Abstract
Pomegranate peel is one of the wastes generated in large quantities in the food industry and ellagic acid is a natural bioactive phenolic compound in pomegranate peel. In this study, high-purity and low-cost ellagic acid was produced by acid hydrolyze process from pomegranate peel and characterization, in vitro bioaccessibility, antioxidant, and antidiabetic activity of the ellagic acid nanoencapsulated with maltodextrin and soy lecithin were studied. The extract yield and purity of ellagic acid was 6.47 and 88.74%, respectively. Results showed that lecithin coated samples were more stable with − 43.16 mV zeta potential and had higher encapsulation efficiency (99.29%) compared to maltodextrin. However, coated samples with maltodextrin had higher production efficiency (91.40%) and loading capacity (54.70%). The thermal resistance of ellagic acid after the nanoencapsulation process was significantly raised. The ellagic acid nanoencapsulated with maltodextrin had higher bioaccessibility (80.22%) and solubility in biological buffers (64.00-98.20%). The α-amylase and α-glucosidase inhibitiory activities of the ellagic acid decreased after the encapsulation process. However, the α-amylase inhibitory activities of the nanoencapsulated materials were higher than acarbose as a positive control. As a result, thermal resistance, solubility in a biological medium, and bioaccessibility of ellagic acid after nanoencapsulation with maltodextrin can be increased. Thus, the use of ellagic acid nanoencapsulated with maltodextrin in the food, cosmetic, and pharmacological industries can be diversified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pomegranate (Punica granatum L.) which grows in temperate climates is an economically important fruit. Nowadays, the production and consumption of pomegranate products, especially fruit juice, has substantially increased worldwide due to the health-promoting effect of different components of pomegranate [1]. As a result of the increase in consumption of pomegranate products, the amount of pomegranate peel as a waste has also risen. Peel is 78% of the pomegranate processing waste and is the most valuable by-product in terms of chemical composition. However, it is commonly used for the production of low value products such as animal feed and fertilizers [2].
The pomegranate peel is a rich source of antioxidant polyphenols, especially ellagic acid (EA). Ellagic acid, a hydrolytic product of ellagitannins, is present in pomegranate peel in free form and bound as ellagitannins [3]. Ellagic acid is mainly produced from wood by acid or alkaline hydrolysis. However, the high purity EA with high commercial value can be produced from especially pomegranate peel. Lu and Yuan [4] reported that the EA was produced by hydrolysis after ellagitannins (ETs) extraction from pomegranate peels and the purity value of the EA was 90%. In addition, the pomegranate peel is less toxic than the gallnut, which in the past was the main material of EA production [4].
Ellagic acid has a wide spectrum of benefits for human health such as antiviral, antibacterial, anti-mutagenic, anticancer, anti-inflammatory, anti-diabetic, cardio-protective, and gastro-protective properties [5]. Thanks to the antioxidant activity and whitening function of EA, it is used as a food additive and cosmetic material. However, the low solubility of EA limits its use in the food, cosmetic, and pharmaceutical industries. When EA is taken orally it is rapidly excreted from the body due to its low solubility and it cannot reach sufficient concentration in the plasma and tissue [6]. This unsatisfactory property leads to poor absorption and poor bioavailability and the use of high doses of EA. High dosage also leads to increased side effects [7]. Therefore, its potential as a systemic antioxidant is limited [6, 7].
The encapsulation technique involves the incorporation of active agents within a carrier capsule and it is a useful technique to improve the protection and delivery of bioactive molecules in foods and the human body. Natural polyphenols require safeguarding against physical and chemical stress factors due to their sensitivity to heat, oxygen, light, acidic, and alkaline conditions. Nanoncapsulation prevents breakdown of polyphenols until consumption. It masks the taste of polyphenols and increases their water solubility and bioavailability [8]. In addition, nanoencapsulation could reduce the total volume of transported material and significantly improve the mass transport of essential nutrients to tissue. Nanoencapsulation of EA in chitosan was studied for the efficient use of EA [9]. It was noted that the anticancer property of the ellagic acid was increased after nanoencapsulation with chitosan. In this study, the EA produced by acid hydrolysis from pomegranate peels was nanoencapsulated using maltodextrin and soy lecithin as the coating material, and characterizations, in vitro bioaccessibilities, antioxidant and antidiabetic activities of nanoencapsulated EAs were investigated.
Materials and methods
Samples
The pomegranate sample belonging to the Hicaz variety was purchased from a local market in Van. The peels were separated from the arils and lyophilized at 0.045 mbar and − 54 °C for 72 h by freez-drier (Free Zone 2.5, Labconco, USA). The lyophilized peel was ground using a mill. The powders (60–80 mesh) were stored at − 24 °C for further processing.
