Abstract
In this study, the effects of harvest year and period on the total polyphenol (TP), radical scavenging activity (RSA), total flavonoid (TF) and ascorbic acid (AA) contents of fresh tea leaves were investigated. Extraction of polyphenols from the tea leaf was optimized using the response surface methodology (RSM). The effect of the independent variables ((solvent concentration, extraction time and solid to solvent (S/S) ratio)) on TP and RSA was studied using Box-Behnken design (BBD). The results showed that extraction parameters selected were significant in the extraction procedure regarding TP and RSA (p < 0.05). The optimum extraction conditions were obtained as 1/60.86 of S/S ratio, 84.65% of ethanol concentration and the extraction time of 50 min. As a result of the extraction of tea leaves under optimum conditions, the TP, RSA and TF contents of the leaves harvested in September in 2020 were found to be higher. In addition, the individual phenolic compounds of these leaves were analyzed by high performance liquid chromatography (HPLC) and epigallocatechin gallate (EGCG) was detected the most (57.52 mg/g DM). After in-vitro digestion of the same samples, TP and RSA values were significantly decreased, mostly at intestinal stage (p < 0.05), and the bioaccessibility of the polyphenols was determined as 48.98%. As a result of AA analysis by HPLC of the, the highest contents for 2019 and 2020 were observed in the leaves harvested in May (41.92 mg/100 g DM) and those in July (38.55 mg/100 g DM), respectively.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Recently natural antioxidants have received more attention than synthetic antioxidants [1] since they are safe and do not cause toxicity problems [2]. Therefore, there is an increasing interest in seeking plant possess high antioxidant capacities [3, 4]. Among the bioactive compounds found in plants, phenolic substances are considered to be the most important antioxidants [5]. Tea is a very important source of phenolic substances and it is stated that the polyphenols in tea have stronger antioxidant activity than synthetic antioxidants such as BHA, BHT and DL-alpha-tocopherol [6, 7]. Furthermore, many previous studies [8, 9] reported that tea polyphenols have lots of health benefits such as prevention of cancers, cardiovascular diseases, microbial diseases, diabetes and obesity. Besides, ascorbic acid (AA), which is also one of the bioactive compounds of tea, has antioxidant activity [10] and supports the circulatory system due to its synergistic effect with rutin, one of the phenolic compounds in tea [11]. Although phenolic compounds have strong antioxidant activity, their bioactivity depends on their degree of bioaccessibility. It measures the proportion of a compound consumed in a meal that is released from the food matrix during digestion.
The polyphenol content of tea varies depending on the factors such as variety, season, climate, manufacturing method and analysis method [12]. For example, fermentation reduces the catechins which are the major polyphenols of tea shoots. For this reason, green tea contains higher amounts of catechins than oolong and black tea. On the other hand, compared to fresh tea leaves, the polyphenol content of green tea is lower due to the heat treatment applied for enzyme inactivation [13, 14]. In this case, it is more meaningful to use fresh tea shoots directly instead of green tea for a source of the polyphenols. Although, numerous researches in literature [15,16,17,18,19] were carried out on the polyphenols from different kinds of tea in particular green tea. To the best of our knowledge, there are limited studies on the polyphenols of fresh tea shoots. Therefore, the aims of this study were (1) to optimize the extraction conditions (solvent concentration, time and solid to solvent (S/S) ratio) of phenolics from tea shoots using response surface methodology (RSM) and (2) to determine seasonal variations in bioactive compounds of the shoots using high performance liquid chromatography (HPLC) and (3) to evaluate bioaccessibility of the polyphenols and also, (4) to compare the modifications of Folin-Ciocalteu reagent (FCR) and 2,2,diphenyl-2-picryl-hydrazyl (DPPH) methods used to determine TP and RSA of the shoots.
Materials and methods
Materials
Fresh tea shoots were plucked from a tea garden in Azaklıhoca (Rize) 40° 59’ 35.8584’’ N, 40° 27’ 42.8760’’ E in Turkey in May, July and September in both 2019 and 2020 years. Because in Turkey, there are three harvests of green tea during each year. The shoots were immediately dried in shade under natural air flow and surrounding temperature (mean temperature = 25 °C) for 36 h, filled in the polyethylene bags and kept at 4 ºC before the experiments were conducted. All chemicals and reagents used in the study were either HPLC or analytical grade.
