Abstract
Recently, the importance of medicinal plants such as Salvia species has been increasing both in medicine and in industrial branches, which includes food, feed, and cosmetic raw materials. On the other hand, chia seed is a functional food that has recently increased industrial importance due to its superior nutritional value, phytochemical components, and therapeutic effects. In our study, the antioxidant activity of methanol extracts from the seeds of endemic Salvia cadmica Boiss var. cadmica, and Salvia caespitosa Montbret & Aucher ex Benth., Salvia pisidica Boiss. & Heldr. ex Benth., and Salvia potentillifolia Boiss. & Heldr. ex Benth. collected from Burdur-Antalya/Turkey were determined via 1,1-diphenyl,2-picryl hydrazyl radical scavenging activity, cupric (II) reducing antioxidant capacity, b-carotene/linoleic acid bleaching and total phenolic, and total flavonoid content tests and compared with that of chia seed. Antimicrobial activity was determined according to minimum inhibitory concentration values, on S. aureus, E. coli, S. enterica, L. monocytogenes, C. albicans strains, but it was found negligible. Phenolic and fatty acid contents of the seed extracts were also determined by HPLC and GC–MS, respectively. S. pisidica and S. potentillifolia were found to be highly active. The major fatty acid composition of the chia seed was linolenic acid, linoleic acid, oleic acid, palmitic acid, and stearic acid while the others were linoleic, oleic, palmitic, and stearic acids. Despite fatty acid ratios of chia seed being more favorable; 1,1-diphenyl,2-picryl hydrazyl radical scavenging activity, cupric (II) reducing antioxidant capacity, total phenolic content, and antimicrobial activity of S. pisidica seed extracts and total flavonoid content and inhibition of β-carotene bleaching of S. potentillifolia seed extracts was higher than that of chia seed. These findings suggest seed extracts of these Salvia species are richer in phytochemicals and they are more active as antioxidants when compared to chia seed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salvia genus, which is in the Lamiaceae family, has approximately 1000 taxa widely distributed in the world, 114 of them in Flora of Turkey and 58 of them are endemic to Turkey [1, 2]. Salvia species are used as folk medicine in colds, pains, wounds, and skin infections [3], also used as carminatives, digestive aids, antiseptics, sedatives, anxiolytics and hypnotics [4], because of their essential oils (EOs) and other active metabolites. Salvia species are remarkable in both medical sciences and food, agriculture, and perfumery researches. They have various bioactive properties like antioxidant, antimicrobial, antidiabetic, anticancer, and antiAlzheimer [5]. Many Salvia species are cultivated for their active substances. They are used for the production of EOs, biocides, pharmaceuticals, colorants, dyes, or cosmetics [6]. Therefore, chia seed, another Salvia species, is a functional food that has recently increased in industrial importance due to its superior nutritional value, phytochemical components, and therapeutic effects [7]. Some other Salvia seeds were also investigated in terms of their phytochemistry, bioactivity [8, 9] and nutritional value such as fatty acid content [10, 11] however, these studies are very limited.
Salvia cadmica, S. caespitosa, S. pisidica, and S. potentillifolia, collected from Burdur and Antalya, are perennials, endemic to Turkey and locally known as “kaya şalbası” “kırk şalba” “benli şalba” and “sarı porsuk”. Although the bioactivities and phytochemicals of both aerial parts and leaves of these species were partly investigated, there are limited studies on their seeds. Even though we found some data on the seed FA contents of these four Salvia species, there is no study on their antioxidant and antimicrobial properties or on the phenolic contents of their seeds. In this study, we aim to reveal the phytochemical content and bioactivity of S. cadmica, S. caespitosa, S. pisidica, and S. potentillifolia, and compared them with that of chia seed and hypothesized that seeds of these Salvia taxa are rich in chemical content and are bioactive as much as chia seeds. Additionally, we discussed similarities and differences between the phytochemical properties besides the nutritional values of chia seed and the other Salvia seeds, from the literature.
Materials and methods
Plant material
Mature seeds from at least 10 individuals were collected randomly, between July and September of 2022, from naturally distributed areas (S. cadmica; Burdur, between Halıcılar-Çatağıl villages, 1318 m, rocky places, S. caespitosa; Antalya, Konyaaltı, Feslikan Tableland, Karçukuru position, 1955 m, rocky cliffs, S. pisidica; Burdur, Yakaköy, 1180 m, steppe, S. potentillifolia; Burdur, Çavdır, Bölmepınarı village, 1283 m, Quercus coccifera maquis). Plants were authenticated and deposited in the Medicinal Plants Laboratory of Mehmet Akif Ersoy University as herbarium material. Chia seeds (of Peruvian origin) were supplied by an online market (Doğa Evi Chia Seed – Denfair Gıda Ürünleri San. Tic. Ltd. Şti., İstanbul).