Characterization, purification and extraction of EA
The extraction procedure described by Lu and Yuan [4] was used with slight modifications. The pomegranate peel powder (40 g) was mixed in 200 mL ethanol (60%). The mixture was stirred at 80 °C and 250 rpm for 2 h in an agitated water bath (WSB-30, Daihan, Korea). Then it was centrifuged (NF 1200R, Nüve, Turkey) at 8000 rpm for 4 min. This procedure was repeated using the residue. The supernatants were combined and evaporated with a rotary vacuum evaporator (R100, Buchi, Swiss) until a quarter volume remains.
For purification, the concentrated extract was completed with deionized water to a volume of 150 mL. It was then hydrolyzed at 105 °C for 5 h in the presence of 5% H2SO4 in total volume in an oil bath. The whole reactant was immediately filtered through a Busher funnel after the hydrolyzation. Filter cake to remove impurities was washed with 125 mL of water adjusted to pH 12. For washing, the water (pH 12) was transferred slowly on the filter cake. The washed cake was dried in a lyophilizer at a pressure of 0.045 mbar and at -54 °C for 48 h. Methanol (30 mL per gram) was added to the powder cake and mixed in a glass bottle. The mixture was stirred in a water bath at 45 °C for 2 h and then filtered using filter paper (Macherey & Nagel, No. 1, 106.02.110, Germany). Subsequently, the filter cake was extracted a second time under the same conditions. The supernatants were combined and concentrated with a rotary vacuum evaporator until about 10 mL. The supersaturated sample, which was allowed to cool to room temperature, was gradually crystallized in a flask. Finally, the crystallized EA was filtered and dried in the lyophilizer under the same conditions. The extract yield was calculated as the ratio between the weight of the extract and the weight of the sample on dry basis.
For the characterization of purified EA, 1HNMR and 13CNMR spectra were measured in DMSO-d6 on an Agilent NMR spectrometer (400 MHz). 1H (400 MHz) and 13CNMR (100 MHz). Chemical shifts are expressed in ppm. LC/MS-MS analyses were carried out by a Thermo Scientific Q Exactive instrument. Results were given in supplementary material (Supp 1).
Preparation of EA nanoencapsulated with maltodextrin and lecithin
The EA: liquid ratio (w/v) and the coating material concentrations in water were determined through preliminary experiments. A %1 maltodextrin (dextrose equivalent 4.0–7.0) (419,672, Sigma-Aldrich Co, USA) in distilled water was solved by stirring with a magnetic stirrer at 40–50 °C for 1 h, and then was cooled to room temperature before use. The preparation of a %2 soy lecithin (AE81.1, Carl Roth, Germany) solution as coating material was similar to maltodextrin but the temperature during stirring did not exceed 40 °C. Ellagic acid (0.1 g) and 10 mL of maltodextrin or soy lecithin solution as a coating material were mixed with a magnetic stirrer. The mixture was homogenized with Ultraturrax at 12,000 rpm for 3 min and then sonicated for 20 min at 60% amplitude using an ultrasonic homogenizer (Bandelein, Sonoplus HD 3200, Germany) fitted with a Bandelin UW 3200 ultrasonic probe (maximum 200 W power, 20 kHz, Germany). The nanoencapsulated samples were freeze-dried at 0.045 mbar and − 54 °C for 72 h. The samples coated with maltodextrin and lecithin were mentioned as EAM and EAL, respectively.
Scanning electron microscopy (SEM)
The images of the EA, EAM, and EAL were taken using the Field Emission Scanning Electron Microscope (Zeiss Sigma 300 FESEM Oberkochen, Germany). The 400–500 K times magnification images of the samples coated with gold-palladium were taken at 10 kV accelerating voltage under ultra-high vacuum.
Characterization of nanoparticles
Dynamic light scattering technique was used to detect the zeta size, zeta potential, and polydispersity index (PDI) of the samples. The measurements were performed at 25 °C with a Zetasizer device (Nano-ZS90, Malvern, UK).
Fourier Transform Infrared (FT-IR) analysis
The functional groups and chemical structures of the samples were determined by FT-IR spectroscopy (IRAffinity-1 S, Shimadzu, Japan). The FT-IR spectras were recorded at the wavelength range of 4000 –500 cm− 1, with a resolution of 2 cm− 1 and accumulation of 16 scans.
Thermogravimetric analysis (TGA)
Thermogravimetric analysis of the sample was performed in a Setaram Labsys Evo Thermal Analyzer. The samples (1 g) were hermetically closed in an aluminum pan. The temperature of the pan was increased from 30 °C to 300 °C at a rate of 10 °C / min and the argon flow rate was maintained at 10 mL/min.