Methods
Experimental design for RSM modeling
In this study, optimization of extraction parameters of polyphenols from tea shoots was done using Box-Behnken design (BBD) and response surface methodology (RSM). The current design comprised 30 experimental runs with three levels, − 1 (lower limit), 0 (central point), and + 1 (upper limit) for each factor. Solid/solvent ratio, ethanol concentration and time were chosen as the independent variables whose selection and range were based on previous studies [20, 21] and preliminary study, while response variables were TP and RSA. The experimental design (coded and actual values of the factors) is shown for each run in Table 1. The experiments were performed in duplicates, and the averages of the duplicates have been taken as responses. The experimental data was fitted to the following second-order polynomial model:
Where Y is the predicted response; \({\beta }_{0}\) is the model intercept coefficient; \({\beta }_{1},{\beta }_{2} , {\beta }_{3}\) are the regression coefficients for the linear effect terms; \({\beta }_{1}^{2},{ \beta }_{2}^{2} , {\beta }_{3}^{2}\) are the square effect terms; and \({\beta }_{1}{\beta }_{2}, {\beta }_{1}{\beta }_{3}\) and \({\beta }_{2}{\beta }_{3}\) are the interaction effect terms, respectively in Eq. 1. Analysis of variance (ANOVA) was applied to determine the effect of each factor to analyze the predicted model on the response variable. Furthermore, to evaluate the fitness of the regression model, the regression coefficient (R2) and the p-value of the lack of fit (LOF) were employed. The relationship between the independent variables and the response variables was presented by the response surface and contour plots, and the optimum conditions were determined. In order to find out the accuracy and suitability of the optimized conditions, an additional experiment was performed under optimal conditions. MINITAB 17 software (State College, PA) was used for data analysis.
Extraction of polyphenols
Tea shoots were ground using a coffee grinder (Moulinex Ar1105) and sieved to obtain 150–300 μm particle size. The ground shoots were extracted in a falcon tube with ethanol and its aqueous solutions at a known concentration (B, %) on a mechanical shaker (Memmert WNB 22). Ethanol was chosen because it has been mostly used to extract polyphenols from plant materials [22, 23] and is environmentally friendly [24]. Extraction was performed at a known S/S ratio (A, w/v) defined in Table 1. After extraction time (C, min), the suspension was filtered through Whatman No.1 filter paper, and the extract was stored at − 18 °C until analyzed. The extraction conditions used are presented in Table 1.
Determination of total polyphenol (TP) by Folin-Ciocalteu reagent (FCR)
TP of extracts from the shoots was determined using spectrophotometer (Shimadzu UV-1800) according to ISO 14502-1:2005 [25] called FCR method 1 in this study. The results determined from the regression equation of calibration curve were expressed as mg gallic acid equivalent per gram of dry matter (mg GAE/g DM) (R2 = 0.9968). For comparison of the methods and results, TP of the extracts was also determined according to other four methods called FCR method 2 [26], FCR method 3 [2], FCR method 4 [27] and FCR method 5 [28].
Determination of total flavonoid (TF)
TF of the extracts was determined using the method proposed by Rodrigues et al. [29]. TF content was quantified using calibration curve of rutin (R2 = 0.9997) and expressed as mg rutin equivalent per gram of DM (mg RE/g DM).
Radical scavenging activity (RSA) by DPPH
RSA was measured using the method reported by Türkmen Erol et al. [30] called DPPH method 1 in this study. Other four methods in the literature which are called DPPH method 2 [31], DPPH method 3 [32], DPPH method 4 [33] and DPPH method 5 [34] were also applied to compare the results. RSA was calculated as percentage inhibition of the DPPH radical by the equations described by the methods and converted to ascorbic acid equivalent (AAE) defined as g of AAE per 100 g of DM.
Determination of phenolic profile of the extracts
The identification and quantification of individual polyphenols ((gallic acid (GA), epigallocatechin (EGC), epigallocatechin gallate (EGCG), epicatechin gallate (ECG), quercetin-3-rhamnosylglucoside (Q3RG), quercetin-3-glucoside (Q3G) and kaempferol-3-rhamnosylglucoside (K3RG)) in the extracts with the highest TP were performed on a HPLC system including LC-20 AD Shimadzu pumps, a CTO-10 ASVP column oven and SPD-M20A photodiode array (PDA) detector, a Shimadzu DGU-20A5R degasser and SLC-10 A VP system controller. A computer-controlled system with LC solution software was employed for data analysis. The column used was a C18 reversed phase Nova Select (250 × 4.6 mm ID, 5 μm) and was operated at 25 °C. Peak areas were measured at 270–355 nm. The two mobile phases used for gradient HPLC elution were (A) 0.1% orthophosphoric acid in water (w/v) and (B) acetonitrile. The gradient elution profile was as follows: from 0 to 10 min, 8–11% B; from 10 to 57 min, 11–21% B; from 57 to 62 min, 21–80% B. The column was re-equilibrated with the initial conditions for 5 min before the next injection. The flow rate was 1.0 mL/min. The injection volume was 20 µL.
Chromatographic peaks in the samples were identified by comparing their retention times and UV spectra with those of their reference standards and by co-chromatography with added standards. Quantification was performed from the peak area of each component and its corresponding calibration curve.
