Abstract
Prunus spinosa L., commonly known as blackthorn, holds traditional significance both as a food source and an herbal remedy. This study aims to determine the extract that includes the highest total phenolic content (TPC), antioxidant capacity (AC) using DPPH and CUPRAC assays, and vitamin C from blackthorn using the response surface method in an ultrasonic water bath and to this extract into powder by foam mat drying. The investigation comprises two primary phases. Firstly, the impact of temperature (20–80 °C) and extraction time (5–30 min) on TPC, AC, and vitamin C were systematically explored. Subsequently, the extracts derived from blackthorn fruit were subjected to foam mat drying, utilizing two distinct ratios (7:3 w/w and 8:2 w/w) of foaming agents [maltodextrin (MD) to egg white (EW)], along with three drying techniques (oven, microwave, and natural drying). The optimized extraction parameters were determined as follows: temperature (80 °C) and time (30 min). Furthermore, the results reveal that microwave-dried powders with a low EW ratio exhibit superior preservation of TPC, AC, and vitamin C content. This research underscores the potential utility of foam mat-dried blackthorn powders as functional ingredients and natural colorants within the realm of the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prunus spinosa L., a member of the Rosaceae family commonly referred to as blackthorn or sloe, is a deciduous shrub or small tree that attains a height of 2 to 3 m. This plant species is grows extensively across North America, Asia, Europe, New Zealand, the northern regions of Africa, as well as Tasmania [1]. It is also cultivated in various regions of Turkey and is recognized as ‘guvem’ [2]. Typically inhabiting the fringes of uncultivated and abandoned fields adjacent to pastures, meadows, and forests, P. spinosa thrives under diverse environmental conditions. Upon reaching maturity, the elliptical-shaped blackthorn fruits exhibit a deep blue hue [3, 4]. Research indicates that the fruits of P. spinosa L. are a notable reservoir of antioxidants and natural pigments. Among its constituents, there exist substantial quantities of antioxidant compounds, including cyanidin 3-O–rutinoside and peonidin 3-O–rutinoside, alongside numerous other antioxidative components [5]. In addition, vitamin C and phenolic compounds, especially anthocyanins and neochlorogenic acid (3-O–caffeoylquinic acid, according to IUPAC guidelines), which are phytochemicals with positive effects on health and potential as dietary supplements, are abundant in fruits. Due to their bitterness, the commercial utilization of P. spinosa L. fruits remains limited; however, they are predominantly employed at the local level to craft syrups, juices, jams, compotes, and spirits [3], as well as for specific dietary and medicinal applications [5]. Within the realm of ethnopharmacology, blackthorn fruits find application primarily in treating digestive and respiratory ailments such as diarrhea, colds, flu, and bronchitis, in addition to diabetes, kidney inflammation, and heart disease. Empirical studies have substantiated their antibacterial, anti-inflammatory, anti-proliferative, antioxidant, and enzyme-inhibitory properties. The fruits are rich in phenolic compounds and vitamin C, notably anthocyanins and neochlorogenic acid (3-O–caffeoylquinic acid as per IUPAC nomenclature), both possessing advantageous health benefits and displaying potential as nutraceutical agents [3]. Additionally, the fruits provide 30% of the daily vitamin C requirement. Plums can spoil quickly due to their high water content. Drying plums provides benefits in terms of storage. At the same time, dried plums have many health benefits and are ground into powder and used as an additive [6].
Influencing consumer preferences significantly, color stands out as one of the paramount quality factors. A substantial portion of colorants employed within the food industry are synthetic and pose potential hazards to human health. In recent years, natural colorants have gained escalating favor among consumers, supplanting chemical alternatives, consequently fueling their global industry growth [7]. The methodologies employed for drying hold pivotal importance, ensuring the utilization of the abundant natural pigments found in fruits within the realm of the food industry. A meticulous selection of the drying method is imperative to harness the rich natural pigments, accompanied by their attendant health benefits, for application in the food industry. Various drying techniques can be harnessed, including microwave drying, spray drying, vacuum drying, freezing, drum drying, foam mat drying, and natural drying. Of these, foam mat drying emerges as a more economical option due to its superior drying rate and lower operational temperatures relative to alternative approaches like air drying. Moreover, it curtails the drying duration, thus mitigating the degradation of heat-sensitive food constituents. The abbreviated drying period further averts undesired modifications in the physical attributes of the food. In contrast to methods such as spray drying and freezing, foam mat drying yields powders that are facile to rehydrate and cost-effective. The resulting powders find utility across an array of products, encompassing baked goods, ice cream, snacks, beverages, pastes, and convenience meals [8]. Propelled by its circumvention of inherent limitations associated with conventional dehydration techniques—namely, inadequate rehydration properties, unfavorable sensory profiles, and protracted drying durations—foam-mat drying has garnered considerable attention as an innovative and pragmatic technology. Foams generally manifest as continuous matrices of gas bubbles suspended in liquid or solid mediums, upheld by agents that act upon the matrix surface, encompassing proteins, polymers, surfactants, and particles [9]. Owing to minimal thermal impact, the foam mat drying process effectively preserves the inherent attributes of fresh fruits, including color, flavor, vitamins, and sensory characteristics [10].
Numerous investigations have been undertaken to discern the phenolic compounds, antioxidant capacity, and vitamin C content of P. spinosa [1,2,3, 5, 11]. However, thus far, no study has delved into the optimization of P. spinosa extraction through response surface methodology or its subsequent conversion into powder via foam mat drying (oven, microwave, and natural drying). This study marks the inaugural exploration into the optimization of ultrasonic-assisted extraction (UAE) conditions, targeting a blend of total phenolic compounds (TPC), antioxidant capacities (DPPH, CUPRAC), vitamin C content, and color attributes (L*, a*, b*) within blackthorn extracts. The principal objective of this study is to identify the optimal extract composition yielding elevated levels of TPC, AC, and vitamin C within blackthorn, employing response surface methodology in conjunction with an ultrasonic water bath, and subsequently converting the resultant extract into powder via foam mat drying, leveraging two distinct foaming agent ratios (7:3 w/w and 8:2 w/w, MD: EW) and three diverse drying techniques. Oven, microwave, and natural drying methods were used as drying methods, and the most effective result was determined by comparing these drying methods.