Production efficiency
Production efficiency was defined according to Eq. (1)
Wp is the weight of dry particles, We is weight of EA used for production and Wm is the weight of the coating material used for production [10].
Ellagic acid extraction
Ellagic acid extraction from the sample was performed according to Robert et al. [11] with some modifications. Nanoparticle sample (0.2 g) was added into the 25 mL of methanol: acetic acid: water (50/8/42 v / v / v). The mixture was mixed by a vortex for 1 min, sonicated for 3 min, and then centrifuged for 5 min at 8000xg. The supernatant was used to determine of nanoencapsulated EA by a HPLC.
Encapsulation efficiency
The amount of EA extracted from the nanoparticle was assumed to be the EA loaded into nanoparticles and encapsulation efficiency was calculated using the following Eq. (2):
Where Ai is the amount of EA used for nanoencapsulation and AA is the amount of EA extracted from nanoparticles after the nanoencapsulation process [12].
Loading capacity
The loading capacity of EA into nanoparticle was calculated by the following Eq. (3) [13].
Solubility of nanoparticle
The method developed by Kuck and Norena [14] was modified and used to determine the solubility of the samples in different solvents. Sample (1 g) was added to methanol, water, 0.1 N HCl, acetate buffer (pH 4.5), and phosphate buffer (pH 6.8) and mixed for 5 min in a magnetic stirrer. The mixture was centrifuged (Nüve NF 1200R, Turkey) at 3000xg for 15 min. The supernatant (25 mL) was transferred to a container with a constant weight, kept in an oven at 105 °C for one day, cooled in a desiccator, and then the solubility percentage was determined.
Determination of EA by HPLC
The EA contents of the samples were determined using a HPLC system (LC-20 AD, Shimadzu, Japan). The extracts were filtered through a 0.45 μm PVDF syringe-driven filter. Filtrate was injected into a HPLC system. The EA separation was performed using a Symmetry C18 (250 × 4.6 mm id, particle size 5 μm) column (Waters, USA) at 25 °C. A binary mobile phase (A- 2% acetic acid in water, B- 0.5% acetic acid in water: acetonitrile (1:1, v/v)) was also used at 1.0 mL/min flow rate. The gradient program was as follows: 0 min 75% A; 25 min 63% A; 28 min 0% A. Detection was made at 360 nm. The EA was identified based on its retention times and spectral data by comparison with the EA standard (E2250, Sigma-Aldrich Co, USA) [15].
DPPH and ABTS assays
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was carried out according to the spectrophotometric method of Pyo et al. [16]. The ABTS assay was performed using the method developed by Re et al. [17]. The results of both analyses were expressed as Trolox equivalent antioxidant capacity (mmol Trol. eq./g dw).
Bioaccessibility of nanoparticles
Distilled water (30 mL), 1.5 mL of pepsin (20 g/L in 0.1 mol/L HCl), and 0.5 g of sample were mixed and the mixture was adjusted to pH 2 by adding HCl (6 mol/L). For gastric digestion, the mixture was kept in a water bath at 37 °C for 1 h. Then, the pH of the mixture to stop the digestion was adjusted to 7.2 with 1 mol/L NaHCO3. Bile/pancreatin solution (7.5 mL) (2 g/L of pancreatin and 12 g/L of bile salt in 0.1 mol/L NaHCO3) and 7.5 mL of NaCl/KCl (120 mmol/L NaCl and 5 mmol/L KCl) was added into the mixture and the mixture was kept at 37 °C for 2.5 h. The mixture was centrifuged at 8000xg for 10 min and the supernatant was used for EA analysis [18].
α-amylase and α-glucosidase inhibitory activities of nanoparticles
α-Amylase (250 µL, 0.05 U/mL) was solved in 0.02 M phosphate buffer (pH 6.9). Extract (200 µL) at various concentrations (1–10 mg/mL) was added into the solution. The mixture was pre-incubated at 37 °C for 10 min. Then, 250 µL starch solution (1%) (0.02 M phosphate buffer, pH 6.9) as the substrate was added and kept at 37 °C for 15 min. To stop the reaction, 1% dinitrosalicylic acid (500 µL) was added and kept in boiling water for 10 min. Then, the mixture was cooled at room temperature and diluted with 5 mL of distilled water. Its absorbance was recorded at 540 nm. Acarbose as a commercial standard was used. The α-amylase inhibition activity of the samples was expressed as IC50 [19].