In-vitro gastrointestinal digestion
The in-vitro digestion process was performed according to the method described by Minekus et al. [35]. Blanks were prepared with identical chemicals without the extract and treated under the same conditions as the extract. Finally, gastric and intestinal digestion mixtures were centrifuged (Sigma 3K30) (12000 x g, 10 min, 4 °C) and filtered (Whatman No:1). The samples were stored at − 20 °C until further analysis. After each stage, the amounts of TP and RSA were determined by spectrophotometer and the bioaccessibilities (%) were calculated according to Eq. 2 as follows;
Bioaccessibility (%) = (C digested / C undigested) x 100 (2).
C digested: Concentration in digested sample after gastric/intestinal stage (mg)
C undigested: Concentration in undigested sample (mg)
Ascorbic acid (AA) determination
The sample was mixed with 0.5% metaphosphoric acid solution at a ratio of 1:24 and vortexed for one minute, followed by centrifugation for 10 min at 12,000 x g. The supernatant was filtered through a 0.45 μm membrane and injected into the high performance liquid chromatography (HPLC) equipped with a LC-20 AD Shimadzu pump, a CTO-10 ASVP column oven and SPD-M20A photodiode array detector. The column was Nova Select C18 reversed phase column (100 A, 5µ, 250 × 4.6 mm) and was operated at 25 °C. The solvents used were: (A) 0.1% orthophosphoric acid in water (w/v) and (B) acetonitrile. The elution gradient established was 5–25% B from 0 to 10 min. The column was re-equilibrated with the initial conditions for 5 min before the next injection. The flow rate was 1.0 mL/min. The injection volume was 20 µL. AA was quantified using the calibration curve of AA at 242 nm (R2 = 0.9998).
Statistical analysis
Experimental results were expressed as means ± standard deviation of triplicate measurements and analyzed by SPSS software (SPSS statistics 23, IBM.2015). Analyses of variance were performed by one-way and two-way ANOVA procedures. Means were compared by using Duncan’s multiple comparison test. Values of p < 0.05 were considered as significantly different.
Results and discussion
RSM modelling
The responses of TP and RSA of the ethanolic extracts from fresh tea shoots are shown in Table 1. Ethanol was chosen as extraction solvent since it is cheap, reusable, nontoxic and environmentally friendly organic solvent [22, 24, 36]. According to the results, the values of TP and RSA of the extracts varied from 46.81 to 95.97 mg GAE/g DM and from 8.12 to 20.84 g AAE/100 g DM, respectively (Table 1).
The ANOVA analysis for TP and RSA of the extracts showed that the model was highly significant (p < 0.05) with high values of determination coefficients (R2) (Table 2). This result represented a good correlation between the experimental and predicted data for TP and RSA of ethanolic extracts (Fig. 1). For the model responses, lack of fit was not significant (p > 0.05) indicating that the model could be used to predict responses.
Effect of extraction parameters on TP and RSA of the shoots extracts
According to variance analysis (Table 2), S/S ratio, ethanol concentration and extraction time had significant linear effects on two responses (p < 0.05) but only linear effect of time on RSA was not significant (p > 0.05), which indicated TP and RSA were strongly influenced by these factors. The square terms of ethanol concentration and S/S ratio except extraction time had significant effects on both TP and RSA (p < 0.05) (Table 2). And, also, the interaction between S/S ratio and ethanol concentration was found to be significant for two responses (p < 0.05). The predicted models for TP and RSA of ethanolic extracts were given below, respectively:
The negative values indicate that an increase in the factors tends to decrease the responses; on the other hand, positive values in the models show that an increase in the factors tends to increase the response values [37]. As seen in Eqs. 3 and 4, TP and RSA increased with increasing extraction time, S/S ratio, and ethanol concentration due to the β1, β2 and β3 terms. For two responses, square and interaction terms of these independent variables also had positive and negative values.
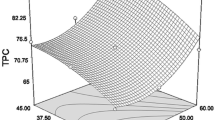
Three dimensional response surface and contour plots were used to represent the relationships between the independent and dependent variables (Figs. 2 and 3). These plots were generated by maintaining one factor at a constant level, whereas the other two factors were varied in their range. These plots were generated by maintaining one factor at a constant level, whereas the other two factors were varied in their range. As seen in Figs. 2a-f and 3a-f, solvent concentration from 100% (code = + 1) to 80% (code = 0) yielded high TP and RSA. However, a decreasing trend towards 60% (code = -1) was observed. As an agreement with this result, Lee et al. [38] showed that the TP content of soybean extracts obtained with three different concentrations of ethanol solutions increased when the ethanol concentration increased from 50 to 75% but it decreased when 95% ethanol was used. The researchers stated that this was due to bioactive compounds in plants have different polarity and solubility. It has also been reported by other study [39] that ethanol concentration affects the extraction of polyphenols.
