Abstract
Glycidol fatty acid esters (GEs) and 3-monochloro-1,2-propanediol fatty acid esters (3-MCPDEs) are formed at high temperatures during edible oil production. In this study, ground meat (beef, pork, and chicken) patties, cookies, and cupcakes were heated under various conditions using a temperature-controlled device to determine the amount of GEs and 3-MCPDEs produced. GE and 3-MCPDE were not detected in any meat heated in the oven (250 °C). When heating all meats using a muffle furnace, GEs were not detected at 400 °C but they were detected at 500 °C and 600 °C. At 600 °C, 3-MCPDEs were detected in all meats. When cookie and cupcake doughs prepared with rapeseed oil were heated in the oven, GEs were formed in both samples, reaching a maximum at 210 °C. 3-MCPDEs were detected only in cookies, reaching its maximum at 250 °C. The percentage of each GE in heated cookies and cupcakes was related to the fatty acid composition of the rapeseed oil. These results suggest that the formation of GEs and 3-MCPDEs may be affected by food ingredients, cooking device, and heating time, as well as heating temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat-generated food toxicants are toxic chemicals produced by heating during food processing and cooking that can be present in foods. These include acrylamide, polycyclic aromatic hydrocarbons, heterocyclic amines, advanced glycation end products [1,2,3,4,5] as well as glycidol fatty acid esters (GEs) and 3-monochloropropanediol fatty acid esters (3-MCPDEs) [6]. The latter two can be formed during deodorization at high temperatures in the refining process of edible oil [7]. The majority of glycidol and MCPD is bound to a variety of fatty acids, such as glycidyl esters and 3-MCPD diesters of palmitate (C16:0), stearate (C18:0), oleate (C18:1), linoleate (C18:2), and linolenate (C18:3) (Fig. 1). When ingested as part of the diet, under the action of lipases GEs and 3-MCPDEs are believed to release glycidol and 3-MCPD, respectively [8, 9].
In 2009, the German risk assessment agency, Bundesinstitut fur Risikobewertung (BfR), found that GEs in infant formula when measuring 3-MCPDEs [6]. The amount of GEs and 3-MCPDEs produced can be affected by the presence of chloride ions and glycerol, as well as heating conditions, such as heating temperature and time. During the deodorization of edible oil, more GEs than 3-MCPDEs are produced when chloride ions are few [10]. In contrast, Frank et al. reported that heating palm oil at high temperature and for long times produced more 3-MCPDEs [10]. Similarly, Kuhlmann et al. revealed an increase in the formation of GEs and 3-MCPDEs with palm oil deodorization at high temperatures and over a long time, namely, at 180 °C or 230 °C for 1–5 h [11]. In potato chips, the concentration of 3-MCPDEs decreased with frying time but increased with heating temperature and NaCl concentration whereas the amounts of GEs produced increased with temperature, time, and NaCl concentration [12]. However, it has also been reported that 3-MCPDE increases and GE decreases in potato chips as frying oil temperature increases and 3-MCPDE decreases and that GE increases as frying time increases [13]. In addition, the amount of 3-MCPDE produced in foods such as cheese, bread, and meat can be affected by the cooking method [14]. Many researchers have speculated on the formation of GEs and 3-MCPDE, but there are no clear conclusions or evidence regarding the detailed mechanisms of these formations [15].
In our previous study, it was reported that GEs are produced when meat is cooked over gas or charcoal flames and that GEs and 3-MCPDEs are formed when fish is cooked over a gas flame. The temperature under the charcoal flames condition was higher than that under pan-frying condition. The range of cooking temperature using charcoal flames was 350–600 °C [16]. However, the effect of temperature and heating time on GEs and 3-MCPDEs formation in these meats is not evaluated yet. Moreover, the temperature ranges in which these substances are formed and their amount in processed foods composed of edible oils remain unclear. In this study, three types of meat (beef, pork, and chicken) patties were heated under various conditions using a temperature-controlled device to examine the formation of 3-MCPDEs and GEs. As the temperatures in charcoal flames cooking ranges from 350 to 600 °C [16], the muffle furnace was used to replicate the temperature condition of charcoal flames cooking (400 °C, 500 °C, and 600 °C). In addition, cookies and cupcakes made with rapeseed oil were heated at different temperatures over different time periods to determine the amount of GEs and 3-MCPDEs produced.