α-Glucosidase (60 µL, 1 U/mL) was solved in phosphate buffer (0.1 M, pH 6.8). and 120 µL extract of various concentrations (1–10 mg/mL) was added into the solution. The mixture was pre-incubated at 37 °C for 10 min. 5 mM 4-nitrophenyl α-D-glucopyranoside (120 µL) as a substrate was added and kept at 37 °C for 15 min. 0.1 M Na2CO3 (300 µL) was added into the mixture to stop the reaction. The absorbance at 405 nm was measured. α-glucosidase inhibition activity was expressed as IC50 [20].
Statistical analysis
Ellagic acid production trials were performed in triplicates. Statistical Package for the Social Sciences (SPSS) statistics program (version 18.0) was used to perform the one-way ANOVA tests. Duncan’s multiple range test to identify the difference among the means was applied at 5% significant level.
Results and discussion
Purification of ellagic acid
Acid hydrolyze is a mainly used process for ellagic acid production from natural sources. In this study, the extract yield and purity of EA produced by the acid hydrolyze process was 6.47% on dry matter and 88.74%. Lu and Yuan [4] reported that EA obtained by acid hydrolysis from pomegranate peels had % 90 purity and 3.5% extraction yield. While the purity value in this study was very close to the result reported by Lu and Yuan [4], the extract yield was approximately 1.8 times higher than the reported result. It may be due to differences in hydrolysis parameters and washing steps. The process steps and parameters applied in the acid hydrolysis can reduce the yield of the extract while ensuring high purity. Goriparti et al. [21] applied a recrystallization process using pyridine to EA obtained by acid hydrolysis from pomegranate peels and EA with > 99% purity was achieved. The high purity may be due to the crystallization process with pyridine which has a few instances of its toxic effect on humans [22].
Scanning electron microscope
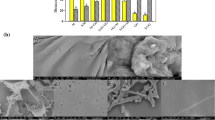
The SEM images obtained from the EA, EAM, and EAL are given in Fig. 1. The average molecular diameter of EA, EAM, and EAL was 203.75, 263.52, and 376.60 nm, respectively. It was detected that EA was already nano size. Thanks to the proportions used with both the coating materials and the applied process parameters, it resulted in obtaining nano scale structures from EA. The particle diameters of nanoparticle coated with lecithin were larger compared to nanoparticle coated with maltodextrin. It may be due to the higher concentration of soy lecithin solution compared to maltodextrin solution. While EA and the nanoparticles coated with maltodextrin were spherical, the samples coated using lecithin were amorphous. The differences in the morphological structure of the encapsulated particles are due to the coating material used, the rate of use, the applied process parameters, and the drying method. Pomegranate seed oil was microencapsulated in a spray dryer using a mixture of maltodextrin, N-LOK, and whey protein concentrate. It was observed that the microcapsules were generally spherical [23].
Zeta potential, zeta size, and PDI
The zeta potential results showed that the use of lecithin as a coating material gave more positive results on the stability compared to maltodextrin (Table 1). The zeta potential value of EA was − 32.71 mV. The zeta potential value (-43.16 mV) of EAL was higher than that of EA and EAM (-28.20 mV). The difference detected between the samples was statistically significant (p < 0.05). The high zeta potential, which is an indicator of electrostatic repulsion, reduces the Vander Waals force between particles, ensuring the stability of nanoparticles [24]. Therefore, EAM was prone to coagulation, whereas EAL was more stable structures. As a result, to avoid coagulation, it is more convenient to keep the samples coated with maltodextrin in solid-powder form and dissolve in a suitable solvent before use. The initial zeta size value of EA is 249.03 nm and there was a significant increase in the size values of the samples after the nanoencapsulation process. However, both the EAM and the EAL were nanosized. The particle size (321.90 nm) of the EAM was lower than the particle size (402.20 nm) of the EAL (p < 0.05). The zeta size values of samples were also higher than the results obtained by SEM. Scanning electron microscope analyzes the morphological size of the particle in the solid state, but the zeta sizer measures the hydrodynamic diameter of the particles in aqueous solutions. Bryła et al. [25] reported that the zeta potential value and average dimension of elderberry extracts coated with soybean lecithin were 36.4 mV and 205 nm, respectively. The nanoparticle coated with soybean lecithin was the most uniform in terms of both size and structure [25]. The PDI values of the EA, EAM, and EAL were determined as 0.29, 0.20, and 0.25, respectively (Table 1). A significant decrease in PDI values of EAL and EAM samples was detected when compared with the PDI value of EA (p < 0.05). The EAM samples had also lower PDI values than the EAL samples (p < 0.05). The low PDI value is interpreted as the positive effect of the applied nanoencapsulation process on the stability of samples in bulk [26].