Materials and methods
Material, standards, and reagents
The study focused on P. spinosa L. fruits. The fruit harvest took place in the Pazarlar district of Kutahya, Turkey, at coordinates 39° 0′ 0′′ N, 29° 8′ 0′′ E, with an elevation of 950 m, at the end of September. Following harvest, the fruits underwent cleaning, draining, and subsequent freezing at -20 °C. All substances employed in the assays were of analytical grade and were procured from Merck (Darmstadt, Germany). The standards used encompassed 6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) (CAS No: 53188-07-1) and gallic acid (CAS No: 149-91-7) from Sigma-Aldrich (Germany), while ascorbic acid (C6H8O6) standard (CAS No: 50-81-7) was acquired from Carlo Erba (France). Folin–Ciocalteau (CAS No: 12111-13-6) was purchased from Sigma-Aldrich (Germany). Maltodextrin (DE 20) and egg white (%79.3 protein) were purchased from Tito (Turkey) and Alfasol (Italy), respectively.
Statistical model and experimental design
The optimization of P. spinosa L. extracts through Ultrasonic-Assisted Extraction (UAE) was conducted utilizing response surface methodology, and central composite design. The influence of temperature (20–80 °C) and extraction time (5–30 min) on total phenolic compounds (TPC), antioxidant capacity (AC), vitamin C, and color values (L*, a*, b*) was investigated. Employing Design-Expert 7.0.0 (Minneapolis, USA), a total of 11 experiments were devised. Table 1 presents the outcomes of the experimental design and optimization. To mitigate the effects of random variables on the results, the tests were conducted in a randomized sequence.
Extraction process
Extraction process studies were determined by comparing the highest phenolic and antioxidant values according to the preliminary study parameters. Fruits were retrieved from the − 20 °C freezer and thawed prior to extraction. Using an ultrasonic water bath (GREATSONIC GS -DS360-P, China), the fruits were subjected to extraction with distilled water (10 g/100 ml) for 5–30 min at temperatures ranging from 20 to 80 °C, as per the settings computed through Response Surface Methodology (RSM). The resultant extracts were then passed through 0.45 µm mixed cellulose esters (MCE) membrane filters (hydrophilic) without centrifugation and stored in bottles at − 20 °C until further analysis.
Determination of TPC and AC (DPPH, CUPRAC)
The quantification of TPC in the fruit extracts was carried out spectrophotometrically using the Folin-Ciocalteu method [11]. A calibration curve was established utilizing gallic acid (50–500 mg/l) as a reference. The TPC data were expressed in mg (GAE)/g of gallic acid equivalents. Assessment of radical scavenging capacity (DPPH) was performed following the methodology elucidated by Singh et al. [12] using a spectrophotometer (Shimadzu UV-1800, Japan) at a wavelength of 517 nm. The percentage inhibition of the DPPH radical for each sample was calculated using the provided equation.
Ac: control absorbance, As: sample absorbance.
To determine the copper-reducing capacity (CUPRAC), the methodology established by Apak et al. [13] was adopted. The absorbance at 450 nm of the resultant solution was measured utilizing a Shimadzu UV-1800 spectrophotometer (Japan). The data were expressed in mg (TE)/g of Trolox equivalents. Each experiment was conducted in triplicate.
Analysis of vitamin C (ascorbic acid)
A 0.004% oxalic acid solution was added to the sample to stop the oxidation of vitamin C and provide the acid needed for the reaction. Vitamin C is decolorized by reducing the oxidation–reduction dye 2,6 dichloroindophenol. The color of the unreduced dye at the end of the reaction was measured with a UV spectrophotometer at 518 nm. A calibration curve was created by making ascorbic acid standard (Carlo Erba, France) solutions with 2, 2,5, 5, 10, 20, 30, and 40 ppm concentrations [14].
Physicochemical analysis
Utilizing a portable refractometer (Atago PAL-1 Pocket, Japan) calibrated with distilled water, the soluble solids content of the extracts was determined and expressed in oBrix. The pH of the extracts was assessed using a pH meter with buffer solution calibration (Starter-3100, Ohaus, USA). The color characteristics were gauged through a CIELAB colorimeter (MiniScan XE, HunterLab, USA), wherein the L*, a*, and b* values represented color brightness, red-green, and yellow-blue attributes, respectively. The moisture content of the powders was determined using a moisture analyzer (RADWAG, AB-0066-K), and the water activity (aw) was measured using Novasina LabMaster equipment (Novasina AG Ltd., Lachen SZ, Switzerland). The measurements were conducted in triplicate, and the mean values were calculated based on the results’ average.
Drying process
Preparation of foam
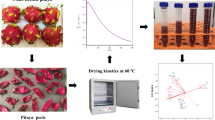
Following the washing and seed removal from blackthorn fruits, extraction was performed (seedless blackthorn fruits: water; 1:3) as per the optimal test conditions (80 °C and 30 min). The resultant blackthorn fruit extracts (7.7 oBrix, pH 3.37) were mixed with a 5% maltodextrin: egg white mixture, at ratios of 7:3 w/w (G1, G2, G3) and 8:2 w/w (G4, G5, G6). The control group (C1, C2, C3) did not receive any foaming agent. The foaming agent contents and drying processes are detailed in Table 2. The foam was generated by whipping the samples using a mixer (Emsan, Model Pro-Multimax-1001, Turkey) at 1000 rpm for 15 min. The density and expansion of foam resulting from different foaming agent ratios were assessed.