Response surface plots (a,c and e) and contour plots (b, d and f) of TP from tea shoots as a function of time, S/S ratio and solvent concentration. Extraction time was kept at constant at 35 min (a and b); solvent concentration was kept at 80% (c and d) and S/S ratio was kept at constant at 1/50 of ratio (e and f)
Response surface plots (a,c and e) and contour plots (b, d and f) of RSA from tea shoots as a function of time, S/S ratio and solvent concentration. Extraction time was kept at constant at 35 min (a and b); solvent concentration was kept at 80% (c and d) and S/S ratio was kept at constant at 1/50 of ratio (e and f)
Among the extraction parameters, S/S ratio significantly affected TP and RSA (p < 0.05). As seen in Figs. 2a-d and 3a-d, increasing S/S ratio from 1/25 (code = -1) to 1/50 (code = 0) increased TP and RSA and between the center point and the ratio of 1/75 (code = + 1) the highest TP and RSA values were obtained. Similarly, Zhou et al. [21], in their study on the extraction of polyphenols from seed coat of red bean, antioxidant activity increased as the S/S ratio increased from 1/10 to 1/25 and reached the maximum level between 1/25 and 1/40. The researchers have determined the ratio of 1/29.3 as the optimal point.
Optimization conditions
Optimum factor levels were determined by optimization with the Box-Behnken design to obtain the highest TP value from fresh tea leaves. As a result of the optimization, the S/S ratio of 1/60.86, the ethanol concentration of 84.65% and the extraction time of 50 min were concluded as the optimum conditions. Under these optimum extraction conditions, the predicted value of TP was 92.74 mg GAE/g DM for ethanolic extraction Afterwards, the validity of the model was analyzed; and the experimentally observed value was 95.79 mg GAE/g DM for the ethanol extract. This indicated that predicted result matched well with the experimental result obtained at optimal extraction conditions. In literature, there have been a lot of researches about the optimization of extraction of polyphenols from different sources. However, it is very difficult to compare the optimum extraction conditions from this study with those from previous limited studies [20, 21] that reported the same independent variables to optimize the extraction method. The main reasons for that are differences in extraction methods applied and materials used.
TP, RSA, TF and AA of fresh tea leaves plucked in different periods
The TP content of the extracts varied significantly depending on the harvest year and period (74.10-108.19 mg GAE/g DM) (Table 3). Roshanak et al. [2] reported that TP contents of fresh tea leaves dried by different drying methods after blanching at 90 °C for 1 min were between 109.85 mg GAE/g dw (drying in the shade) and 143.09 mg GAE/g dw (freeze drying). Also, TP content of fresh tea leaves dried in hot air (36 h at 45 °C) ranged from 33.00 mg GAE/ g dw to 291.18 mg GAE/g dw [40]. These results are in consistent with the result of this study. On the other hand, Somsong et al. [14] determined 379.01 mg GAE/g dw of TP in freeze-dried fresh tea leaves, which is more than the result obtained from this study. The difference between the results may be due to differences in the extraction method and the variety.
The TP values obtained from this study were also compared with those of green tea which is known to be rich in polyphenols. Accordingly, in some previous studies on green tea, TP values of 88–173 mg GAE/g dw [13], 116.30 mg GAE/g dw [10], 66.91-139.24 mg GAE/g [41], 1345.41-1765.12 mg/L (corresponding to 76.88 -100.86 mg GAE/g dw) [11] and 45.7 mg GAE/g dw [42] have been reported. These results show that fresh tea leaf can be considered as a more economical source of polyphenols, since it was unprocessed compared to green tea.
The TP content of the leaves plucked in 2020 was generally found to be higher compared to 2019. Harvest period significantly affected the TP content of the leaves (p < 0.05) and the highest TP was determined in the leaves plucked in September for both years. Similarly, Erturk et al. [40] also observed an increase in the TP content of fresh tea leaves from May, which is the first harvest period, to September, which is the last harvest period. The researchers stated that this situation may be due to more sun exposure of the region in September and the stress conditions caused by the high temperature difference between day and night and the irregular rains in this month. Y. Chen et al. [18], also, reported that when tea leaves plucked in autumn were used for oolong tea production, total catechin content of oolong tea was found to be higher compared to the leaves plucked in spring.
The RSA values of the extracts varied between 12.70 and 21.80 g AAE/100 g DM, depending on the harvest year and period (Table 3). There have been limited studies in the literature on the antioxidant activity of fresh tea leaves. Moreover, in these studies, the DPPH method was applied with different modifications, different standards were used, and the results were expressed in different units. For this reason, it is hard to compare the present results with literature values directly. However, when compared with the antioxidant activity of green tea; for example, Ramírez-Aristizabal et al. [43] found lower antioxidant activity (22.36-110.01 mg trolox equivalent (TE)/g tea) in water extracts of green tea than the results of this study. The highest RSA was found in the tea leaves harvested in September of 2020, which was in agreement with the TP amounts. This can be explained by the fact that polyphenols, which are known to have antioxidant activity, were detected the most in the leaves harvested in September. In many previous studies [44, 45], it was determined that there was a strong relationship between the total phenolic contents of plant materials and their antioxidant activities. As agreement with the result of this study, Erturk et al. [40] stated that, fresh tea leaves harvested in September contained the highest antioxidant activity compared to May and July.