Molecular characterization by FT-IR
Fouirer Transform Infrared Spektrofotometre (FT-IR) spectrums of the samples were given in Fig. 2. In the EA sample, the signals at 1724, 3309, 2922, and 1193 cm− 1 indicate the carbonyl, the phenolic -OH, the aromatic C-H, and the –O– (etheric oxygen) groups, respectively. The carbonyl group is due to the ketone-type carbonyl groups in the structure of the ellagic acid, while the phenolic -OH groups arise from the phenolic hydroxy groups in the structure of the ellagic acid. In the EAM, the signals at 3412, 2924, and 1697 cm− 1 represent the phenolic -OH or hydroxy, the aromatic C-H, and the carbonyl groups from the EA, respectively. The signal at 2888 cm− 1 represents the aliphatic C-H groups coming from the -CH2 group of maltodextrins. The 1195 cm− 1 and 1111 cm− 1 signals indicate the presence of etheric oxygen groups. In the EAL, while the 3554 and 3273 cm− 1 signals represent the phenolic -OH groups, the 2922 cm− 1 represents the aromatic C-H groups. The aliphatic C–H group at 2852 cm− 1 originated from the straight aliphatic chains of lecithin in EAL samples. The 1697 cm− 1 signal belongs to the carbonyl group and originates from both the EA and lecithin. The 1224 cm− 1 and 1195 cm− 1 signals indicate the presence of etheric oxygen. Erdik [27] reported that carbonyl (-C = O) functional group signals occur in the band range of 1650–1750 cm− 1. While sharp or broad signals of –OH phenolic groups come up in a band range of 3200–3500 cm− 1, –O– (etheric oxygen) group signals appear in the range of 1300 –1000 cm− 1. The signals of the aromatic C – H groups appear in the 2900–3050 cm− 1 [27]. Goriparti et al. [21] reported that in the IR spectrum of ellagic acid, the bands in the range of 2800–3700 cm− 1 were caused by -OH stretching, the signal at 1725 cm− 1 is based on C = O stretching. The bands observed in the range of 1669 –1500 cm− 1 have been attributed to aromatic ring vibrations. The bands at 1190 and 1052 cm− 1 are from ester links and the signal at 751 cm− 1 belongs to the aromatic C-H vibration [24]. The results were similar to the results reported by Goriparti et al. [21] and Erdik [27].
Production efficiency, encapsulation efficiency, and loading capacity
The production efficiencies of EAM and EAL samples were 91.40% and 82.20%, respectively (Table 1). The tested coating materials had a significant effect on the production efficiency (p < 0.05). It was determined that samples coated with soy lecithin tended to stick to the drying container walls during the freeze-drying process. The lower yield in EAL samples may be associated with the use of higher lecithin concentration and their tendency to stick. Tolun et al. [28] reported that the yield obtained from microencapsulation of grape polyphenols using maltodextrin and spray dryer was 59.52%. This difference may be due to differences in the core or coating material ratio, the drying method and drying temperature. Encapsulation efficiency is the percentage of material successfully entrapped into a micelle or nanoparticle. In this study, it was determined that the samples had high encapsulation efficiency in both coating materials. The encapsulation efficiency of the EAM (99.13%) and EAL (99.29%) samples were found to be close to each other (Table 1). However, the difference between the samples was found to be statistically significant (p < 0.05). Kaderides et al. [29] reported that the microencapsulation efficiency for pomegranate peel extract microencapsulated with maltodextrin (12DE), skimmed milk powder, whey protein isolate, and gum arabic ranged from 69.80 to 99.80%. Loading capacity is the amount of material loaded per unit weight of nanoparticle. The loading capacity of EAM and EAL samples was measured as 54.70% and 40.55%, respectively (Table 1). The EAM had higher loading capacity values than the EAL (p < 0.05). The EAM samples with lower PDI values showed more homogeneous distribution compared to lecithin, and this may increase the unit weight of the EA molecules loaded into the nanoparticle.
Thermogravimetric analysis
Thermogravimetric analysis thermogram of EA, EAM, and EAL samples is given in Fig. 3. The loss of weight in the range of 100–200 °C is caused by water molecules moving away from the structure. The loss of weight above 200 °C is due to the cleavage of phenolic or biphenyl groups. After nanoencapsulation process, the breaking points in the weight loss of samples occurred at higher temperatures. Therefore, it can be said that the coating materials increased the thermal stability of EA. The EAM and EAL samples are more stable at high temperatures when compared to the EA. The decrease in weight losses of the EAM and EAL samples were found to be close to each other. As a thermal food process, pasteurization is applied at temperatures below 100 °C, and sterilization is applied at 121 °C under 1 atm Hg pressure. Deep frying is also carried out in the range of 175–195 °C. The results showed that the nanoencapsulation process provides an important advantage in terms of its use in especially thermal food processes. Tavakolipour et al. [30] also reported that the thermal stability of pomegranate seed oil increased after coating with zein.