Extraction
Powdered seeds were extracted with methanol at 35–40 °C by using an ultrasonic bath for 30 min. Extracts were filtered with Whatman no: 1 filter paper and the solvents were evaporated. Methanol extracts were used in the antioxidant and antimicrobial activity tests and HPLC analysis of phenolic compounds. Plant extracts and the solvents used in the assays were prepared daily.
Powdered seeds were extracted preliminary with both hexane and chloroform–methanol (2:1, v/v) at room temperature for 24 h. Chloroform–methanol mixture was preferred for the FA extraction due to higher fat yield compared to hexane (31% and 28%, respectively). FAs were analyzed in GC–MS after derivatization.
Antioxidant activity
Antioxidant activity of seed extracts were determined by DPPH (1,1-diphenyl,2-picryl hydrazyl) radical scavenging and cupric ion reducing activity (CUPRAC), inhibition power of β-carotene/linoleic acid bleaching, and total phenolic and total flavonoid content tests.
DPPH radical scavenging activity (RSA) potentials of the seed extracts were detected according to Blois’s [12] method. Each sample or control of 200 µL was added DPPH, a stable free radical, (50 µL, 1 mM) at certain concentrations (0.8–500 µg/mL) and then mixed well. DPPH RSA was expressed as percent inhibition and the butylated hydroxyanisole (BHA) was used as the positive control. The absorbance was measured at 517 nm. The calculations of DPPH RSA were determined by using the following formula:
An IC50 value is also expressed as the corresponding concentration value to the 50% inhibition on a concentration vs. percent inhibition plot.
CUPRAC assay was conducted by the method of Apak et al. [13], with some modifications. 0.01 M copper chloride, 0.75 M neocuproine, and 1 M ammonia acetate buffer (pH = 7.0), 73 μL each, were mixed. 50 μL antioxidant or standard solution and 30 μL water were added to the initial mixture. 1.5 h later the absorbance was measured at 450 nm. Trolox was the reference standard (0.8–500 μg/mL) (linear equation; y = 0.0075x + 0.1024, R2 = 0.9997) and the results were expressed as μg TE/mL.
To prepare β-carotene/linoleic acid stock solution, 0.5 mg β-carotene dissolved in 1 mL chloroform plus 25 µL linoleic acid and 200 mg Tween 40. After the chloroform was evaporated, 100 ml of oxygenated pure water was added to the mixture and then shaken vigorously. 350 µL extract was added to a 2.5 mL reaction mixture then absorbance was determined at 490 nm. The emulsion was incubated at 50 °C for 120 min. then absorbance was read again [3]. β-Carotene bleaching rate was calculated with the formula below:
where ln is the natural logarithm, a is the initial absorbance, b is the absorbance after 120 min, t: time.
Antioxidant capacity was determined with the following formula:
BHA and Trolox were used as the positive control.
Total phenolic content (TPC)
One hundred and fifty microliters of diluted Folin-Ciocalteu reagent (4:1 water/reagent) was added to 10 µL of sample or standard. After the addition of saturated sodium carbonate (7.5%) and incubation for 2 h at room temperature, the absorbance was measured at 725 nm [14]. TPC was expressed as gallic acid equivalent (0.8–500 µg GAE/mL) (linear equation; y = 0.0025x + 0.0167, R2 = 0.9954).
Total flavonoid content (TFC)
Ten microliters 5% sodium nitrite, 10 µL 10% aluminum chloride, 150 µL 1 M sodium hydroxide, and 50 µL water were added onto the 10 µL sample or standard in the 96-well-plate, respectively. The plate was stirred well and absorbance was read at 510 nm [15]. TFC was expressed as catechin equivalent (0.8–500 µg CE/mL) linear equation; y = 0.0008x + 0.0399, R2 = 0.9999).
