and non-diabetics can affect the results. For example, one important pillar of the treatment for type 2 diabetes is the reduction of diabetic dyslipidemia with prescription of statins. Within our morbidly obese population, diabetic patients were more likely to use statins and therefore their mean LDL cholesterol level was significantly lower than that in the non-diabetic group. The differences in cIMT and PWV between diabetics and non-diabetics may be limited due to the extensive use of statins in diabetics. However, a previous study showed that statin use does not influence cIMT in diabetic patients without previous cardiovascular disease [29] and only aggressive treatment goals, such as LDL-cholesterol ≤ 70 mg/dl, can influence the progression of cIMT [34]. Another important target in the treatment of diabetic patients is the prevention of micro- and macrovascular complications by administration of antihypertensive drugs. Within our cohort, the diabetic patients were more likely to be diagnosed with hypertension and to be treated with antihypertensive drugs. Diabetic patients with hypertension are known to have increased cIMT values and increased cIMT progression over time [35, 36]. Even though the actual value of systolic and diastolic blood pressures was not significantly different between the diabetic and non-diabetic subjects in our cohort, it was previously shown that treatment with antihypertensive drugs had a positive influence on cIMT and progression of cIMT, independent of the level of blood pressure [35]. Therefore, the effect of type 2 diabetes on cIMT in this study may be limited due to the excessive use of antihypertensive agents within this group.
In conclusion, although T2DM negatively affects the vasculature in morbid obesity, age and hypertension seem to be the main risk factors independent from the presence of T2DM.
References
Kim SH, Lee SJ, Kang ES, et al. Effects of lifestyle modification on metabolic parameters and carotid intima-media thickness in patients with type 2 diabetes mellitus. Metabolism. 2006;55(8):1053–9.
Miyamoto M, Kotani K, Okada K, et al. Arterial wall elasticity measured using the phased tracking method and atherosclerotic risk factors in patients with type 2 diabetes. J Atheroscler Thromb [Internet]. 2013;20(8):678–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23648429
Gordon SM, Davidson WS, Urbina EM, et al. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62(8):2958–67.
Teoh WL, Price JF, Williamson RM, et al. Metabolic parameters associated with arterial stiffness in older adults with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. J Hypertens. 2013;31(5):1010–7.
Liu JJ, Sum CF, Tavintharan S, et al. Obesity is a determinant of arterial stiffness independent of traditional risk factors in Asians with young-onset type 2 diabetes. Atherosclerosis. 2014;236(2):286–91.
Su T-C, Chien K-L, Jeng J-S, et al. Age- and gender-associated determinants of carotid intima-media thickness: a community-based study. J Atheroscler Thromb [Internet]. 2012;19(9):872–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22972311
Kablak-Ziembicka A, Przewlocki T, Tracz W, et al. Gender differences in carotid intima-media thickness in patients with suspected coronary artery disease. Am J Cardiol [Internet]. 2005;96(9):1217–22. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002914905012580
Kotb NA, Gaber R, Salama M, et al. Clinical and biochemical predictors of increased carotid intima-media thickness in overweight and obese adolescents with type 2 diabetes. Diab Vasc Dis Res [Internet]. 2012;9(1):35–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21985955
Kawamoto R, Tomita H, Ohtsuka N, et al. Metabolic syndrome, diabetes and subclinical atherosclerosis as assessed by carotid intima-media thickness. J Atheroscler Thromb [Internet]. 2007;14(2):78–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17485892
Canepa M, AlGhatrif M, Pestelli G, et al. Impact of central obesity on the estimation of carotid-femoral pulse wave velocity. Am J Hypertens. 2014;27(9):1209–17.
Nordstrand N, Gjevestad E, N-Dinh K, et al. The relationship between various measures of obesity and arterial stiffness in morbidly obese patients. BMC Cardiovasc Disord [Internet]. 2011;11(1):7. Available from: http://www.biomedcentral.com/1471-2261/11/7
Dalmas E, Kahn JFJ-F, Giral P, et al. Intima-media thickness in severe obesity: links with BMI and metabolic status but not with systemic or adipose tissue inflammation. Diabetes Care [Internet]. 2013;36(11):3793–802. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc13-0256
Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55.
American Diabetes Association - Standards of Medical Care in Diabetes, 2014. Diabetes Care [Internet]. 2014 Jan 1;37(Supplement_1):S14–80. Available from: http://care.diabetesjournals.org/cgi/doi/10.2337/dc14-S014
van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology. 2016;55(7):1210–6.