In vitro solubility
In in vitro solubility tests, the solubility of compounds in an environment such as an artificial stomach or intestine is determined under certain experimental conditions. 1.2, 4.6, and 6.8 pH values were generally used for representing the stomach, small intestine, and large intestine mediums, respectively [31]. In this study, the solubility of samples was determined in methanol, water, and biological buffers (0.1 N HCl (pH 1), pH 4.5 acetate buffer, and pH 6.8 phosphate buffer) (Table 2). The solubility of EA in water, pH 4.5 acetate and pH 6.8 phosphate buffers were increased by nanoencapsulation processes (p < 0.05). Especially EAL has the highest solubility in water. In pH 4.5 acetate and pH 6.8 phosphate buffer, solubility values of EAL and EAM samples were quite close to each other(p > 0.05). The highest solubility in 0.1 N HCl (pH 1) was found in the EAM sample. Lei et al. [32] reported that after oral administration of pomegranate leaf extract, most of the EA was absorbed from the stomach. It may be said that the absorption and solubility of nanoparticles in the stomach (pH 1) increases by coating with maltodextrin. The encapsulation of ellagic acid with maltodextrin was a more successful application and had high solubility in all biological buffers compared to EA and EAL. However, EAL had the lowest solubility of nanoparticles in the stomach (pH 1) and lecithin as a coating material can be used to dissolve the nanoparticle in the intestine. Avachat and Patel [33] reported that the solubility of EA - phospholipid complex in n-octanol, water, 0.1 N HCl, pH 4.5 acetate buffer, and pH 6.8 phosphate buffer increased compared to EA.
Bioaccessibility of ellagic acid and antioxidant activities
The EA contents and antioxidant activities of the samples at the initial and after digestion were given in Table 3. The EA and both antioxidant activity results at the initial were determined in the following order: EA > EAM > EAL. For EA acid content and antioxidant activities, the differences among EA, EAM, and EAL were statistically significant (p < 0.05). The EA content and antioxidant activity values of all samples decreased after in vitro digestion. The decrease in the EA is due to the degradation of this compound during digestion and leads the decreases in the antioxidant activities. Kamiloglu et al. [34] stated that after in vitro digestion, there is a general loss of phenolic compounds, which are antioxidant sources. The bioaccessibility values for EA and antioxidant activities were ranked in the following order: EAM > EAL > EA. Both nanoencapsulation processes increased bioaccessibilty values of EA and antioxidant activities (p < 0.05). Especially, nanoencapsulation with maltodextrin provided better protection for EA during digestion compared to lecithin. While the EA sample had the lowest bioaccessibility values (4.74%) in terms of the EA content of the sample, EAM had the highest bioaccessibility values (80.22%). There were some studies indicating that nanocarriers are a good method of improving the bioavailability of phenolics and other bioactive compounds [35].
Antidiabetic activity of nanoparticles
Type 2 diabetes mellitus is an increasing health problem worldwide and characterized by dysregulation of carbohydrate, lipid, and protein metabolism [36]. In the digestive system, dietary carbohydrates are disintegrated to monosaccharides by α-amylase activity. α-Glucosidase also converts these monosaccharides into glucose and then glucose molecules are directed into the bloodstream by absorption [37]. α-Glucosidase and α-amylase inhibitors restrict the carbohydrate metabolism in the intestines and decrease glucose levels in the blood. Therefore, these inhibitors can be used to treat type 2 diabetes mellitus and plant phenolics are important α-amylase and α‐glucosidase enzyme inhibitors [19]. In this study, α-amylase and α-glucosidase enzyme inhibitions of the samples were given in Table 4. The α-amylase and α-glucosidase enzyme inhibition activities of the samples decreased after the encapsulation process. The presence of the coating material along with the core in the coated nanoparticle may cause the reduced antidiabetic activity. For α-amylase and α-glucosidase enzymes, enzyme inhibition activities of EAM and EAL samples were quite close to each other (p > 0.05). In addition, all samples had higher α-amylase inhibition activities than the positive control acarbose (p < 0.05). For α-glucosidase, IC50 values of EA and acarbose were similar. While EAM and EAL had high α-glucosidase inhibitory activity, their inhibitory activities were lower than acarbose. Barathikannan et al. [38] reported that pomegranate peel extract has a significant effect on reducing the long-term complications of diabetes patients due to their α-glucosidase inhibition activity.