Antimicrobial activity
Antimicrobial activity was determined based on Minimum Inhibition Concentration (MIC) values [16]. Using Mueller Hinton Broth II (MHBII) medium, methanol extracts dissolved in dimethyl sulfoxide (DMSO) were mixed into the medium in at least eight different concentrations (0.625–20 mg/mL). The media were transferred to a 96-well flat-bottomed microplate, on which fresh bacterial cultures were suspended in a physiological buffer at 0.5 McFarland value and inoculated at 10%. After the microorganisms were incubated at the optimum temperature and time suitable for the species, absorbance was measured at 600 nm wavelength. MIC values were determined according to absorbance values.
Phytochemical analyses
HPLC–DAD detection of phenolic compounds
HPLC–DAD detection of phenolic compounds (phenolic acids; 3,4-dihydroxy benzoic acid, caffeic acid, chlorogenic acid, cinnamic acid, ferulic acid, p-coumaric acid, rosmarinic acid, syringic acid, flavonoids; quercetin, rutin) were done at a system: Shimadzu Prominence, CBM: 20ACBM, Detector: DAD (SPD-M20A), Pomp: LC20 AT, Autosampler: SIL 20ACHT, Column Oven: CTO-10ASVp, Computer Programme: LC Solution, Mobile Phase A: 3% Formic acid, Mobile Phase B: Methanol. The elution gradient was applied at a flow rate of 1 mL/min: 95%A/5%B for 3 min, 80%A/20%B for 2 min, 60%A/40%B for 10 min, 50%A/50%B for 10 min, 100%B for 10 min until the end of the run. 10 µL methanol samples were injected into the column [17].
Gas chromatography–mass spectrometry analysis of FAs
Fats were analyzed in GC–MS after derivatization. Derivatization was done with 1.5 M methanolic HCl. Fatty acid compositions of samples were determined by GC–MS system Agilent 5975 C Agilent 7890A GC–MS. The column was DB WAX (50 × 0.20 mm, 0.20 µm), the initial temperature was 60 °C, which was increased to 175 °C with 13 °C/min then increased to 215 °C with 4 °C/min and held for 35 min. The injector and detector temperature was 250 °C [18].
Statistical analysis
One-way variance analysis (ANOVA) before Tukey’s HSD test was done to evaluate differences among groups. The level of significance was set at p < 0.05. IBM SPSS Statistics software version 25 was used for statistical analysis.
Results and discussion
Antioxidant activity
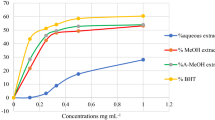
Salvia pisidica seed extract has a higher DPPH RSA than that of S. potentillifolia (p < 0.05) whereas other seed extracts have negligible DPPH RSA (IC50 > 1000 µg/mL) (Table 1). It is well established that the antioxidant capacity of the aerial extracts of Salvia species, however, there are limited studies in the concern of their seeds. For example, S. officinalis seeds have high antioxidant capacity because of their higher content of vitamin E and carotenoids [19].
CUPRAC assay is one of the methods to measure the reducing power of antioxidant compounds [20]. Some metal ions such as Cu2+ can induce free radical oxidation. Therefore, antioxidants reducing Cu2+ to Cu+ in the presence of neocuproine also decrease free radical oxidation. A chromogen of Cu(1)-neocuproine is produced in the CUPRAC redox reaction as phenolic hydroxiles are converted to the corresponding quinones [13]. CUPRAC activity of the extracts was determined as Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalent which is a water-soluble analog of vitamin E. S. pisidica seed extract exhibited higher CUPRAC activity than that of the others (p < 0.05). CUPRAC of the other seed extracts were about of the chia seed extract (Table 1.).
Phenolic compounds are structurally variant active metabolites. Structural variation in polyphenols influences their solubility and bioactivity [21]. Phenolics are potential antioxidant agents because the hydroxyl groups bind an aromatic ring and they can be grouped such as phenolic acids, flavonoids, coumarins, and lignins, according to the carbon skeleton structure [22]. Salvia species are a rich source of polyphenols (caffeic acids, phenolic glucosides, flavonoids, anthocyanins, proanthocyanidins) with more than 160 polyphenols having been identified, some of which are unique to the genus [23]. A very limited number of studies was found in the literature on the phenolic compounds of these four species. In this study, the TPC and TFC of the extracts were determined gallic acid and catechin equivalent, respectively. S. pisidica seed extract had high TPC (176.28 µg/mL); while S. potentillifolia seed extract had high TFC (28.87 µg/mL) (p < 0.05). Caffeic acid was found as the most abundant phenolic acid in the seed extracts of S. potentillifolia (2.03 µg/mL), and quercetin was the highest flavonoid in S. pisidica seeds (9.60 µg/mL).
