Abstract
Introduction
There is an ongoing debate on which procedure provides the best treatment for type 2 diabetes. Furthermore, the pathomechanisms of diabetes improvement of partly anatomically differing operations is not fully understood.
Methods
A loop duodenojejunostomy (DJOS) with exclusion of one third of intestinal length, a sleeve gastrectomy (SG), or a combination of DJOS + SG was performed in 8-week-old male ZDF rats. One, three, and six months after surgery, an oral glucose tolerance test and measurements of GLP-1, GIP, insulin, and bile acids were conducted.
Results
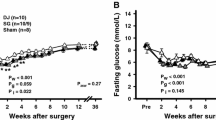
After an initial (4 weeks) equal glucose control, DJOS and DJOS + SG showed significantly lower glucose levels than SG 3 and 6 months after surgery. There was sharp decline of insulin levels in SG animals over time, whereas insulin levels in DJOS and DJOS + SG were preserved. GIP levels were significantly larger in both groups containing a sleeve at all three time points, whereas GLP-1 was equal in all groups at all time. Bile acid levels were significantly higher in the DJOS compared to the SG group at all time points. Interestingly, the additional SG in the DJOS + SG group led to lower bile acid levels 1 and 6 months postoperatively.
Conclusion
The effect of SG on glucose control was transient, whereas a duodenal exclusion was the more effective procedure in this model due to a sustained pancreatic function with a preserved insulin secretion.






Similar content being viewed by others
References
WHO | Diabetes [Internet]. WHO. [cited 2016 Apr 19]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. https://doi.org/10.1371/journal.pmed.0030442.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–52. https://doi.org/10.1097/00000658-199509000-00011.
Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(3):437–55. https://doi.org/10.1007/s11695-013-1160-3.
Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013 Jun 5;309(21):2250–61. https://doi.org/10.1001/jama.2013.4851.
Lee W-J, Hur KY, Lakadawala M, et al. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16(1):45–52. https://doi.org/10.1007/s11605-011-1740-2.
Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–85. https://doi.org/10.1056/NEJMoa1200111.
Chai J, Zhang G, Liu S, et al. Exclusion of the distal ileum cannot reverse the anti-diabetic effects of duodenal-jejunal bypass surgery. Obes Surg. 2016;26(2):261–8. https://doi.org/10.1007/s11695-015-1745-0.
Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. https://doi.org/10.1038/ncomms8715.
Kohli R, Setchell KD, Kirby M, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154(7):2341–51. https://doi.org/10.1210/en.2012-2069.
Laessle C, Michelmichel S, Marjanovic G, Kuesters S, Seifert G, Hopt UT, et al. Common channel length in bypass surgery does not impact T2DM in diabetic Zucker rats. Obes Surg [Internet]. 2017 Mar 9 [cited 2017 Mar 27]; Available from: http://link.springer.com/10.1007/s11695-017-2611-z.
Switzer NJ, Prasad S, Debru E, et al. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review of long-term outcomes. Obes Surg. 2016;26(7):1616–21. https://doi.org/10.1007/s11695-016-2188-y.
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429–42. https://doi.org/10.1007/s11695-015-1996-9.
Aminian A, Brethauer SA, Andalib A, et al. Can sleeve gastrectomy “‘cure’” diabetes? Long-term metabolic effects of sleeve gastrectomy in patients with type 2 diabetes. Ann Surg. 2016 Oct 4;264(4):674–81. https://doi.org/10.1097/SLA.0000000000001857.
Peterli R, Steinert RE, Woelnerhanssen B, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8. https://doi.org/10.1007/s11695-012-0622-3.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9. https://doi.org/10.1007/s00464-012-2166-y.
Farey JE, Preda TC, Fisher OM, et al. Effect of laparoscopic sleeve gastrectomy on fasting gastrointestinal, pancreatic, and adipose-derived hormones and on non-esterified fatty acids. Obes Surg. 2017;27(2):399–407. https://doi.org/10.1007/s11695-016-2302-1.
Mallipedhi A, Prior SL, Barry JD, et al. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(5):860–9. https://doi.org/10.1016/j.soard.2014.02.038.
Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. 2016;39(6):893–901. https://doi.org/10.2337/dc16-0145.
Marjanovic G, Holzner P, Kulemann B, et al. Pitfalls and technical aspects during the research of intestinal anastomotic healing in rats. Eur Surg Res. 2010;45(3–4):314–20. https://doi.org/10.1159/000320768.