Materials and methods
Chemicals
The standard materials, glycidyl palmitate (C16:0-GE, purity 98.0%), glycidyl stearate (C18:0-GE, purity 98.0%), glycidyl oleate (C18:1-GE, purity 98.0%), glycidyl linoleate (C18:2-GE, purity 90.0%), glycidyl linolenate (C18:3-GE, purity 85.0%), 3-MCPD dipalmitate (purity 95.0%), 3-MCPD distearate (purity 98.0%), 3-MCPD dioleate (purity 98.0%), 3-MCPD dilinoleate (purity 98.0%), and 3-MCPD dilinolenate (purity 98.0%), were purchased from Fujifilm Wako Pure Chemicals Co., Ltd. (Osaka, Japan). Each standard was diluted using methanol (Kanto Chemical Co., Inc., Tokyo, Japan)/2-propanol (Fujifilm Wako Pure Chemicals Co., Ltd.) (1:1 v/v). The standard materials of methyl palmitate (purity 98.0%), methyl stearate (purity 99.5%), and methyl oleate (purity 99.0%) were purchased from Fujifilm Wako Pure Chemicals Co., Ltd. (Osaka, Japan). Methyl linoleate (purity 99.0%), methyl linolenate (purity 98.0%), and methyl laurate (C12:0, purity 99.5%) were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Each standard was diluted using dichloromethane (Kanto Chemical Co., Inc., Tokyo, Japan). All other reagents used in this study were of analytical grade.
Preparation of meat patties
After kneading commercially available edible ground beef, pork, and chicken about 200 times, 100 g of each ground meat was packed in a cellule to form patties (100 × 15 mm) for oven heating, as shown in Fig. 2A. For heating in a muffle furnace, 25 g of each ground meat was kneaded and shaped into patties in the same way. One of the patties was placed on a heat-resistant tray and heated in an oven and muffle furnace. The heating conditions for each sample were as follows: oven heating (250 °C, 10 and 20 min), muffle furnace heating 400 °C (0, 5, and 10 min), 500 °C (0, 5, and 10 min), and 600 °C (5 min) (Fig. 2B). Each heated sample was ground using a mixer, transferred to an eggplant flask, frozen in a freezer, and dried in a freeze dryer.
Pre-treatment method for determination of GEs and 3-MCPDEs in meat patties. A Meat patties before heating. B Pre-treatment schemes for GE and 3-MCPDE measurements. After kneading commercially available ground beef, pork, and chicken, 100 g of each ground meat was packed in a cell and formed into patties for oven heating. For heating in a muffle furnace, 25 g of each ground meat were shaped into patties
The baking process of cookies and cupcakes with added rapeseed oil
Cookie preparation
The cookie sample preparation method is shown in Fig. 3A. A parchment paper was placed on a baking sheet and the oven was preheated to the respective heating temperatures (170 °C, 210 °C, and 250 °C). First, 25 g rapeseed oil (Fujifilm Wako Pure Chemicals Co., Ltd.), 50 g granulated sugar, and 20 g eggs were placed in a bowl, and mixed. The flour (100 g) and baking powder (3.5 g) were sifted together twice, added to the mixture, and then mixed well. The dough (20 g) was measured out and shaped into a circle 5.5 cm in diameter and 4 mm thick. We prepared six samples per heating condition. The samples were placed on a baking sheet and baked in the oven for 12 min at each temperature (170 °C, 210 °C, and 250 °C). Each heated sample was then crushed in a mortar, transferred to an eggplant flask, frozen in a freezer, and dried in a freeze dryer.