Conclusion
Pomegranate peel is generated as waste in especially fruit juice production and a rich source of EA that has many beneficial effects on health. In this study, high purity EA was produced by simple processes applied to pomegranate peels and was successfully nanoencapsulated using maltodextrin and lecithin. It was determined that both nanoencapsulation processes significantly increased the thermal resistance of EA. The nanoparticles coated with lecithin were more stable in a liquid medium than maltodextrin. However, the samples coated with maltodextrin have higher efficiency and loading capacity values. The EA nanoencapsulated with maltodextrin had high bioaccessibility as it was better preserved during the digestion process. The EAM sample was also better dissolved in biological buffers, especially in pH 1 which represented the stomach medium. Therefore, the absorption of EA in the human body, especially in the stomach may be increased by nanoencapsulation of EA with maltodextrin. As a result, pomegranate peels with acid hydrolysis and nanoencapsulation techniques can be easily processed into high value-added products. Future studies should focus on developing the possibilities of using EA nanoencapsulated with maltodextrin as a systemic antioxidant in the food, cosmetic, and pharmaceutical industries.
References
R.R. Mphahlele, O.A. Fawolea, M.A. Stander, U.L. Opara, Sci. Hort. 178, 114–123 (2014). https://doi.org/10.1016/j.scienta.2014.08.010
N. Hasnaoui, B. Wathelet, A. Jimenez-Araujo, Food Chem. 160, 196–203 (2014). https://doi.org/10.1016/j.foodchem.2014.03.089
M.I. Gil, F.A. Tomas-Barberan, B. Hess-Pierce, D.M. Holcroft, A.A. Kader, J. Agric. Food Chem. 48(10), 4581–4589 (2000). https://doi.org/10.1021/jf000404a
J. Lu, Q. Yuan, J. Food Process Eng. 31(4), 443–454 (2008). https://doi.org/10.1111/j.1745-4530.2007.00169.x
A. Galano, M. Francisco-Márquez, A. Pérez-González, Chem. Res. Toxicol. 27(5), 904–918 (2014). https://doi.org/10.1021/tx500065y
V. Murugan, K. Mukherjee, K. Maiti, P.K. Mukherje, J. Agric. Food Chem. 57, 4559–4565 (2009). https://doi.org/10.1021/jf8037105
I. Nyamba, A. Lechanteur, R. Semdé, B. Evrard, Eur. J. Pharm. Biopharm. 159, 198–210 (2021). https://doi.org/10.1016/j.ejpb.2020.11.004
W. Zam, G. Bashour, W. Abdelwahed, W. Khayata, Brazilian J. Pharm. Sci. 50(4), 741–748 (2014). https://doi.org/10.1590/S1984-82502014000400009
V. Arulmozhi, K. Pandian, S. Mirunalini, Colloids Surf., B 110, 313–320 (2013). https://doi.org/10.1016/j.colsurfb.2013.03.039
Z. Li, S.S. Percival, S. Bonard, L. Gu, Mol. Nutr. Food Res. 55, 1096–1103 (2011). https://doi.org/10.1002/mnfr.201000528
P. Robert, T. Gorena, N. Romero, E. Sepulveda, J. Chavez, C. Saenz, Int. J. Food Sci. Technol. 45, 1386–1394 (2010). https://doi.org/10.1111/j.1365-2621.2010.02270.x
D.M. Casa, T.C.M.M. Carraro, L.E.A. Camargo, N.M. Khalil, R.M. Mainardes, J. Nanosci. Nanotechnol. 14, 1–7 (2014). https://doi.org/10.1166/jnn.2015.9177
Y.B. Kılınç, Türk Mikrobiyoloji Cemiyeti Dergisi. 49(1), 47–54 (2019). https://doi.org/10.5222/TMCD.2019.047
L.S. Kuck, C.P.Z. Norena, Food Chem. 194, 569–576 (2016). https://doi.org/10.1016/j.foodchem.2015.08.066
S. Uğurlu, E. Okumuş, E. Bakkalbaşı, Ultrason. – Sonochemistry. 66, 1050942 (2020). https://doi.org/10.1016/j.ultsonch.2020.105094
Y.H. Pyo, T.C. Lee, L. Logendra, R.T. Rosen, Food Chem. 85, 19–26 (2004). https://doi.org/10.1016/S0308-8146(03)00294-2