Lee W-J, Chong K, Ser K-H, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus a randomized controlled trial. Arch Surg. 2011;146(2):143–8. https://doi.org/10.1001/archsurg.2010.326.
Eickhoff H, Louro TM, Matafome PN, et al. Amelioration of glycemic control by sleeve gastrectomy and gastric bypass in a lean animal model of type 2 diabetes: restoration of gut hormone profile. Obes Surg. 2015;25(1):7–18. https://doi.org/10.1007/s11695-014-1309-8.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal–jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75. https://doi.org/10.1007/s11695-015-1835-z.
Wang K, Zhou X, Quach G, et al. Effect of sleeve gastrectomy plus side-to-side Jejunoileal anastomosis for type 2 diabetes control in an obese rat model. Obes Surg. 2016;26(4):797–804. https://doi.org/10.1007/s11695-015-1811-7.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. AJP Endocrinol Metab. 2014;306(4):E424–32.
Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg. 2012;256(6):1023–9. https://doi.org/10.1097/SLA.0b013e318262ee6b.
Buchwald H, Menchaca HJ, Michalek VN, et al. Ileal effect on blood glucose, HbA1c, and GLP-1 in Goto-Kakizaki rats. Obes Surg. 2014;24(11):1954–60. https://doi.org/10.1007/s11695-014-1307-x.
Ye J, Hao Z, Mumphrey MB, et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. AJP Regul Integr Comp Physiol. 2014;306(5):R352–62.
Rao RS, Kini S. GIP and Bariatric surgery. Obes Surg. 2011;21(2):244–52. https://doi.org/10.1007/s11695-010-0305-x.
Kindel TL, Yoder SM, D’Alessio DA, et al. The effect of duodenal–jejunal bypass on glucose-dependent Insulinotropic polypeptide secretion in Wistar rats. Obes Surg. 2010;20(6):768–75. https://doi.org/10.1007/s11695-010-0095-1.
Sun D, Liu S, Zhang G, et al. Type 2 diabetes control in a nonobese rat model using sleeve gastrectomy with duodenal–jejunal bypass (SGDJB). Obes Surg. 2012;22(12):1865–73. https://doi.org/10.1007/s11695-012-0744-7.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8. https://doi.org/10.1038/nature13135.
Prawitt J, Caron S, Staels B. Glucose-lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol Metab. 2014;25(5):235–44. https://doi.org/10.1016/j.tem.2014.03.007.
Trabelsi M-S, Lestavel S, Staels B, Collet X. Intestinal bile acid receptors are key regulators of glucose homeostasis. Proc Nutr Soc. 2017;76(3):192–202.
Speck M, Cho YM, Asadi A, et al. Duodenal-jejunal bypass protects GK rats from -cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. AJP Endocrinol Metab. 2011;300(5):E923–32.
Zhou X, Qian B, Ji N, et al. Pancreatic hyperplasia after gastric bypass surgery in a GK rat model of non-obese type 2 diabetes. J Endocrinol. 2016;228(1):13–23.
Zhong M-W, Liu S-Z, Zhang G-Y, et al. Effects of sleeve gastrectomy with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats. World J Gastroenterol. 2016;22(32):7332–41. https://doi.org/10.3748/wjg.v22.i32.7332.
Shimabukuro M, Zhou Y-T, Levi M, et al. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci. 1998;95(5):2498–502. https://doi.org/10.1073/pnas.95.5.2498.
Fed Glucose and Insulin Values for Obese Male ZDF Rats Fed Purina 5008 (January 2010 – July 2010) [Internet]. [cited 2016 Jun 3]. Available from: http://www.criver.com/files/pdfs/rms/zdf/rm_rm_r_glucose_insulin_zdf_july_2010.aspx.
Acknowledgements
The authors thank Silke Hempel for the outstanding work in assistance with ELISA measurements. Moreover, we thank Claudia Bravo and Monika Kolterjahn for the excellent work with animal care.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Laessle, C., Nenova, G., Marjanovic, G. et al. Duodenal Exclusion but Not Sleeve Gastrectomy Preserves Insulin Secretion, Making It the More Effective Metabolic Procedure. OBES SURG 28, 1408–1416 (2018). https://doi.org/10.1007/s11695-017-3045-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3045-3