Cupcake preparation
The cupcake sample preparation method is shown in Fig. 3B. A parchment paper was placed on a baking sheet and the oven was preheated to the respective heating temperatures (170 °C, 210 °C, and 250 °C). First, 50 g rapeseed oil (Fujifilm Wako Pure Chemicals Co., Ltd.) were mixed with 100 g granulated sugar, followed by addition of two egg yolks, and 30 mL milk and mixing; then, the beaten egg whites for those two eggs were added, and mixed. The flour (100 g) and baking powder (3.5 g) were sifted together twice, added to the mixture, and then mixed well. Then, each portion of dough (25 g) was placed in a baking cup to prepare three samples per heating condition. The samples were placed on a baking sheet and baked in the oven for 20 min at the corresponding temperature (170 °C, 210 °C, and 250 °C). Finally, each heated sample was crushed in a mortar, transferred to an eggplant flask, frozen in a freezer, and dried in a freeze dryer.
Heat treatment of the rapeseed oil used for cookies and cupcakes
To determine the amount of GEs and 3-MCPDEs produced when rapeseed oil alone was heat-treated, rapeseed oil was treated at various heating temperatures (Fig. 3C). First, the oven was preheated to the respective heating temperatures (170 °C, 210 °C, and 250 °C). Then, about 5 g of rapeseed oil were poured into a heatproof dish and heated at each temperature for 12 min. Finally, the heated sample was used for solid phase extraction.
Soxhlet extraction
Each dry sample (5 g) was placed in cylindrical filter paper (ϕ25 × ϕ28 × 100 mm, Advantec MFS Inc., Tokyo, Japan) and 80 mL of diethyl ether was added and incubated for 8 h for Soxhlet oil extraction. The entire diethyl ether extract was dehydrated using sodium sulfate (Kanto Chemical Industry) and concentrated under reduced pressure in a rotary evaporator. The crude fat sample obtained was stored at − 80 °C.
Solid phase extraction
Each crude oil sample (1 g) was diluted to 10 mL with a t-butyl methyl ether Fujifilm Wako Pure Chemicals Co., Ltd.)/ethyl acetate (Fujifilm Wako Pure Chemicals Co., Ltd.) (4:1, v/v) solution. A reversed-phase solid phase extraction (SPE) column (Sep-Pak Vac RC C18 cartridge 500 mg, Waters, Milford, MA, USA) was pre-treated with 2 mL of methanol and then 1.0 mL of crude sample solution was loaded onto the column. Methanol (2 mL) was added to the column and the eluted solution was collected. The same procedure was repeated three times. The eluate (total of 6 mL) was purged with a nitrogen stream. Next, the normal-phase SPE column (Sep-Pak Vac RC Silica cartridge 500 mg, Waters) was pre-treated with 4 mL of an n-hexane (Fujifilm Wako Pure Chemicals Co., Ltd.)/ethyl acetate (Fujifilm Wako Pure Chemicals Co., Ltd.) (95:5 v/v) solution. The residue after purging was dissolved in 2 mL of the n-hexane/ethyl acetate (95:5 v/v) solution and loaded onto the normal-phase SPE column. Then, 2 mL of the n-hexane/ethyl acetate (95:5 v/v) solution were added to the column and the eluted solution was collected. The same procedure was repeated three times. The eluate (total of 6 mL) was purged with a stream of nitrogen. The dried residue was dissolved in 0.3 mL of methanol/2-propanol (1:1 v/v) solution. This solution was used for the GE and 3-MCPDE analysis by LC–MS/MS.