Foam density
The foam’s weight and volume were recorded after placement in a measuring cylinder. The mass-to-volume ratio, termed foam density, was calculated using the formula [15]:
Foam expansion
Foam expansion, indicating the volume increment due to introduced air during whipping, was calculated as a percentage increase. Employing the equation below, the difference in extract volume before and after foaming was assessed, as per Abd El-Salam et al. [16]:
Vo: extract volume before foaming (ml), V1: extract volume after foaming (ml).
Drying of foams by oven, microwave, and natural drying
Oven (Memmert UN55, Germany) drying was carried out for samples C1, G1, and G4; microwave (Beko MD1610, Turkey) drying for C2, G2, and G5; and natural drying (C3, G3, G6) was performed. The foam, spread about 5 mm thick on a stainless steel tray with greaseproof paper, was dried at approximately 70 ± 5 °C for 90–150 min in the oven. Microwave drying was conducted at 300 W for about 6–8 min, while natural drying occurred over 150–330 min at an ambient temperature of 20–25 °C and humidity at 50%. Subsequent to milling the dried products with a grinder (Siemens MC23200, Germany), the powders were packed in polypropylene bags and stored at room conditions for further analysis.
Water solubility (%) of the powder
Water solubility was determined with minor modifications based on the method proposed by Bayram et al. [17]. Approximately 0.3 g of powder was vortexed for 10 min in 30 ml of deionized water. The solution was then centrifuged at 4000 rpm for 15 min. The supernatant was transferred to preweighed petri dishes and dried at 105 °C for 20 h in an oven. The solubility percentage was calculated using the formula:
The TPC, AC (DPPH, CUPRAC, and ascorbic acid of powders
The extraction of powder samples was performed the method proposed by Khamjae and Rojanakorn [18]. Dried samples (0.2 g) were extracted with 20 ml of 50% methanol in an ultrasonic bath for 30 min, followed by filtration. The Total Phenolic Compounds (TPC) were determined utilizing the Folin Ciocalteu (FC) reagent based on Singleton et al.’s [11] method, using gallic acid as the standard. DPPH radical scavenging activity [12] and Copper Reduction Capacity (CUPRAC) were assessed as per Apak et al.’s [13] methodology. The results were expressed in mg TE/g Trolox equivalent. Absorbance measurements were conducted at wavelengths of 760 nm, 517 nm, and 450 nm using a Shimadzu UV-1800 spectrophotometer (Kyoto, Japan). The quantification of ascorbic acid in dried foam samples followed the protocol outlined by Araujo et al. [19]. Powder samples (0.1 g) were mixed with 10 ml of a 0.004% oxalic acid solution. After extraction in an ultrasonic bath for 20 min, the solution was filtered (0.45 µm). Subsequently, 1 ml of the filtrate was mixed with 9 ml of 2,6-dichloroindophenol and subjected to vortexing The absorbance of the mixture was determined at 518 nm using a spectrophotometer. By utilizing a standard curve, the amount of ascorbic acid was determined and expressed as mg/100 g.
Statistics analyzes
SPSS Statistics was used to conduct the statistical analysis (PASW Statistics 18, USA). When the one-way analysis of variance (ANOVA) yielded a significant result, Tukey’s multiple comparison tests with a 95% confidence interval were utilized to assess the significant differences among the samples. Optimization of extraction parameters and analysis of variance were carried out using Design-Expert 7.0.0 (Minneapolis, USA).
Results and discussion
Fitting the model
The extraction temperature (X1: 20–80 °C) and extraction time (X2: 5–30 min), which are significant variables, were employed to obtain the extract with the highest TPC and AC (DPPH and CUPRAC) content. This was achieved through the utilization of a two-factor RSM (Response Surface Methodology) with central composite design. A total of 11 experiments were conducted in alignment with the central composite design to determine the optimal conditions (Table 1). The optimal conditions for maximizing TPC and AC (DPPH, CUPRAC) were predicted by overlaying the outcomes of simultaneous optimization utilizing the desirability function approach and each individual optimal condition. Under ideal circumstances, with a temperature of 80 °C and an extraction time of 30 min, the TPC, DPPH, and CUPRAC values were 11.88 mg GAE/g, 78.6%, and 535.69 mg TE/g, respectively. These findings demonstrated concurrence between experimental and predicted values, indicating the precision and reliability of the model parameters derived using RSM (Table 3).
Response surface 3D graphics (Fig. 1) illustrate the influence of process parameters. The TPC and AC levels in the extracts exhibited significant variations due to the effects of time and temperature process parameters. The ANOVA results presented in Table 4 indicate that among the examined factors, the linear effects of extraction temperature were highly significant (P < 0.001). The experiments revealed an increase in temperature-dependent extraction efficiency. Another indicator of model adequacy was the absence of a significant lack of fit (P > 0.05). The recovery of biologically active compounds should be achieved with minimal degradation by employing an effective extraction procedure [20]. With rising temperatures, TPC and AC significantly increased (P < 0.001). The influence of temperature on the rate of extraction of bioactive compounds has been reported in previous studies [20]. Temperature affects the mass transfer process, enhancing solvent penetration. Higher temperatures lead to a quicker breakdown of plant matrix and cell structures, rendering cells more permeable [7]. As temperature rises, the connections between polysaccharides, proteins, and phenolic compounds weaken, leading to an increased rate of phenolic compound diffusion [21].
As far as is known, this is the inaugural study of the maximum determination of total polyphenols and antioxidant capacity using RSM optimization.