As seen in Table 3, the TF content of tea leaves varied from 63.69 to 91.16 mg RE/g DM depending on the harvest year and period. However, Roshanak et al. [2] reported that TF contents of tea leaves dried after blanching were between 14.30 mg quercetin/g dw (microwave drying) and 38.18 mg quercetin/g dw (hot air drying at 100 °C). Although these results are lower than those obtained in this study, in agreement with the result of our study their TF values were lower than their TP ones. In terms of the harvest period, the highest TF content was determined in the tea leaves plucked in September of both years, as in the TP and RSA results. This might be due to the meteorological parameters in September, as mentioned for the TP results. In addition, it was observed that the TF content of the leaves harvested in September in 2020 was higher than those in 2019.
Although fresh tea leaves are mainly rich in polyphenols, it has been stated that they are also an important source of vitamins and minerals [2]. AA is one of the important components of tea leaf due to its antioxidant activity [10] and synergistic effect on the circulatory system with rutin which is among the phenolic compounds of tea leaf [11]. AA content of tea leaves was determined by HPLC analysis and varied between 33.54 and 41.92 mg/100 g DM, depending on the harvest year and period (Table 3). For 2019 and 2020, the highest AA content was observed in the tea leaf harvested in May and July, respectively. Lower amounts of AA (10.00-16.36 mg/100 g DM) were detected in tea leaves dried after harvest [2] compared to the results obtained in this study. On the other hand, Jakubczyk et al. [11] found 32.12–44.80 mg/L (corresponding to 183.54–256.00 mg/100 g dw) of AA in matcha tea, which was higher than our results. The differences between the results may be due to the differences in leaf type, extraction method and analysis method (such as spectrophotometric or chromatographic). For example; since the sensitivity of the spectrophotometric method is lower than that of the HPLC method, this can cause the result to be higher than it is. Additionally, it was reported that AA content of tea decreased with an increase in the extraction time and temperature during the extraction [46], because, it can be easily oxidized in the presence of moisture and oxygen [47].
Phenolic profile of tea leaves
HPLC analysis was carried out to determine the individual phenolic compounds of the tea leaf harvested in September in 2020 with the highest TP. As seen in Table 4, the phenolic compounds of the tea leaf are given in comparison with the green tea samples obtained from the market as a reference. In the tea leaf, GA from phenolic acids, EGC, EGCG and ECG from catechins (flavan-3-ols) and Q3RG, Q3G and K3RG from flavonol glycosides were identified and quantified (Table 4).
The lowest level of GA (0.15 mg/g DM) among the phenolic compounds investigated in tea leaves was detected. On the other hand, Jiang et al. [48] found lower amount of GA (0.07 mg/g) in fresh tea leaf than that obtained from this study. Among the catechins in the tea leaf, EGCG was detected the most (57.52 mg/g DM) followed by EGC and ECG, respectively, which is in agreement with the previous studies [49,50,51]. Similarly, Jiang et al. [48] found the most EGCG in fresh tea leaf, but its amount (30.9 mg/g) was lower than the result from this study. EGCG is generally accepted as a quality indicator in tea products because it has the highest antioxidant activity among all catechins [52,53,54]. Among the flavonol glycosides in the tea leaf, the most K3RG was detected, followed by Q3RG and Q3G, respectively. Although there is no study on the flavonol glycosides of tea leaves, Somsong et al. [14] determined the most quercetin in fresh tea leaves as flavonol aglycones, followed by kaempferol and myricetin, respectively. The discrepancy may be due to differences in the variety, region, soil composition and climate. In addition, Veljković et al. [55] found a lower level of rutin (1.091 g/kg) in the water extract of green tea than the value obtained from this study. As seen in Table 4, the fact that the highest total amount of individual phenolic compounds was detected in the tea leaf is quite significant in terms of showing that the tea leaf can be evaluated as a rich source of phenolic compounds without processing into green tea.
Effect of in-vitro digestion on TP and RSA of tea leaf
The TP and RSA values of tea leaf harvested in September in 2020 before and after digestion are given in Table 5. TP and RSA values showed a similar trend after digestion and both significantly decreased according to the initial values (p < 0.05). The highest decrease was detected in the intestinal stage. This is associated with the lower stability of polyphenols due to the alkaline environment during intestinal digestion [56]. To our knowledge, there are no studies on the change of polyphenols and antioxidant activity of tea leaves after digestion. On the other hand, compared to the studies focused on the change of green tea polyphenols, Rodrigues Silva et al. [57] and Shu et al. [58] determined the bioaccessibility of green tea polyphenols as 25.9% and 9.69–15.57%, respectively, lower than the result obtained from this study (48.98%). Green et al. [50] also reported that after gastric and intestinal digestions, a reduction of approximately 80.4% in total catechins was observed and the greatest reduction was in the intestinal stage.