Measurement of GEs
The AOCS/JOCS approved Cd28-10 method is used for analysis in this study [17]. The measurement of GEs was performed using an LC–MS system comprising an HPLC Prominence system (Shimazdu, Kyoto, Japan) connected to the triple quadruple mass spectrometer API2000 (AB SCIEX, Tokyo, Japan). We used the L-column ODS (Chemicals Evaluation and Research Institute, 5 µm, 150 × 4.6 mm, i.d.). The mobile phase solvent A was 3 mM ammonium acetate in methanol:water = 98:2 and B was 3 mM ammonium acetate in 2-propanol:water = 98:2. The gradient elution program was as follows: 0–1 min, 0% A; 1–30 min, 0%–100% A; 30–60 min, isocratic elution 100% A, return to initial conditions. LC–MS acquisition parameters (selected ion monitoring mode) for GEs are shown in Table 1. The limit of detection (LOD) and the limit of quantification (LOQ) were set using the signal-to-noise (S/N) ratio (LOD: 3, LOQ: 10).
Measurement of 3-MCPDEs
The measurement of 3-MCPDEs was performed using an LC–MS/MS system comprising an HPLC Prominence system (Shimazdu, Kyoto, Japan) connected to the triple quadruple mass spectrometer API2000 (AB SCIEX, Tokyo, Japan). The column was CAPCELL PAKC 18 (3 μm, 100 × 4.6 mm i.d., Shiseido). The mobile phase solvent A was 3 mM ammonium acetate in methanol:water = 98:2 and B was 2-propanol. The gradient elution program was as follows: 0–10 min, 0% A; 10–110 min, 0%–100% A; 110–120 min, isocratic elution 100% A, return to initial conditions. LC–MS/MS acquisition parameters (multiple reaction monitoring mode) for 3-MCPDEs are shown in Table 1. The 3-MCPDEs analytical method, which included methanol and ammonium acetate, was used as mobile phase to detect the NH4+-added ions. The LOD and LOQ were set using the S/N ratio (LOD: 3, LOQ: 10).
Determination of the fatty acid content
An internal standard solution was prepared by adding 2 mg of lauric acid (Tokyo Chemical Industry Co., Ltd.) to 1.5 mL of a 0.5 M sodium hydroxide-methanol solution. Rapeseed oil (20 mg) was aliquoted into a screw-capped test tube and 1.5 mL of the internal standard solution was added. Then, the tube was heated at 100 °C for 9 min. Thereafter, a boron trifluoride methanol complex methanol solution (2 mL; Fujifilm Wako Pure Chemicals Co., Ltd., for gas chromatography) was added and heated again at 100 °C for 7 min. After cooling, 2 mL of dichloromethane was added followed by sonication for 3 min. After mixing, 5 mL of saturated brine was added and centrifuged at 1500 rpm for 10 min. Then, the dichloromethane layer was collected and diluted tenfold with dichloromethane to prepare a test solution. The GC-FID system used was a GC-18A-FID (Shimadzu Corporation, Kyoto, Japan) equipped with an autosampler (AOC-20 series; Shimadzu Corporation) and a hydrogen generator (OPGU-2200S; Shimadzu Corporation). Separations were performed using an SLB-IL100 Capillary Column (60 m × 0.25 mm × 0.2 μm; Supelco, Bellefonte, PA, USA). Helium (99.999%) was used as carrier gas at a constant flow rate mode of 0.4 mL/min. Injection (1.0 μL) was performed at 240 °C in split mode (1.5:4.1). The oven temperature was maintained at 140 °C for 5 min. The temperature was then increased to 240 °C at a rate of 2 °C/min (maintained for 10 min.) Hydrogen gas was generated by a hydrogen generator for FID at a flow rate of 2.0 kgf/cm2. The air flow rate for FID was 4.0 kgf/cm2 and that of the primary gas (nitrogen) was 3.0 kgf/cm2. Fatty acid species were identified using retention times of standard fatty acid methyl esters. The content of each fatty acid was calculated using GC-FID chromatograms. Obtained analytical curves were linear from 0.1 up to 50 ppm. The linearity was thereafter assessed by the linear regression equation. The LOD and LOQ were set using the S/N ratio (LOD: 3, LOQ: 10). Overall, obtained LOD values ranged from 0.10 (linolenic acid) to 0.23 ppm (oleic acid), while the range of LOQ was from 0.30 (linolenic acid) to 0.69 ppm (oleic acid).