Effect of extraction parameters on TPC, AC (DPPH, CUPRAC), vit C, and the CIELab values (L*, a*, b*)
The extract with the highest TPC content, AC, and color values were determined through UAE under optimal conditions with RSM (Table 1). The experimentally obtained TPC, DPPH, CUPRAC, a*, and b* values were fitted to a quadratic polynomial model, and an analysis of variance (ANOVA) was used to examine the effects of the variables under study, their interactions, and the statistical significance of the model. L* value was fitted to a linear model and Vit C to a mean model. Tables 4 and 5 present the significance of each coefficient as determined by the F-test and P-value. A higher F-value compared to the P-value for any model term indicates a greater impact on the associated response variables [22]. The results demonstrate that the evaluated parameters significantly influenced the responses (P < 0.01).
The P values of the regression coefficients for each of the examined responses for TPC and AC (DPPH, CUPRAC) are presented in Table 4. The quadratic models for TPC and AC were found to be suitable approximations for the analyzed responses, as indicated by the statistically significant P-values. The coefficients of multiple determinations for TPC and AC (DPPH, CUPRAC) were quite high, with values of 0.90, 0.95, and 0.96, respectively (R2). The model’s adequacy was further confirmed by the absence of significant lack of fit (P > 0.05). The following quadratic equations elucidate the influence of extraction factors on TPC (Eq. 5), AC (DPPH (Eq. 6), CUPRAC (Eq. 7).
where x1 and x2 represent temperature and time, respectively. The optimal conditions for achieving maximum TPC and AC in the present study were a 30 min extraction period and a temperature of 80 °C. This demonstrates that the chosen RSM model was successfully used to make extracts of blackthorn with the highest TPC and AC concentration using UAE.
The blackthorn extracts obtained via the UAE method exhibited varying values for TPC, AC (DPPH and CUPRAC), ranging from 7.02 to 11.97 mg GAE/g, 20.40% to 79.60% ARA, and 182.94 to 537.20 mg Trolox/g, respectively (Table 1). The TPC values detected in this study were consistent with the findings of Miodragović et al. [23] (2.30–7.59 mg GAE/g) and Popović et al. [24] (11.10–30.43 mg GAE/g). However, they were lower than the values reported by Magiera et al. [1] (26.8–124 mg GAE/g) and higher than those by Aliyazıcıoğlu et al. [25] (4.198 mg GAE/g). The results highlight a significant correlation between antioxidant capacities and total polyphenolic content. The substantial AC of the blackthorn extracts was verified by both assays. Similar to our study, Sabatini et al. [26] demonstrated the antioxidant capacity of P. spinosa, with ethanolic extracts exhibiting 75% DPPH activity at a concentration of 0.6 mg/ml. Capek and Košťálová [4] observed even higher (~ 92%) activity in blackthorn extracts extracted with alkali compared to the present study. The DPPH scavenging activity of ethanolic extracts of P. spinosa, expressed in terms of IC50, was found to be 0.62–3.46 mg dw/ml [24]. Celik et al. [2] reported a TAC assay result of 1.021 mmol TE/kg fw in their antioxidant study of blackthorn. Differences among the results might be attributed to variables such as the extraction method employed, choice of solvent, fruit part utilized, and variations in soil and weather conditions during the harvest year.
Vitamin C, a naturally occurring water-soluble antioxidant, is found abundantly in many fruits. Due to its limited stability after heat processing, it is utilized as an indicator of dried food quality [27]. Vitamin C was demonstrated to be represented by a mean model for the examined responses and exhibited no significant lack of fit (P > 0.05). In this study, the vitamin C content in blackthorn extracts was found to range from 54.05 to 140.00 mg/100 ml (Table 1). These results exceeded values of 19–26 (mg/100 g fw) reported by Ozkan [28] and 25.5 (mg/100 g fw) reported by Celik et al. [2].
Color optimization was a pivotal analysis, given that the powdered products derived from the extracts would be employed for both coloring purposes and functional properties. The CIELab values a* value represents redness/greenness and the b* value represents blue/yellowness. The whiteness and brightness increase as the L* value approaches 100 [29]. The blackthorn extracts obtained using the UAE method exhibited color parameters (L*, a*, and b*) ranging from 26.46 to 45.07, 4.90 to 26.21, and 8.11 to 16.70, respectively (Table 1). The P-values of the regression coefficients are displayed in Table 5 for each examined response of color values L*, a*, and b*. The linear model for L* and the quadratic models for a* and b* were suitable for the investigated responses, as indicated by the statistically significant P-values. With respective values of 0.77, 0.96, and 0.94, the coefficients of multiple determinations for L*, a*, and b* were determined (R2). Temperature significantly impacted the response variables (P < 0.001). The following linear and quadratic equations elucidate how extraction parameters influenceL* (Eq. 8), a* (Eq. 9), and b* (Eq. 10).
where, x1 and x2 stand for temperature, and time, respectively. Extraction temperature exhibited a significant (P < 0.01) effect on L*, a*, and b* (Table 5). Figure 2 shows the Response surface-3D graphics of process parameters on L*, a*, and b*. The ‘a*’ values underwent significant alterations due to the influence of process parameters. This phenomenon can be attributed to the impact of temperature, which renders molecular interactions more time-dependent.
This study marks the first attempt to optimize the UAE conditions for a simultaneous enhancement of TPC, DPPH, and CUPRAC alongside color attributes (L*, a*, and b*) in blackthorn extracts.
Physicochemical characteristics of blackthorn extracts
Table 1 displays the physicochemical characteristics of the blackthorn extracts. Soluble solids (°Brix) ranged from 2.00 to 2.40, while pH values varied between 3.37 and 3.47. Comparable findings have been reported previously for P. spinosa fruits, with pH values spanning 3.43 to 3.92 [30, 31]. StankoviĆ et al. [32] found a higher pH value (4.08) for P. spinosa fruit extracts compared to this study. There were statistical differences in pH and oBrix values between the extracts. Assessing physical–chemical properties is pivotal for quality evaluation and standardized extraction procedures. Notably, the temperature and time parameters employed during the extraction were observed to influence extract bioactivity through their effects on phenolic compounds.