The decrease in TP and thus antioxidant activity after gastrointestinal digestion has also been demonstrated in previous studies with different foods. Bouayed et al. [59] stated that the TP content of four different apple cultivars containing 44.42 mg/100 g fresh weight (fw) on average, decreased to 35.95 mg/100 g fw after gastric stage and 21.84 mg/100 g fw after pancreatic stage. TP and RSA values of ten different walnut varieties decreased by an average of 74.1% and 77%, respectively, after in-vitro digestion compared to their initial values [45]. Similar results were also observed for grape pomace [60] and pomegranate by-products [56].
Comparison of different modifications of the methods used to determine TP and RSA of fresh tea leaf
The FCR and DPPH methods are widely used for TP and RSA analyses in biological materials, respectively, due to their some properties such as simplicity, not requiring any equipment, good reproducibility and high radical stability [5, 61, 62]. However, there are many modifications of them in the literature, which makes it difficult to compare the results of the studies conducted. Therefore, in this study, different modifications of each method were applied to the same sample and the obtained results were compared. As a result of the application of different modifications of FCR to the tea leaf, the amounts of TP varied from 108.19 to 158.00 mg GAE/g DM (Table 6). This could be due to the differences in the reaction such as the order and concentration of the chemical and FC reagent constituting the reaction medium, incubation time and temperature [63]. Furthermore, in addition to the advantages of the FCR method mentioned above, it has the disadvantage of poor specificity to phenolic compounds. In other words, FC reagent can not only react with phenols, but also with other reducing compounds such as organic acids, sugars, proteins, iron ions and sulphites, which are not phenolic substances and commonly found in foods [59, 64]. This phenomenon causes the TP value to be higher than the actual value. For all these reasons, the TP results obtained as a result of the application of five different methods were compared with the HPLC result of the same sample shown in Table 4, since the HPLC method is more sensitive. The total amount of phenolic compounds detected in the tea leaf by HPLC is 90.05 mg/g DM, which is lower than the TP results found spectrophotometrically. This result is consistent with the results of previous studies [10, 55]. Since FCR Method 1 gave the closest result spectrophotometrically to that found by HPLC, this method was considered to be more reliable. Therefore, in this study, it was used to determine TP contents of the samples.
As a result of the application of different modifications of the DPPH method to the tea leaf, the RSA values ranged from 74.33 to 96.33% (Table 6). This might be due to the differences in the reaction conditions, as in the FCR methods applied for TP. Marinova & Batchvarov [62] reported that the DPPH method was applied in different conditions (solvent, the ratio of radical solution/extract, the concentration of radical solution, time and wavelength etc.) and the solvent and ratio of radical solution/extract had a significant effect on the accuracy of the method. In this study, DPPH Method 1 was used to determine the antioxidant activity of leaf samples, since it gave results consistent with the amounts of TP in previous studies [30, 65].
Conclusions
In this study, the effect of the harvest year and period on the bioactive compounds of tea leaves was determined. Among these compounds, extraction of polyphenols was optimized using RSM and the optimum conditions were determined. According to the results, regarding TP and RSA, the extraction parameters were found significant (p < 0.05). The TP, RSA and TF values of leaves harvested in September of 2020 were found to be higher. It was determined that these leaves contained the most EGCG among the phenolic compounds detected. The amount of AA, another bioactive compound found in tea leaves, also varied depending on the harvest year and period. The TP and RSA values of the tea leaf were significantly decreased after in-vitro digestion, mostly at the intestinal stage (p < 0.05). In order to determine the TP and RSA of the samples, different modifications of the most commonly used FCR and DPPH methods in the literature were also compared and significant differences were observed between the methods (p < 0.05). This study showed that the tea leaf harvested in the appropriate period can be used as an important source of polyphenols without processing into green tea.
Data availability
Data will be made available on request.