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. 26, 1231–1237 (1999). https://doi.org/10.1016/s0891-5849(98)00315-3
D. Vitali Čepo, I. Vedrina Dragojević, B. Šebečić, Food Chem. 114, 1462–1469 (2009). https://doi.org/10.1016/J.FOODCHEM.2008.11.032
M.I. Kazeem, J.O. Adamson, I.A. Ogunwande, Biomed. Res. Int. 527–570 (2013). https://doi.org/10.1155/2013/527570
N. Zahratunnisa, B. Elya, A. Noviani, Pharmacognosy J. 9(2), 273–275 (2017). https://doi.org/10.5530/pj.2017.2.46
S. Goriparti, M.N.K. Harish, S. Sampath, Chem. Commun. 49, 7234–7236 (2013). https://doi.org/10.1039/C3CC43194K
L.J. Pollock, I. Finkelman, A.J. Arieff, Arch. Intern. Med. (Chic). 71(1), 95–106 (1943). https://doi.org/10.1001/archinte.1943.00210010101008
N.H. Şahin, M.A. Ozen, Eur. J. Lipid Sci. Technol. 116, 847–856 (2014). https://doi.org/10.1002/ejlt.201300355
B. Schäfer, M. Hecht, J. Harting, H. Nirschl, J. Colloid Interface Sci. 349, 186–195 (2010). https://doi.org/10.1016/j.jcis.2010.05.025
A. Bryła, G. Lewandowicz, W. Juzwa, J. Food Eng. 167, 189–195 (2015). https://doi.org/10.1016/j.jfoodeng.2015.07.025
S. Kalimouttou, M. Lahiani-Skiba, N. Naouli, V.S.Z. Lin, M. Skiba, N.S.T.I. Nanotech, Technical Proceedings 2, 407–410 (2008)
E. Erdik, Organik Kimyada Spektroskopik yöntemler, 5th edn. (Gazi Kitapevi, Turkey, 2007)
A. Tolun, Z. Altintas, N. Artik, J. Biotechnol. 239, 23–33 (2016). https://doi.org/10.1016/j.jbiotec.2016.10.001
K. Kaderides, A.M. Goula, K.G. Adamopoulos Innovative Food Science & Emerging Technologies 31, 204–215 (2015). https://doi.org/10.1016/j.ifset.2015.08.006
H. Tavakolipour, L. Bagheri, A. Madadlou, J. Food Process Eng. 38(1), 49–56 (2015). https://doi.org/10.1111/jfpe.12125
A.B. Iskit, 8. Ulusal Kemik İliği Transplantasyonu ve Kök Hücre Tedavileri Kongresi, Antalya, Turkey, 51–52 (2014)
F. Lei, D. Xing, L. Xiang, Y. Zhao, W. Wang, L. Zhang, L.J. Du, Chromatogr. B 796, 189–194 (2003). https://doi.org/10.1016/s1570-0232(03)00610-x
A.M. Avachat, V.G. Patel, Saudi Pharm. J. 23(3), 276–289 (2015). https://doi.org/10.1016/j.jsps.2014.11.001
S. Kamiloglu, A.A. Pasli, B. Ozcelik, E. Capanoglu, LWT - Food Sci. Technol. 56, 284–289 (2014). https://doi.org/10.1016/j.lwt.2013.11.040
J. Liang, H. Yan, P. Puligundla, X. Gao, Y. Zhou, X. Wan, Food Hydrocoll. 69, 286–292 (2017). https://doi.org/10.1016/J.FOODHYD.2017.01.041
R.A. DeFronzo, E. Ferrannini, L. Groop, R.R. Henry, W.H. Herman, J.J. Holst, F.B. Hu, C.R. Kahn, I. Raz, G.I. Shulman, D.C. Simonson, M.A. Testa, R. Weiss, Nat. Reviews Disease Primers. 1, 15019 (2015). https://doi.org/10.1038/nrdp.2015.19
C. Peyrot des Gachons, P.A.S. Breslin, Curr. Diab. Rep. 16, 102 (2016). https://doi.org/10.1007/s11892-016-0794-7
K. Barathikannan, B. Venkatadri, A. Khusro, N.A. Al-Dhabi, P. Agastian, M.V. Arasu, Y.O. Kim, BMC Complement. Altern. Med. 16(1), 264 (2016). https://doi.org/10.1186/s12906-016-1237-3
Acknowledgements
Financial support provided by Van Yüzüncü Yıl University Research Fund (Project No: FDK-2019-8121) is gratefully acknowledged and appreciated.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okumuş, E., Bakkalbaşı, E. & Gümüş, A. Characterization, in vitro bioaccessibility and antidiabetic activities of maltodextrin and lecithin nanomaterials loaded with ellagic acid. Food Measure (2024). https://doi.org/10.1007/s11694-024-02673-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02673-w