Foam and powder analyses
Foam properties
The density and expansion capacity of foam, produced using varying maltodextrin (MD): egg white (EW) concentrations (7:3 w/w, 8:2 w/w) as foaming agents, were evaluated. Foam density and expansion were determined as 0.12 g/ml (742%) and 0.16 g/ml (645%) for 7:3 w/w and 8:2 w/w MD: EW ratios, respectively. Notably, increasing EW concentration led to reduced foam density and enhanced volume expansion. This behavior is attributed to augmented protein absorption at the liquid–air interface, contributing to decreased surface tension and increased volume expansion. Proteins exhibit effective foaming and stabilization properties as they adsorb at the liquid–air interface, reducing surface tension [8]. Proteins adsorb at the liquid–air interface, lowering a liquid’s surface tension. Proteins are hence effective foaming and stabilizing agents [33]. Similar results (639.3 ml) were reported for the foam volume of foam-dried mulberry with a carrier additive ratio of 6% albumin, 0.3% CMC and 1.5% RDM (w/w), and a whipping time of 10 min [34]. Low-density foams were found to dry faster. Higher-density foams take a while to dry out, which causes thermal damage and lower product quality [35].
Physicochemical properties of blackthorn powder
Moisture content (% moisture) and water activity (aw) serve as indicators of water’s role in biological activities and product quality, especially during prolonged powder storage. Table 6 presents the effects of different foaming agents and drying techniques on the physicochemical attributes of foam mat-dried blackthorn powders. Among samples with equivalent content, natural drying yielded the highest moisture content and aw values, while oven-dried samples exhibited the lowest values. Moisture content ranged from 3.80% to 9.26%, and aw spanned 0.16 to 0.32, with statistically significant differences observed among samples (P < 0.05). In a similar technique applied to mulberry powder, Thuy et al. [34] reported comparable moisture content (4.11–5.31%) and aw (0.24–0.32) results. Pushkar et al. [36] noted moisture content variations (1.6%–9.3%) among watermelon juice powder produced through different methods (under the sun, under vacuum, spraying, and lyophilization). Franceschinis et al. [37] deemed 6% moisture content suitable for powdered blackberries. Moisture content significantly influences glass transition, crystallization behavior, drying efficiency, powder flowability, stickiness, and storage stability. At aw 0.85, bacteria can’t grow, while at aw 0.70 and 0.65, respectively, yeast and mold can’t develop. Microbial growth, oxidative, and enzymatic activity decrease in 0.2–0.3 water activity [34]. The results of our study were found in this range.
Powder solubility ranged from 56.0% to 82.5% (Table 6), with significant differences observed between the control group and other samples (P < 0.05). However, no statistically significant differences were noted among samples G1 to G6 (P > 0.05). Notably, the addition of foaming agents significantly improved powder solubility in the control group (C1, C2, and C3). This demonstrated how foaming agent additions enhance the powder’s solubility. Increasing maltodextrin concentration in samples dried by the same method corresponded to higher solubility. This can be attributed to maltodextrin’s high solubility in water. The highest solubility (82.50%) was evident in the naturally dried G6 sample. Although the solubility was statistically insignificant (P > 0.05), it showed an increasing trend toward oven drying, microwave drying, and natural drying. This can be attributed to the low ambient temperature (20–25 °C) in natural drying, preventing the collapse of the bubbles. Similarly, Gao et al. [38] reported water solubility of egg white, CMC, and MD foaming agents in microwave-assisted foam-mat dried blueberry pulp powders (51.56%-80.00%). They also found that the resolution of the control group was lower than the other samples in their study. Additionally, the water solubility of foam-mat dried sour cherry powder containing egg white and methylcellulose, as studied by Abbasi and Azizpour [39], ranged from 42.2% to 48.4%, lower than the present findings.
Color is a determining factor for assessing food product quality and enhancing market appeal [40]. Figure 3 illustrates the colors of blackthorn extract powders with and without foaming agents (G1, G2, G3, G4, G5, G6) and controls (C1, C2, C3). L* values, indicating lightness/darkness, revealed darker colors in control samples (lower L* values) (Table 6). The a* values, reflecting green/red variation, exhibited higher redness in samples G1, G2, and G3 (higher a* values). The b* values, corresponding to blue/yellow, demonstrated the highest value in sample G5. Color changes may be attributed to anthocyanin oxidation and the Maillard reaction, contributing to product darkening [34]. Effects of foaming agents on color were evident, with higher L*, a*, and b* values observed in foaming agent-utilizing samples compared to control groups. The L* value was found to be the highest in the oven-dried G4 sample. This can be attributed to the high concentration of maltodextrin used as a foaming agent. The increase in L* value may be due to the white maltodextrin [29]. The lowest L* value was observed in the natural dried G6 sample. This may be due to oxidation due to the long drying time. In the control group, the highest b* value was seen in the naturally dried C3 sample (3.82). Microwave-dried samples displayed increased a* values with higher egg white concentrations. Notably, microwave drying yielded the highest b* value in samples with low egg white concentrations. Microwave drying causes the browning appearance, which can be related to pigment decomposition and the Maillard reaction [38]. The significant influence of foaming agents and drying methods on L*, a*, and b* (P < 0.05) was observed in the color analyses.