References
V. Katalinic, S.S. Mozina, I. Generalic, D. Skroza, I. Ljubenkov, A. Klancnik, Int. J. Food Prop. 16, 45 (2013)
S. Roshanak, M. Rahimmalek, S.A.H. Goli, J. Food Sci. Technol. 53, 721 (2016)
B. Nickavar, N. Esbati, JAMS J. Acupunct. Meridian Stud. 5, 119 (2012)
L. Gu, T. Wu, Z. Wang, Lwt. 42, 131 (2009)
L.Y. Chen, C.W. Cheng, J.Y. Liang, Food Chem. 170, 10 (2015)
O.K. Horbańczuk, M.A. Kurek, A.G. Atanasov, M. Brnčić, S.R. Brnčić, Food Technol. Biotechnol. 57, 439 (2019)
D. Prakash, C. Gupta, J. Complement. Integr. Med. 9, 20 (2012)
X. Zhang, C. Zhang, X. Sun, J. Yang, C. Zhu, RSC Adv. 9, 18971 (2019)
J.M. Lorenzo, P.E.S. Munekata, Asian Pac. J. Trop. Biomed. 6, 709 (2016)
J.H. Park, J.M. Lee, Y.J. Cho, C.T. Kim, C.J. Kim, K.C. Nam, S.C. Lee, J. Food Biochem. 33, 149 (2009)
K. Jakubczyk, J. Kochman, A. Kwiatkowska, J. Kałdunska, K. Dec, D. Kawczuga, K. Janda, Foods 9, (2020)
S.A. Aherne, N.M. O’Brien, Nutrition. 18, 75 (2002)
N. Donlao, Y. Ogawa, Lwt. 89, 648 (2018)
P. Somsong, C. Santivarangkna, P. Tiyayon, C.M. Hsieh, W. Srichamnong, Sustain. 12, (2020)
C.W. Han, M. Ma, H.H. Zhang, M. Li, Q.J. Sun, Food Chem. 308, 125676 (2020)
A. Gramza-Michałowska, J. Kobus-Cisowska, D. Kmiecik, J. Korczak, B. Helak, K. Dziedzic, D. Górecka, Food Chem. 211, 448 (2016)
A. Rashidinejad, E.J. Birch, D.W. Everett, Int. J. Food Sci. Nutr. 67, 624 (2016)
Y. Chen, Y. Jiang, J. Duan, J. Shi, S. Xue, Y. Kakuda, Food Chem. 119, 648 (2010)
W. Dai, D. Qi, T. Yang, H. Lv, L. Guo, Y. Zhang, Y. Zhu, Q. Peng, D. Xie, J. Tan, Z. Lin, J. Agric. Food Chem. 63, 9869 (2015)
I.F. Strati, G. Kostomitsopoulos, F. Lytras, P. Zoumpoulakis, C. Proestos, V.J. Sinanoglou, Foods 7, (2018)
Y. Zhou, X.Y. Xu, R.Y. Gan, J. Zheng, Y. Li, J.J. Zhang, D.P. Xu, and H. Bin Li, Antioxidants 8, 1 (2019)
K.D.P.P. Gunathilake, K.K.D.S. Ranaweera, H.P.V. Rupasinghe, Food Sci. Nutr. 7, 528 (2019)
I. Zardo, A. de Espíndola, L.D.F. Sobczyk, Marczak, J. Sarkis, Waste Biomass Valoriz. 10, 33 (2019)
I.R. Amado, D. Franco, M. Sánchez, C. Zapata, J.A. Vázquez, Food Chem. 165, 290 (2014)
ISO, 14502-1:2005, (n.d.)
M. Obanda, P.O. Owuor, S.J. Taylor, J. Sci. Food Agric. 74, 209 (1997)
S. Barreira, C. Moutinho, A.M.N. Silva, J. Neves, E.J. Seo, M.E.F. Hegazy, T. Efferth, L.R. Gomes, Phytomedicine Plus 1, (2021)
T. Ren, P. Zheng, K. Zhang, J. Liao, F. Xiong, Q. Shen, Y. Ma, W. Fang, X. Zhu, Plant. Physiol. Biochem. 159, 363 (2021)
M.J. Rodrigues, A. Soszynski, A. Martins, A.P. Rauter, N.R. Neng, J.M.F. Nogueira, J. Varela, L. Barreira, L. Custódio, Ind. Crops Prod. 77, 315 (2015)
N. Türkmen Erol, F. Sari, E. Çalikoǧlu, Y.S. Velioǧlu, Turkish J. Agric. For. 33, 353 (2009)
A. Topuz, C. Dinçer, M. Torun, I. Tontul, H. Şahin-Nadeem, A. Haznedar, F. Özdemir, Turkish J. Agric. For. 38, 233 (2014)
L.S. Lee, N. Lee, Y.H. Kim, C.H. Lee, S.P. Hong, Y.W. Jeon, Y.E. Kim, Molecules. 18, 13530 (2013)
F. Qu, W. Zeng, X. Tong, W. Feng, Y. Chen, D. Ni, Lwt. 117, 108646 (2020)
A. Sentkowska, K. Pyrzynska, J. Pharm. Biomed. Anal. 159, 393 (2018)
M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. MacIerzanka, A. MacKie, S. Marze, D.J. McClements, O. Ménard, I. Recio, C.N. Santos, R.P. Singh, G.E. Vegarud, M.S.J. Wickham, W. Weitschies, A. Brodkorb, Food Funct. 5, 1113 (2014)
V. Sablania, S.J.D. Bosco, M. Bashir, J. Food Sci. Technol. 56, 5500 (2019)
É.O. Barizão, A.C. Martins, L. Ercoli, M.V. Kvitschal, R. Silva, O. Pezoti Junior, J.V. Visentainer, and V. de Cinque Almeida, Food Anal. Methods 6, 1407 (2013)