Relationship between GE and 3-MCPDE content in foods
To determine the relationship between GE and 3-MCPDE content in food, the Pearson correlation coefficient (r) and probability p-value were calculated by using Microsoft Excel 2019 (Microsoft, Redmond, WA, USA). Mean values of GEs and 3-MCPDEs production in meat (beef, pork, and chicken) heated above 500 °C (500 °C for 5 and 10 min, 600 °C for 5 min, n = 9) and cookies heated at 170 °C, 210 °C, and 250 °C for 12 min (n = 3) were used. Cupcakes were excluded from the analysis because 3-MCPDEs were not detected.
Results
GE content in meat patties heated under various conditions
The amount of GEs in meat patties heated in a temperature-controlled oven and muffle furnace was determined using LC–MS, and calculated using a calibration curve (Table 1) based on the chromatogram of the standard solution. The meat patties heated in the oven at 250 °C are shown in Fig. 4. The weight of each meat patty after oven heating and freeze drying as well as the amount of oil extracted from the heat-treated meat are shown in Table S1. Figure 4 shows the meat patties heated using the muffle furnace at 400 °C, 500 °C, and 600 °C. The amount of GEs in each meat heated under different conditions is shown in Table 2. GEs were not detected in any beef, pork, or chicken patties heated in the oven at 250 °C or in the muffle furnace at 400 °C. However, GE formation was observed in beef patties heated at 500 °C and 600 °C for 5 min, and in pork and chicken patties heated at 500 °C for 10 min and 600 °C for 5 min using a muffle furnace. In beef and pork patties, more GEs were produced with increasing heating time and temperature. However, in chicken patties, more GEs were formed heating at 500 °C for 10 min than at 600 °C for 5 min. Comparing each GE production with its constituent fatty acid, glycidyl oleate was the most abundant in beef (500 °C for 5 min; 58.0% [17.4 ± 5.5 ng/g meat sample], 500 °C for 10 min; 44.8% [25.9 ± 4.1 ng/g meat sample], and 600 °C for 5 min; 51.6% [163.9 ± 76.7 ng/g meat sample]) and in pork patties (500 °C for 10 min; 55.4% [33.5 ± 10.1 ng/g meat sample] and 600 °C for 5 min; 47.7% [37.6 ± 6.6 ng/g meat sample]), followed by glycidyl palmitate, glycidyl stearate, and glycidyl linoleate (Table 2). In chicken patties, glycidyl oleate was also the most abundant (500 °C for 10 min; 80.0% [47.1 ± 3.9 ng/g meat sample], and 600 °C for 5 min; 53.6% [9.8 ± 2.3 ng/g meat sample]), followed by glycidyl palmitate, glycidyl linoleate, and glycidyl stearate (Table 2).
3-MCPDE content in meat patties heated under various conditions
The amount of 3-MCPDEs in meat patties heated in temperature-controlled ovens and muffle furnaces was determined using LC–MS/MS and calculated using a calibration curve (Table 1) as made for 3-MCPDEs. The amount of 3-MCPDEs in each meat heated under different conditions is shown in Table 3. 3-MCPDEs were not detected in either beef, chicken, or poultry patties heated in the oven at 250 °C. In beef patties, 3-MCPDE formation was observed only at 600 °C for 5 min whereas in pork and chicken patties they appeared at 400 °C, 500 °C, and 600 °C. The highest amount of 3-MCPDEs was produced in all types of patties by heating to 600 °C for 5 min. Comparing the most common constituent fatty acids in 3-MCPDEs, 3-MCPD dioleate was the most abundant in beef patties (600 °C for 5 min; 79.9% [141.4 ± 105.3 ng/g meat sample]), followed by 3-MCPD dipalmitate and 3-MCPD distearate (Table 3). Pork patties produced the most 3-MCPD dioleate (400 °C for 5 min; 100% [0.6 ± 1.0 ng/g meat sample], 400 °C for 10 min; 98.5% [40.0 ± 7.1 ng/g meat sample], 500 °C for 5 min; 86.7% [1.3 ± 2.0 ng/g meat sample], 500 °C for 10 min; 81.3% [1.3 ± 1.5 ng/g meat sample], 600 °C for 5 min; 77.0% [6.7 ± 3.6 ng/g meat sample]), followed by 3-MCPD dilinoleate (Table 3). In chicken patties, 3-MCPD dioleate was the most abundant when heated at 500 °C (5 min; 100% [0.08 ± 0.05 ng/g meat sample], 10 min; 100% [0.3 ± 0.1 ng/g meat sample]) and at 600 °C (5 min; 74.4% [18.6 ± 19.7 ng/g meat sample]); 3-MCPD dipalmitate was also produced when heating at 600 °C for 5 min (Table 3).