TPC, AC (DPPH, CUPRAC), vitamin C assessment of powders
The outcomes of the comparison of drying conditions and foaming agents on TPC and AC in blackthorn powders are displayed in Table 7. The highest TPC (14.8 mg GAE/g), DPPH (6.45 mg TE/g), and CUPRAC (578.28 mg TE/g) values were ascertained in the powder sample from the control group (without MD + EW) subjected to microwave drying. These results are higher than those of Blagojevi´c et al. [3] who reported the TPC of P. spinosa L. encapsulated in maltodextrin and halloysite-based coating at 4.88–9.47 mg/g. Conversely, the present findings are less than those of Darniadi et al. [41], who identified the TPC of foam-mat freeze-dried blueberry powder as between 29.43 and 31.73 mg/g. Microwave drying has emerged as the most effective method among powders in which foaming agents are used at the same rate. This is attributed to the rapid of microwave drying, leading to reduced oxidation of foams. In instances where foaming agents were employed, TPC and AC levels elevated as egg white (EW) content declined in microwave-dried powder samples. Heightened EW content led to increased foaming and oxidation due to larger surface area exposure, thereby decreasing TPC and AC.
Antioxidant properties play a pivotal role in evaluating powder quality. The results showed that MD + EW (8:2) contains the G5 sample with the highest TPC (14.1 mg GAE/g), DPPH (5.26 mg TE/g), and CUPRAC (465.07 mg TE/g). Microwave drying was observed to better preserve the antioxidant properties of powders. De Carvalho et al. [42] reported lower TPC for jambolan juice powders dried via foam mat (2.28–2.92 mg/g) using 10.0% (w/w) Emustab®, 2.5% (w/w) Super Liga Neutra® and 20.0% (w/w) Maltodextrin 10 DE. It was observed that the antioxidant value increased as the maltodextrin ratio increased in the microwave drying method. Similarly, in their study using maltodextrin and gum arabic as wall materials, Ruengdech et al. [14] reported that the antioxidant value increased with an increasing maltodextrin ratio. Their assessment of powder antioxidant capacity (DPPH and FRAP) stood at 11.7–13.9 (μmol Trolox/g) and 24.7–30.3 (μmol Trolox/g).
Ascorbic acid, being water-soluble and sensitive to heat, deteriorates rapidly, a process hastened by heat, light, and airborne oxygen [43]. As a heat-sensitive component, ascorbic acid is a valuable indicator of fruit drying quality [8]. The effect of diverse drying conditions and wall materials on vitamin C retention was explored. Vitamin C values for powder samples are shown in Table 7. Samples G5 and G6 exhibited maximal vitamin C content (69.22–68.10 mg/100 g). Enhanced vitamin C retention was noted in microwave-dried samples, possibly due to shorter drying times. Microwave drying lasted around 6 min, reducing vitamin C degradation. Oxidative degradation of vitamin C may be accelerated due to prolonged drying times and oxygen in the air [43]. Moreover, vitamin C content declined as egg white increased. Similar to the present study, egg albumin addition during kadam fruit drying with a foam mat led to reduced vitamin C content [8].
Conclusions
This study covers the optimization of P. spinosa L. juice extraction using the Response Surface Methodology and the conversion of the optimum extract into powder products by foam mat drying. RSM proved invaluable for studying component interactions and optimizing processes. Optimal extraction conditions were established at a temperature of 80 °C and a duration of 30 min.
In the subsequent phase, blackthorn powder was produced via the foam drying method. To achieve powdered products with heightened total phenolic compound, antioxidant capacity, and vitamin C content, two foaming agent ratios (7:3 w/w and 8:2 w/w) (MD: EW), and three drying methods (oven, microwave, and natural drying) were employed. As EW content increased from 1% to 1.5%, all properties (TPC, AC, and Vit C) exhibited a declining trend. Remarkably, microwave-dried products exhibited the highest TPC, AC, and vitamin C values. This study underscores the successful utilization of maltodextrin: egg white (8:2) in combination with microwave drying for producing blackthorn extracts in powdered form. In conclusion, this study underscores the rich bioactive components within fruit extracts and powdered blackthorn products, highlighting their potential to enhance the nutritional value of various foods. These products hold potential applications as flavoring agents, functional ingredients, and natural colorants in powdered beverages, pastries, and confectionery items. Additionally, this research prompts further exploration into the stability of blackthorn powder products concerning color and bioactive components, expanding their possible applications in various sectors.
