Abstract
Background
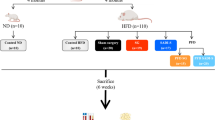
Sleeve gastrectomy plus side-to-side jejunoileal anastomosis (JI-SG), a relatively new approach to bariatric surgeries, has shown promising results for treating obesity and metabolic comorbidities. This study investigated the feasibility and safety of JI-SG in weight loss and diabetes remission compared with sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB).
Methods
Forty 10-week-old male Zucker diabetic fatty rats were randomly assigned to four groups: control, SG, JI-SG, and RYGB. Their body weights, food intake, and levels of gut hormones (ghrelin, insulin, and glucagon-like peptide-1 (GLP-1)) and lipids were measured.
Results
Rats in the SG, JI-SG, and RYGB groups demonstrated lower food intake and more weight loss 2 weeks postoperatively compared with control rats. Furthermore, rats in the JI-SG group achieved more weight loss (mean 242.7 ± 11.2 g) compared with those in the SG and RYGB groups (SG, 401.4 ± 15.1 g and RYGB, 298 ± 12 g, both P < 0.01). All surgery groups demonstrated a decreased fasting insulin, serum glucose, lipid levels, and increased GLP-1 postoperatively. The JI-SG group had lower fasting ghrelin levels than the RYGB group (168 ± 19.8 ng/L vs. 182 ± 16.7 ng/L, P < 0.01) and higher fasting GLP-1 levels than the SG group (1.99 ± 0.11 pmol/L vs. 1.71 ± 0.12 pmol/L, P < 0.01) at 12 weeks postoperatively. Over the experimental period, the ghrelin levels slowly increased in all surgical groups but remained lower than the preoperative and control levels.
Conclusions
JI-SG induced higher ghrelin and GLP-1 levels and improved glycemic control in Zucker diabetic fatty rats. Compared with SG and RYGB, JI-SG appeared to be a simple, relatively safe, and more effective procedure for treating type 2 diabetes and obesity in this animal model.







Similar content being viewed by others
References
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med. 2014;370(21):2002–13.
Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–25.
Rosenthal RJ. International Sleeve Gastrectomy Expert Panel, Diaz AA, et al. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19.
Li JF, Lai DD, Lin ZH, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
DePaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of metabolic syndrome in patients with type 2 diabetes mellitus. Surg Endosc. 2008;22(12):2670–8.
Raj PP, Kumaravel R, Chandramaliteeswaran C, et al. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: preliminary results of a prospective series from India. Surg Endosc. 2012;26(3):688–92.
Li JF, Lai DD, Lin ZH, et al. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24(1):1–11.
Li P, Fu P, Chen J, et al. Laparoscopic Roux-en-Y gastric bypass vs. laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus: a meta-analysis of sixteen recent studies. Hepato-Gastroenterology. 2013;60(121):132–7.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-Kakizaki rats through an enhanced Proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Melissas J, Peppe A, Askoxilakis J, et al. Sleeve gastrectomy plus side-to-side jejunoileal anastomosis for the treatment of morbid obesity and metabolic diseases: a promising operation. Obes Surg. 2012;22(7):1104–9.
Lifante JC, Milone L, Korner J, et al. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg. 2012;22(7):1110–6.
Patel RT, Shukla AP, Ahn SM, et al. Surgical control of obesity and diabetes: the role of intestinal vs. gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring). 2014;22(1):159–69.
Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19(1 Suppl):I110–115.
Lifante JC, Milone L, Korner J, et al. Sleeve gastrectomy improves glucose homeostasis in Zucker diabetic fatty rats. Obes Surg. 2012;22(7):1110–6.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19.
Jurgens CA, Toukatly MN, Fligner CL, et al. B-cell loss and B-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–40.
Trung VN, Yamamoto H, Yamaguchi T, et al. Effect of sleeve gastrectomy on body weight, food intake, glucose tolerance, and metabolic hormone level in two different rat models: Goto-Kakizaki and diet-induced obese rat. J Surg Res. 2013;185(1):159–65.
Peng Y, Murr MM. Roux-en-Y gastric bypass improves hepatic mitochondrial function in obese rats. Surg Obes Relat Dis. 2013;9(3):429–35.
Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–304.
Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258(4):628–37.
Nestvold TK, Nielsen EW, Lappegård KT. Bariatric surgery reduces risk factors for development of type 2 diabetes mellitus in morbidly obese, nondiabetic patients. Metab Syndr Relat Disord. 2013;11(6):441–6.
DePaula AL, Stival AR, Halpern A, et al. Surgical treatment of morbid obesity: mid-term outcomes of the laparoscopic ileal interposition associated to a sleeve gastrectomy in 120 patients. Obes Surg. 2011;21(5):668–75.
Santoro S, Malzoni CE, Velhote MCP, et al. Digestive adaptation with intestinal reserve: a neuroendocrine-based operation for morbid obesity. Obes Surg. 2006;16:1371–9.
Tvarijonaviciute A, Ceron JJ, Holden SL, et al. Effects of weight loss in obese cats on biochemical analytes related to inflammation and glucose homeostasis. Domest Anim Endocrinol. 2012;42(3):129–41.
Samat A, Malin SK, Huang H, et al. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 months in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15(10):963–6.
Cruz-Domínguez MP, Cortés DH, Zarate A, et al. Relationship of ghrelin, acid uric and proinflammatory adipocytokines in different degrees of obesity or diabetes. Int J Clin Exp Med. 2014;7(5):1435–41.
Tamboli RA, Breitman I, Marks-Shulman PA, et al. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity (Silver Spring). 2014;22(7):1617–22.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52.
Aroor A, McKarns S, Nistala R, et al. DPP-4 inhibitors as therapeutic modulators of immune cell function and associated cardiovascular and renal insulin resistance in obesity and diabetes. Cardiorenal Med. 2013;3(1):48–56.
Svane MS, Madsbad S. Bariatric surgery-effects on obesity and related comorbidities. Curr Diabetes Rev. 2014;10(3):208–14.
Chambers AP, Smith EP, Begg DP, et al. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):424–32.
Disclosures
Authors’ contributions
KJW and XGZ were responsible for the study conception and design, analyses and interpretation of the data, and drafting of the manuscript. GQ, JJL, WG, and AAX generated the experimental data. KJW and JFZ provided advice on the study concept, conducted specific analyses, and critically revised the manuscript. JFZ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was funded by the Health Bureau of Pudong New Area (Shanghai, pw2013A-3).
Conflict of Interest
The authors declare that they have no competing interests.
Statement of Animal Rights
The Animal Care and Utilization Committee of Tongji University approved all animal experiments in this study. All animals were housed under standard conditions with free access to water and food.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kaijing Wang and Xiaogang Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, K., Zhou, X., Quach, G. et al. Effect of Sleeve Gastrectomy Plus Side-to-Side Jejunoileal Anastomosis for Type 2 Diabetes Control in an Obese Rat Model. OBES SURG 26, 797–804 (2016). https://doi.org/10.1007/s11695-015-1811-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1811-7