GE content in cookies and cupcakes heated under various conditions
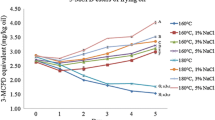
The weight of cookies and cupcakes before and after freeze drying as well as that of the extracted oil are shown in Table S2. With increasing temperature, the weight decreased for cookies and cupcakes but the weight of the extracted oil was not affected. The cookies and cupcakes heated at various temperatures are shown in Fig. 5. The amount of each GE produced in the heated cookies is shown in Table 4. GEs were detected in cookies heated at 170 °C, 210 °C, and 250 °C for 12 min; the highest concentration was detected at 210 °C. With respect to individual fatty acids, glycidyl oleate was the most frequently detected (170 °C; 64.4% % [66.3 ± 5.1 ng/g sample], 210 °C; 61.5% [111.4 ± 12.7 ng/g sample], 250 °C; 67.9% [59.5 ± 9.8 ng/g sample]), followed by glycidyl linoleate and glycidyl linolenate. Glycidyl palmitate and glycidyl stearate levels were below the detection limit (Table 4). The amount of each GE formed and the total amount in cupcakes are shown in Table 4. In cupcakes, GEs were detected at 170 and 210 °C, but not at 250 °C. With respect to individual fatty acids, glycidyl oleate was the most frequently formed (170 °C: 77.2% [126.0 ± 68.9 ng/g sample], 210 °C: 76.6% [148.0 ± 101.6 ng/g sample]), followed by glycidyl linoleate and glycidyl linolenate. Glycidyl palmitate and glycidyl stearate levels were below the detection limit (Table 4).
3-MCPDE content in cookies and cupcakes heated under various conditions
The amount of each 3-MCPDE produced and their total amount in cookies are shown in Table 5. 3-MCPDEs were produced at 170 °C, 210 °C, and 250 °C for 12 min, being especially abundant when heating at 250 °C. With respect to individual fatty acids, 3-MCPD dilinoleate (170 °C:100% [111.7 ± 16.5 ng/g sample], 210 °C:100% [137.8 ± 17.6 ng/g sample], 250 °C:96.0% [164.1 ± 14.2 ng/g sample]) was the most commonly produced at all heating temperatures; in contrast, 3-MCPD dioleate was detected only at 250 °C. In the cupcakes, 3-MCPDEs were not detected at any heating temperatures (Table 5).