References
A. Magiera, M.E. Czerwińska, A. Owczarek, A. Marchelak, S. Granica, M.A. Olszewska, Polyphenols and maillard reaction products in dried Prunus spinosa fruits: quality aspects and contribution to anti-inflammatory and antioxidant activity in human immune cells ex vivo. Molecules 27(10), 3302 (2022). https://doi.org/10.3390/molecules27103302
F. Celik, M. Gundogdu, S. Alp, F. Muradoglu, S. Ercişli, M.K. Gecer, I. Canan, Determination of phenolic compounds, antioxidant capacity and organic acids contents of Prunus domestica L., Prunus cerasifera Ehrh. and Prunus spinosa L. fruits by HPLC. Acta Chromatogr. 29(4), 507–510 (2017). https://doi.org/10.1556/1326.2017.00327
B. Blagojević, D. Četojević-Simin, S. Djurić, G. Lazzara, S. Milioto, D. Agić et al., Anthocyanins and phenolic acids from Prunus spinosa L. encapsulation in halloysite and maltodextrin based carriers. Appl. Clay Sci. 222, 106489 (2022). https://doi.org/10.1016/j.clay.2022.106489
P. Capek, Z. Košťálová, Isolation, chemical characterization and antioxidant activity of Prunus spinosa L. fruit phenolic polysaccharide-proteins. Carbohydr. Res. 515, 108547 (2022). https://doi.org/10.1016/j.carres.2022.108547
E. Backes, M.G. Leichtweis, C. Pereira, M. Carocho, J.C. Barreira, A.K. Genena et al., Ficus carica L. and Prunus spinosa L. extracts as new anthocyanin-based food colorants: a thorough study in confectionery products. Food Chem. 333, 127457 (2020). https://doi.org/10.1016/j.foodchem.2020.127457
S.A. Sifat, A.T. Trisha, N. Huda, W. Zzaman, N. Julmohammad, Response surface approach to optimize the conditions of foam mat drying of plum in relation to the physical–chemical and antioxidant properties of plum powder. Int. J. Food Sci. (2021). https://doi.org/10.1155/2021/3681807
S. Karasu, Y. Bayram, K. Ozkan, O. Sagdic, Extraction optimization crocin pigments of saffron (Crocus sativus) using response surface methodology and determination stability of crocin microcapsules. J. F. Meas. Charact. 13(2), 1515–1523 (2019). https://doi.org/10.1007/s11694-019-00067-x
K. Osama, K. Younis, O.S. Qadri, S. Parveen, M.H. Siddiqui, Development of under-utilized kadam (Neolamarkia cadamba) powder using foam mat drying. LWT 154, 112782 (2022). https://doi.org/10.1016/j.lwt.2021.112782
M. Azizpour, M. Mohebbi, M.H.H. Khodaparast, Effects of foam-mat drying temperature on physico-chemical and microstructural properties of shrimp powder. Innov. Food Sci. Emerg. Technol. 34, 122–126 (2016). https://doi.org/10.1016/j.ifset.2016.01.002
N.A. Shaari, R. Sulaiman, R.A. Rahman, J. Bakar, Production of pineapple fruit (Ananas comosus) powder using foam mat drying: effect of whipping time and egg albumen concentration. J. Food Process. Preserv. 42(2), e13467 (2018). https://doi.org/10.1111/jfpp.13467
V.L. Singleton, R. Orthofer, R.M. Lamuela-Raventos, Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzym. 299, 152–178 (1999)
R. Singh, K. Chidambara Murthy, G. Jayaprakasha, Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agri. F. Chem. 50, 81–86 (2002). https://doi.org/10.1021/jf010865b
R. Apak, K. Guclu, M. Ozyurek, S.E. Karademir, Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agri. and F. Chem. 52, 7970–7981 (2004). https://doi.org/10.1021/jf048741x
Pearson, D. The Chemical Analysis of Foods. Chem. Pub. New York. 1970. at:https://books.google.gg/books/about/The_chemical_analysis_of_foods. Accessed 21 March 2022
A. Ruengdech, U. Siripatrawan, Improving encapsulating efficiency, stability, and antioxidant activity of catechin nanoemulsion using foam mat freeze-drying: the effect of wall material types and concentrations. LWT 162, 113478 (2022). https://doi.org/10.1016/j.lwt.2022.113478
E.A.E.S. Abd El-Salam, A.M. Ali, K.S. Hammad, Foaming process optimization, drying kinetics and quality of foam mat dried papaya pulp. J. Food Sci. Technol. 58, 1449–1461 (2021). https://doi.org/10.1007/s13197-020-04657-2
Y. Bayram, K. Ozkan, O. Sagdic, Valorization of viticulture waste as verjuice powders: their characterization, storage stability and application in beverage formulation. Eur. Food Res. Technol. 249, 2897–2910 (2023). https://doi.org/10.1007/s00217-023-04336-4
T. Khamjae, T. Rojanakorn, Foam-mat drying of passion fruit aril. Int. Food Res. J. 25(1), 204–212 (2018)
C.D.S. Araujo, J.L.G. Corrêa, S. Dev, L.L. Macedo, W.C. Vimercati, C.R. de Oliveira, L.A.S. Pio, Influence of pretreatment with ethanol and drying temperature on physicochemical and antioxidant properties of white and red pulp pitayas dried in foam mat. Dry. Technol. 40(3), 484–493 (2022). https://doi.org/10.1080/07373937.2020.1809446
Y. Bayram, C.E. Karabacak, Characterization of unripe grapes (Vitis vinifera L.) and its use to obtain antioxidant phenolic compounds by green extraction. Front. Sustain. Food Syst. 6, 909894 (2022). https://doi.org/10.3389/fsufs.2022.909894
J. Živković, K. Šavikin, T. Janković, N. Ćujić, N. Menković, Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 194, 40–47 (2017). https://doi.org/10.1016/j.seppur.2017.11.032
A. Esmaeili, M. Gholami, Optimization and preparation of nanocapsules for food applications using two methodologies. Food Chem. 179, 26–34 (2015). https://doi.org/10.1016/j.foodchem.2015.01.115
M. Miodragović, N. Magazin, Z. Keserović, B. Milić, B. Popović, B. Blagojević, J. Kalajdžić, The early performance and fruit properties of apricot cultivars grafted on Prunus spinosa L. interstock. Sci. Hortic. 250, 199–206 (2019). https://doi.org/10.1016/j.scienta.2019.02.042