Elte JWF, Castro Cabezas M, Vrijland WW, Ruseler CH, Groen M, Mannaerts GHH. Proposal for a multidisciplinary approach to the patient with morbid obesity: the St. Franciscus Hospital morbid obesity program. Eur J Intern Med [Internet]. 2008 [cited 2014 Sep 17];19(2):92–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18249303
Bovenberg S a, Klop B, Alipour A, et al. Erythrocyte-associated apolipoprotein B and its relationship with clinical and subclinical atherosclerosis. Eur J Clin Invest [Internet]. 2012;42(4):365–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21913916
Klop B, van de Geijn G-JM, Birnie E, Njo TL, Janssen HW, Jansen HG, et al. Vitamin D3 mediated effects on postprandial leukocyte activation and arterial stiffness in men and women. Eur J Clin Nutr [Internet]. 2014 12;68(5):635–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24619107
Rogowicz-Frontczak A, Araszkiewicz A, Pilacinski S, Zozulinska-Ziolkiewicz D, Wykretowicz A, Wierusz-Wysocka B. Carotid intima-media thickness and arterial stiffness in type 1 diabetic patients are dependent on age and mean blood pressure. Exp Clin Endocrinol Diabetes [Internet]. 2011 119(5):281–285. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21031337
Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA J Am Med Assoc [Internet]. 2003;290(17):2277–83. Available from: papers2://publication/doi/10.1001/jama.290.17.2277
Baldassarre D, Nyyssonen K, Rauramaa R, et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J [Internet]. 2010;31(5):614–22. https://doi.org/10.1093/eurheartj/ehp496.
Sipilä K, Moilanen L, Nieminen T, et al. Metabolic syndrome and carotid intima media thickness in the Health 2000 Survey. Atherosclerosis [Internet]. 2009;204(1):276–81. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0021915008006102
Paul TK, Chen W, Srinivasan SR, et al. Contrast of the impact of multiple cardiovascular risk factors on the femoral and carotid intima-media thickness in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis [Internet]. 2011;216(2):359–64. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0021915011001791
Fitch KV, Stavrou E, Looby SE, et al. Associations of cardiovascular risk factors with two surrogate markers of subclinical atherosclerosis: endothelial function and carotid intima media thickness. Atherosclerosis [Internet]. 2011 217(2):437–440. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21570076
Yokoyama H, Kuramitsu M, Kanno S, et al. Relationship between metabolic syndrome components and vascular properties in Japanese type 2 diabetic patients without cardiovascular disease or nephropathy. Diabetes Res Clin Pract [Internet]. 2007;75(2):200–6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168822706002452
Lundby-Christensen L, Tarnow L, Hansen DL, et al. Carotid intima-media thickness is reduced 12months after gastric bypass surgery in obese patients with type 2 diabetes or impaired glucose tolerance. J Diabetes Complications [Internet]. 2014;28(4):517–22. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1056872714000609
Crouse JR, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA [Internet]. 2007 28;297(12):1344–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17384434
Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet (London, England) [Internet]. 2001;357(9256):577–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11558482
Beishuizen ED, van de Ree MA, Jukema JW, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care [Internet]. 2004;27(12):2887–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15562202
Sipilä K, Koivistoinen T, Moilanen L, et al. Metabolic syndrome and arterial stiffness: the Health 2000 Survey. Metabolism [Internet]. 2007;56(3):320–6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0026049506003775
Lundby-Christensen L, Tarnow L, Hansen DL, et al. Carotid intima-media thickness is reduced 12 months after gastric bypass surgery in obese patients with type 2 diabetes or impaired glucose tolerance. J Diabetes Complicat. 2014;28(4):517–22.
Shamai L, Lurix E, Shen M, Novaro GM, Szomstein S, Rosenthal R, Hernandez AV, Asher CR Association of body mass index and lipid profiles: evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg [Internet]. 2011;21(1):42–47. Available from: http://link.springer.com/10.1007/s11695-010-0170-7
Drapeau V, Lemieux I, Richard D, et al. Metabolic profile in severely obese women is less deteriorated than expected when compared to moderately obese women. Obes Surg [Internet]. 2006;16(4):501–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16608618
Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol [Internet]. 2008;52(25):2198–205. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19095139
Zheng L, Hodis HN, Buchanan TA, et al. Effect of antihypertensive therapy on progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Am J Cardiol [Internet]. 2007;99(7):956–60. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002914906024994
Wang J-G, Staessen JA, Li Y, et al. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke [Internet]. 2006;37(7):1933–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16763185
Acknowledgements
The authors thank all study subjects for their participation in this study. This study was supported by the research Foundation of the Department of Internal Medicine of the Sint Franciscus Gasthuis Rotterdam.
Author information
Authors and Affiliations
Contributions
SvM has a substantial contribution in the conception and design of the study, participated in analysis and interpretation of data, and drafted the manuscript. LB has a substantial contribution to the conception and design of the study and revising the manuscript for intellectual content. GJvdG participated in study conception, in delivering laboratory data, and in revision of the manuscript. EB participated in the statistical analysis and in revision of the manuscript. MD and JIJ contributed to revising the manuscript for intellectual content. NvdM participated in study conception and in delivering cIMT and PWV data. GM and MCC had a substantial contribution to the conception and design of the study and revising the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Human and Animal Right Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Mil, S.R., Biter, L.U., van de Geijn, GJ.M. et al. Contribution of Type 2 Diabetes Mellitus to Subclinical Atherosclerosis in Subjects with Morbid Obesity. OBES SURG 28, 2509–2516 (2018). https://doi.org/10.1007/s11695-018-3196-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3196-x