C.H. Lee, K.E. Hwang, H.W. Kim, D.H. Song, Y.J. Kim, Y.K. Ham, Y.S. Choi, S.J. Jang, T.J. Jeong, C.J. Kim, Korean J. Food Sci. Anim. Resour. 36, 359 (2016)
Y. Riciputi, E. Diaz-de-Cerio, H. Akyol, E. Capanoglu, L. Cerretani, M.F. Caboni, V. Verardo, Food Chem. 269, 258 (2018)
Y. Erturk, S. Ercisli, M. Sengul, Z. Eser, A. Haznedar, M. Turan, Pak J. Pharm. Sci. 23, 69 (2010)
L. Guo, J. Guo, W. Zhu, X. Jiang, Food Bioprod. Process. 100, 303 (2016)
H. Cho, E. Cho, H. Jung, H.C. Yi, B. Lee, K.T. Hwang, Food Sci. Biotechnol. 23, 1295 (2014)
L.S. Ramírez-Aristizabal, A. Ortíz, M.F. Restrepo-Aristizabal, and J. F. Salinas-Villada, Vitae. 24, 132 (2017)
M. Lutz, J. Hernández, C. Henríquez, CYTA - J. Food. 13, 541 (2015)
F. Figueroa, J. Marhuenda, P. Zafrilla, A. Martínez-Cachá, J. Mulero, B. Cerdá, J. Food Nutr. Res. 55, 229 (2016)
P.R. Das, J.B. Eun, Food Chem. 253, 22 (2018)
L. Zhao, F. Li, G. Chen, Y. Fang, X. An, Y. Zheng, Z. Xin, M. Zhang, Y. Yang, Q. Hu, Int. J. Food Sci. Technol. 47, 572 (2012)
H. Jiang, F. Yu, L. Qin, N. Zhang, Q. Cao, W. Schwab, D. Li, C. Song, J. Food Compos. Anal. 77, 28 (2019)
Y.L. Lin, I.M. Juan, Y.L. Chen, Y.C. Liang, J.K. Lin, J. Agric. Food Chem. 44, 1387 (1996)
R.J. Green, A.S. Murphy, B. Schulz, B.A. Watkins, M.G. Ferruzzi, Mol. Nutr. Food Res. 51, 1152 (2007)
G. Rusak, D. Komes, S. Likić, D. Horžić, M. Kovač, Food Chem. 110, 852 (2008)
C.J. Dufresne, E.R. Farnworth, J. Nutr. Biochem. 12, 404 (2001)
J.V. Higdon, B. Frei, Crit. Rev. Food Sci. Nutr. 43, 89 (2003)
A.J. Stewart, W. Mullen, A. Crozier, Mol. Nutr. Food Res. 49, 52 (2005)
J.N. Veljković, A.N. Pavlović, S.S. Mitić, S.B. Tošić, G.S. Stojanović, B.M. Kaličanin, D.M. Stanković, M.B. Stojković, M.N. Mitić, J.M. Brcanović, J. Food Nutr. Res. 52, 12 (2013)
O.A. Fawole, U.L. Opara, BMC Complement. Altern. Med. 16, 1 (2016)
F.M. Rodrigues Silva, L.M. Ribeiro da Silva, A.S. Gomes Duarte, C.E. da Silva Monteiro, A.R. Campos, D.K. Rodrigues Holanda, Silva De Lima, L. Bruno De Sousa Sabino, and R. Wilane De Figueiredo. Food Biosci. 42, 101190 (2021)
Y. Shu, J. Li, X. Yang, X. Dong, X. Wang, J. Food Sci. Technol. 56, 1127 (2019)
J. Bouayed, H. Deußer, L. Hoffmann, T. Bohn, Food Chem. 131, 1466 (2012)
S. Wang, M. Amigo-Benavent, R. Mateos, L. Bravo, B. Sarriá, Int. J. Food Sci. Nutr. 68, 188 (2017)
D. Chen, G. Chen, Y. Sun, X. Zeng, H. Ye, Food Res. Int. 137, 109584 (2020)
G. Marinova, V. Batchvarov, Bulg. J. Agric. Sci. 17, 11 (2011)
N. Cicco, M.T. Lanorte, M. Paraggio, M. Viggiano, V. Lattanzio, Microchem J. 91, 107 (2009)
L.M. Magalhães, F. Santos, M.A. Segundo, S. Reis, and J. L. F. C. Lima, Talanta 83, 441 (2010)
N. Turkmen, F. Sari, Y.S. Velioglu, Food Chem. 99, 835 (2006)
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Hind Chebbi: Investigation, Formal analysis, Validation. Nihal Turkmen Erol: Supervision, Conceptualization, Methodology, Writing - original draft. Bige Incedayi: Data curation, Resources, Project administration, Writing, review & editing. Ferda Sari: Formal analysis, Methodology, Validation, Visualization. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chebbi, H., Turkmen Erol, N., Incedayi, B. et al. Bioactive compounds of fresh tea shoots plucked in different seasons: optimization of extraction of polyphenols. Food Measure 18, 4192–4203 (2024). https://doi.org/10.1007/s11694-024-02486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02486-x