β-Carotene/linoleic acid bleaching assay evaluates the capacity of the extracts to reduce the oxidative loss of β-carotene in a β-carotene/linoleic acid emulsion. In this coupled oxidation model, linoleic acid is thermally induced to oxidation, thus producing free radicals, which make β-carotene undergo rapid discoloration in the absence of an antioxidant [24]. S. caespitosa and S. potentillifolia seed extracts were more active in the inhibition of β-carotene bleaching than those of the other seed extracts (p < 0.05). Inhibition of the lipid peroxidation activity of the other seed extracts was about that of the chia seed extract while positive controls BHA and Trolox were 88–105% in the assays, respectively (Table 1).
Various results of the antioxidant activity assays were obtained in this study probably because of various components responsible for different bioactivities. Antioxidant activity not only deals with the numbers and positions of hydroxyl groups of the phenolic compounds but also the other components such as carotenoids, ascorbates, terpenes, and pigments [25].
Antimicrobial activity
The antimicrobial activity of the seed extracts was determined on the widely used pathogen microorganisms, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 35150, Salmonella enterica subsp. enterica ATCC 700408, Listeria monocytogenes RSKK 472, Candida albicans ATCC 10231. Seed extracts were not active in inhibiting the growth of the microorganisms, as some literature data stated [8], while methanol extract of the chia seed was more efficient on the S. aureus, S. enterica and L. monocytogenes strains than the other seed extracts. MIC values are given in Table 2. There are some Salvia species like S. verbenaca whose seeds have EOS—especially camphor rich—and it’s thought that they could be used as antimicrobial agents [9].
Phytochemical analyses
The main chemical constituents of Salvia species are polyphenols and terpenoids [26]. In this study, phenolic acids (3,4-dihydroxy benzoic acid, caffeic acid, chlorogenic acid, cinnamic acid, ferulic acid, p-coumaric acid, rosmarinic acid, syringic acid), and flavonoids (quercetin, rutin) were determined by using HPLC–DAD detection (Sample chromatogram is shown in Fig. 1). Caffeic acid was found in the highest levels of the seed extracts of S. potentillifolia (2.03 µg/mL), ferulic acid was found in chia seed (1.82 µg/mL), rosmarinic acid was found in S. pisidica seeds (1.74 µg/mL) (p < 0.05). Other phenolic acids were found in nanogram levels in the seed extracts. On the other hand, S. pisidica seeds contained higher levels of quercetin (9.60 µg/mL). Quercetin was not determined in chia seeds contrary to some data [7], whereas, rutin (2.78 µg/mL) and ferulic acid (1.82 µg/mL) levels in chia seeds were higher than in others (p < 0.05) (Table 3). Therefore S. cadmica was highly rich in benzoic acid (0.079 µg/mL), caffeic acid 1.97 µg/mL), and quercetin (5.27 µg/mL).
Phytochemical studies have been broadly carried out in Salvia species and caffeic acid and derivatives have been found in this genus [26]. In this study, it has been determined that seeds of S. pisidica and S. potentillifolia contained remarkably high levels of phenolic acids and flavonoids.