B.M. Popović, B. Blagojević, R.Ž Pavlović, N. Mićić, S. Bijelić, B. Bogdanović et al., Comparison between polyphenol profile and bioactive response in blackthorn (Prunus spinosa L.) genotypes from north Serbia-from raw data to PCA analysis. Food Chem. 302, 125373 (2020). https://doi.org/10.1016/j.foodchem.2019.125373
R. Aliyazicioglu, O. Yildiz, H. Sahin, O.E. Eyupoglu, M.T. Ozkan, S.A. Karaoglu, S. Kolayli, Phenolic components and antioxidant activity of Prunus spinosa from Gumushane. Turkey. Chem. Nat. Compd. 51(2), 346–349 (2015). https://doi.org/10.1007/s10600-015-1278-8
L. Sabatini, D. Fraternale, B. Di Giacomo, M. Mari, M.C. Albertini, B. Gordillo et al., Chemical composition, antioxidant, antimicrobial and anti-inflammatory activity of Prunus spinosa L. fruit ethanol extract. J. Funct. Foods. 67, 103885 (2020). https://doi.org/10.1016/j.jff.2020.103885
A. Chang, X. Zheng, H. Xiao, X. Yao, D. Liu, X. Li, Y. Li, Short-and medium-wave infrared drying of cantaloupe (Cucumis melon L.) slices: Drying kinetics and process parameter optimization. Processes 10(1), 114 (2022). https://doi.org/10.3390/pr10010114
G. Ozkan, Phenolic compounds, organic acids, vitamin C and antioxidant capacity in Prunus spinosa L. C. R. Acad. Bulg. Sci. 72(2), 267–273 (2019). https://doi.org/10.7546/crabs.2019.02.17
Y. Bayram, O. Sagdic, Antioxidant, color, and sensory properties of apple juices colored with saffron microcapsules. Lat. Am. Appl. Res. 52(4), 379–386 (2022). https://doi.org/10.52292/j.laar.2022.922
R. Ganhao, M. Estévez, P. Kylli, M. Heinonen, D. Morcuende, Characterization of selected wild Mediterranean fruits and comparative efficacy as inhibitors of oxidative reactions in emulsified raw pork burger patties. J. Agric. Food Chem. 58(15), 8854–8861 (2010). https://doi.org/10.1021/jf101646y
P. Morales, I.C. Ferreira, A.M. Carvalho, V. Fernández-Ruiz, M.C. Sánchez-Mata, M. Cámara, J. Tardío, Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 115(2), 176–185 (2013). https://doi.org/10.1002/ejlt.201200162
M.I. Stankovi, V.L. Savi, J.V. Živkovi, V.M. Tadi, I.A. Arsi, Tyrosinase inhibitory and antioxidant activity of wild Prunus spinosa L. fruit extracts as natural source of bioactive compounds. Not. Bot. Horti. Agrobot. 47(3), 651–657 (2019). https://doi.org/10.15835/nbha47311425
J. Dehghannya, M. Pourahmad, B. Ghanbarzadeh, H. Ghaffari, Heat and mass transfer modeling during foam-mat drying of lime juice as affected by different ovalbumin concentrations. J. Food Eng. 238, 164–177 (2018). https://doi.org/10.1016/j.jfoodeng.2018.06.014
N.M. Thuy, V.Q. Tien, N.N. Tuyen, T.N. Giau, V.Q. Minh, N.V. Tai, Optimization of mulberry extract foam-mat drying process parameters. Molecules 27(23), 8570 (2022). https://doi.org/10.3390/molecules27238570
Z. Izadi, M. Mohebbi, F. Shahidi, M. Varidi, M.R. Salahi, Cheese powder production and characterization: A foam-mat drying approach. Food Bioprod. Process. 123, 225–237 (2020). https://doi.org/10.1016/j.fbp.2020.06.019
S. Pushkar, N.K. Sachan, S.K. Ghosh, Pharmacotechnical assessment of processed watermelon flesh as novel tablet disintegrant. Chem. Phytopotentials Health Energy Environ. Perspect. (2012). https://doi.org/10.1007/978-3-642-23394-4_34
L. Franceschinis, D.M. Salvatori, N. Sosa, C. Schebor, Physical and functional properties of blackberry freeze- and spray-dried powders. Dry. Technol. 32, 197–207 (2014). https://doi.org/10.1080/07373937.2013.814664
R. Gao, L. Xue, Y. Zhang, Y. Liu, L. Shen, X. Zheng, Production of blueberry pulp powder by microwave-assisted foam-mat drying: Effects of formulations of foaming agents on drying characteristics and physicochemical properties. LWT 154, 112811 (2022). https://doi.org/10.1016/j.lwt.2021.112811
E. Abbasi, M. Azizpour, Evaluation of physicochemical properties of foam mat dried sour cherry powder. LWT-Food Sci. Technol. 68, 105–110 (2016). https://doi.org/10.1016/j.lwt.2015.12.004
C. Gu, H. Ma, J.A. Tuly, L. Guo, X. Zhang, D. Liu et al., Effects of catalytic infrared drying in combination with hot air drying and freeze drying on the drying characteristics and product quality of chives. LWT 161, 113363 (2022). https://doi.org/10.1016/j.lwt.2022.113363
S. Darniadi, I. Ifie, P. Ho, B.S. Murray, Evaluation of total monomeric anthocyanin, total phenolic content and individual anthocyanins of foam-mat freeze-dried and spray-dried blueberry powder. J. Food Meas. Charact. 13, 1599–1606 (2019). https://doi.org/10.1007/s11694-019-00076-w
T.I.M. De Carvalho, T.Y.K. Nogueira, M.A. Mauro, S. Gómez-Alonso, E. Gomes, R. Da-Silva et al., Dehydration of jambolan [Syzygium cumini (L.)] juice during foam mat drying: quantitative and qualitative changes of the phenolic compounds. Int. Food Res. J. 102, 32–42 (2017). https://doi.org/10.1016/j.foodres.2017.09.068
T. Sarkar, M. Salauddin, H.I. Sheikh, S. Pati, R. Chakraborty, Effect of drying on vitamin, carotene, organic acid, mineral composition, and microstructural properties of mango (Mangifera indica). J. Food Process. Preserv. 46(2), e16237 (2022). https://doi.org/10.1111/jfpp.16237
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work received support from TÜBİTAK—ULAKBİM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bayram, Y. Optimizing the extraction of polyphenols from Prunus spinosa L. fruit using response surface methodology and production of powders from optimized extracts by foam mat drying. Food Measure (2024). https://doi.org/10.1007/s11694-024-02681-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02681-w