Lipid molecules are important for bearing a high energy yield, forming the main structure of the cell membrane, and their role in signal transduction [27]. Plant seeds are recommended for cardiovascular health because they are rich in unsaturated FAs [28]. Chia seed, is a functional food that has recently increased in industrial importance due to its superior nutritional value, phytochemical components, and therapeutic effects [7], being a strong reason that the phytochemical content and bioactivity of S. cadmica, S. caespitosa, S. pisidica and S. potentillifolia were compared to chia seeds, in this study. The major FA components of the chia seed were linolenic acid (53.516%), linoleic acid (24.865%), oleic acid (6.991%), palmitic acid (8.894%), and stearic acid (3.753%) while the others’ were linoleic, oleic, palmitic and stearic acids (Table 4) as Kılıç et al. [10] established. (Sample chromatogram is shown in Fig. 2). Studies in recent years showed that ω3 α-linolenic acid (18:3) to ω6 linoleic acid (18:2) ratio is considered the most important factor more than the total amount of FAs in the context of nutritional quality of lipid fraction. Besides, an unbalanced ω3 ω6−1 ratio with a high content of ω6 polyunsaturated FAs may cause some health disorders such as obesity, diabetes, and atherosclerosis [7]. Chia seed oil is well known as a rich source of polyunsaturated FAs mainly linolenic acid (~ 60%) and linoleic acid (~ 20%) [29]. FA ratios of other Salvia seeds we studied were not in the chia seed. Gören et al. [11] and Kılıç et al. [10] determined the FA composition of some Salvia species including S. cadmica, S. caespitosa and S. potentillifolia from various regions of Turkey, and found linoleic acid (18:2; 24.3 to 69.2%), linolenic acid (18:3; 0.6 to 40.8%), oleic acid (18:1; 8.3 to 31.0%), palmitic acid (16:0; 3.8 to 21.0%) and stearic acid (18:0; 1.8 to 5.2%) as main components. Researchers stated that the FA composition of Salvia seed oils could be used as a chemotaxonomic marker [11]. In the limited literature data, FA ratios of only a few other Salvia seeds were found similar to that of chia seeds. For example 18:3/18:2 ratio of S. staminea Montbret & Aucher ex Benth and S. sclarea L. were found above 2 similar to that of chia seed [10, 11, 19, 30]. Farida et al. [31] also investigated FA contents of some Salvia seeds from Iran and found that the 18:3/18:2 ratio of S. sclarea was above 3, while some of them (like S. numerosa L. and S. spinosa L.) were above 2. Iran is one of the regions that has a high endemism ratio in Salvia genus like Turkey [31] and we encountered that most of the studies on the seeds of Salvia species from Iran.
Most of the literature data is concentrated on the nutritional value of the chia seed. Chia seeds possess excellent nutritional value as they are good sources of carbohydrates, fats, proteins, ash, and dietary fibers with contents of 41%, 30%, 23%, 4%, and 18–30%, respectively [7] Reports regarding nutritional values and bioactive properties of other Salvia seeds are very limited in the literature. Tocopherols (another lipid group) [32] were determined in some Salvia seeds. S. przewalskii seeds have high crude protein content [33], S. leriifolia Benth. seeds have anti-inflammatory and antinociceptive activity [9], S. macrosiphon Boiss. seeds has rich phytosterol content and concluded that it could be used as a dietary supplement [34], S. sclarea L. seeds has antiradical properties [35]. In this study, we determined antioxidant and antimicrobial properties and phenolic contents of the seeds of S. cadmica, S. caespitosa, S. pisidica, and S. potentillifolia, from Turkey, for the first time.
Conclusion
These findings suggest that seed extracts of especially S. pisidica and S. potentillifolia are richer in phytochemicals like phenolic substances and they are more active with antioxidants when compared to chia seed. The potential they bear in medicine, perfumery, and other industrial branches with their antioxidant activities is not lower than they have in the food industry because of FA compositions. The literature shows Salvia seeds like S. sclarea have similar FA content as chia seed has, besides their bioactive properties. Hence, those Salvia species could be new functional food candidates when their detailed phytochemical and bioactive properties are revealed. Furthermore, not only the phytochemical diversity, bioactivity and FA compositions but also other nutritional parameters such as dietary fibers, proteins, and carbohydrates of Salvia seeds could be better investigated.
Data Availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
F. Celep, T. Dirmenci, Ö. Güner, Phytotaxa 227(3), 289–294 (2015)
https://bizimbitkiler.org.tr. Accessed 19 Jan 2023
İ Kivrak, M.E. Duru, M. Öztürk, N. Mercan, M. Harmandar, G. Topçu, Food Chem. 116, 470–479 (2009)
S.F. Askari, R. Avan, Z. Tayarani-Najaran, A. Sahebkar, S. Eghbali, Phytochemistry 183, 112619 (2021)
V. Adımcılar, Z. Kalaycıoğlu, N. Aydoğdu, T. Dirmenci, A. Kahraman, F.B. Erim, J. Pharm. Biomed. Anal. 175, 112763 (2019)
A. Lubbe, R. Verpoorte, Ind. Crop Prod. 34(1), 785–801 (2011)
Z.U. Din, M. Alam, H. Ullah, D. Shi, B. Xu, H. Li, C. Xiao, Food Hydrocoll. 1, 100010 (2021)
H. Hosseinzadeh, H.R. Sadeghnia, S.M. Imen, B.B. Fazli, Iran. J. Basic Med. Sci. 12(1), 1–8 (2009)
M.B. Taârit, K. Msaada, K. Hosni, B. Marzouk, Adv. Chem. 2014, 838162 (2014)
T. Kılıç, T. Dirmenci, A.C. Gören, Rec. Nat. Prod. 1(1), 17–23 (2007)
A.C. Gören, T. Kiliç, T. Dirmenci, G. Bilsel, Biochem. Syst. Ecol. 34, 160–164 (2006)
M.S. Blois, Nature 181, 1199–1200 (1958)
R. Apak, K. Güçlü, M. Özyürek, S.E. Karademir, J. Agric. Food Chem. 52(26), 7970–7981 (2004)
V.L. Singleton, J.A. Rossi Jr., Am. J. Enol. Vitic. 16, 144–158 (1965)
J. Zhishen, T. Mengcheng, W. Jianming, Food Chem. 64, 555–559 (1999)
A. Klančnik, S. Piskernik, B. Jeršek, S.S. Možina, J. Microbiol. Methods 81, 121–126 (2010)
F. Caponio, V. Alloggio, T. Gomes, Food Chem. 64, 203–209 (1999)
B. Bardakçı, H. Seçilmiş, Süleyman Demirel Univ. Fen Dergisi 1(1), 64–69 (2006)
J. Živković, M. Ristić, J. Kschonsek, A. Westphal, M. Mihailović, V. Filipović, V. Böhm, Chem. Biodivers. 14(12), e1700344 (2017)
Y. Cai, Q. Luo, M. Sun, H. Corke, Life Sci. 74(17), 2157–2184 (2004)
D. Pathiraja, J.P. Wanasundara, F.M. Elessawy, R.W. Purves, A. Vandenberg, P.J. Shand, Food Chem. 407, 135145 (2023)
K. Robards, M. Antolovich, Analyst 122, 11R-34R (1997)
Y. Lu, L.Y. Foo, Phytochemistry 59(2), 117–140 (2002)
F. Wang, J.A. Yang, LWT Food Sci. Technol. 46(1), 239–244 (2012)
F.M. Awah, P.N. Uzoegwu, P. Ifeonu, J.O. Oyugi, J. Rutherford, X. Yao, F. Fehrmann, K.R. Fowke, M.O. Eze, Food Chem. 131(4), 1279–1286 (2012)
S. Rungsimakan, M.G. Rowan, Phytochemistry 108, 177–188 (2014)
R. Acharya, S.S. Shetty, S.N. Kumari, Chem. Phys. Lipids 250, 105269 (2023)
M. Kozłowska, E. Gruczyńska, I. Ścibisz, M. Rudzińska, Food Chem. 213, 450–456 (2016)
A.E. Di Marco, V.Y. Ixtaina, M.C. Tomás, Food Hydrocoll. 108, 106030 (2020)
N. Azcan, A. Ertan, B. Demirci, K.H.C. Baser, Chem. Nat. Compd. 40, 218–221 (2004)
S.H.M. Farida, T. Radjabian, M. Ranjbar, S.A. Salami, N. Rahmani, A. Ghorbani, Chem. Biodivers. 13, 451–458 (2016)
G. Özkan, R.S. Göktürk, M. Kıralan, M.F. Ramadan, Riv. Ital. Sostanze Grasse 95, 17–21 (2018)
T. Wang, L. Wang, Y. Jiang, L. Yu, L. Zhang, Y. Zhou, R. Yang, C. Ding, X. Wang, Asian J. Chem. 26(16), 4971–4974 (2014)
A. Hamedi, A. Jamshidzadeh, S. Ahmadi, M. Sohrabpour, M.M. Zarshenas, Res. J. Pharmacogn. 3(4), 27–37 (2016)
M. Aćimović, B. Kiprovski, M. Rat, V. Sikora, V. Popović, A. Koren, M. Brdar-Jokanović, J. Agron. Technol. Eng. Manage. (JATEM) 1(1), 18–28 (2018)
Acknowledgements
This study was financially supported by the Research Fund of Mehmet Akif Ersoy University (Project ID: 0724-MP-21), Burdur–Turkey.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karadeniz-Pekgöz, A., Turgut, A.C., Çinbilgel, İ. et al. Phytochemical contents and bioactivity of four endemic Salvia seeds from Turkey: a comparative study to chia seed. Food Measure (2024). https://doi.org/10.1007/s11694-024-02594-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11694-024-02594-8