Fatty acid composition of rapeseed oil and GE composition of cookies and cupcakes
The fatty acid composition of GE is directly correlated with the fatty acid composition of rapeseed oil. In this study, we also determined the fatty acid composition in rapeseed oil using GC-FID. In rapeseed oil, the fatty acid profile included: palmitic acid: 1.5%, stearic acid: 0.3%, oleic acid: 65.8%, linoleic acid: 27.8%, and linolenic acid: 4.6%; oleic acid being the most abundant (Fig. 6A). The GE composition in heated rapeseed oil is shown in Fig. 6B. With respect to individual fatty acids in GE, glycidyl oleate (unheated: 62.1%, 170 °C: 72.1%, 210 °C: 67.7%, 250 °C: 68.1%) was the most common, followed by glycidyl linoleate (unheated: 26.7%, 170 °C: 21.0%, 210 °C: 24.7%, 250 °C: 25.2%), and glycidyl linoleate (unheated: 11.2%, 170 °C: 6.9%, 210 °C: 7.5%, 250 °C: 6.8%). Glycidyl palmitate and glycidyl stearate were below the detection limit. 3-MCPDEs were not detected at any heating temperature. With respect to the percentage of each GE in cookies and cupcakes prepared with rapeseed oil, the GE ratios varied depending on heating temperature and confectionery type. In particular, in cookies heated at 250 °C, only glycidyl oleate and glycidyl linoleate were detected.
Relationship between GEs and 3-MCPDEs content in foods
As shown in Table 6, no correlation between GEs and 3-MCPDs was observed in case of cookies, but a correlation was observed for meat. The plot in Fig. 7 summarizes the amount of GEs and 3-MCPDEs in meat and cookies (n = 12). There was a correlation between the amounts of GEs and 3-MCPDEs in some foods (r = 0.781, p < 0.005).
The scatter plot of the amount of GEs and 3-MCPDEs in meat patties and cookies. Mean values of GEs and 3-MCPDEs production in meat (beef, pork, and chicken) heated above 500 °C (500 °C for 5 and 10 min, 600 °C for 5 min, n = 9) and cookies heated at 170 °C, 210 °C, and 250 °C for 12 min (n = 3) were used
Discussion
In this study, three types of meat (beef, pork, and chicken) patties were heated at various temperatures in a temperature-controlled oven or muffle furnace to determine the amounts of GEs and 3-MCPDEs produced. GEs were not formed in meat patties heated in the oven for 5 or 10 min at 250 and 400 °C. In comparison, heating at higher temperatures (500 °C or 600 °C) using a muffle furnace resulted in GE formation in all meat patties (Table 2). In beef patties, GE formation was observed only heating at 600 °C whereas in beef and pork patties, more GEs were produced heating at 600 °C than at 500 °C. In contrast, in chicken patties, more GEs were formed at 500 °C. The amount of GEs detected in meat patties heated at 600 °C in this study was about 1/2 to 1/10 of that detected in meat patties heated with charcoal [16]. In previous studies, GEs were also produced when beef and pork were gas-heated at 250 °C. However, it was postulated that the moisture in the meat patties did not evaporate easily when heated in an oven or muffle furnace, unlike the case when heated with gas or charcoal. Therefore, it is possible that the oil in the meat patties had difficulty reaching the temperature at which GE is produced.
3-MCPDEs were not produced in any of the meat patties with oven cooking (250 °C, 5 or 10 min). Previous studies confirmed the production of 3-MCPDEs in meat heated with gas-fired cooking (approximately 250 °C [16]. The highest 3-MCPDEs concentrations were detected at 600 °C, particularly in beef patties (Table 3). However, in this type of meat, no 3-MCPDE formation was observed at other heating temperatures. Therefore, there must be an optimum heating temperature for 3-MCPDE formation in each meat.
Similar to previous studies, the GEs and 3-MCPDEs detected were thought to be related to the fatty acid composition of the meat [16]. In addition, GEs tended to be produced more readily than 3-MCPDEs when meat was heated under various conditions. In normal cooking, the presence of oil may result in locally high temperatures. Therefore, the formation of GEs and 3-MCPDEs may change significantly due to localized flame exposure of the meat surface and the correspondingly local high temperature. Thus, the formation of GEs and 3-MCPDEs during meat heating may depend on the type of meat, its components, heating temperature, flame position, and oil location.
In comparison to when cookies were heated at 170 °C, the GE production increased at 210 °C but decreased at 250 °C (Table 4). 3-MCPDE formation in heated cookies increased with rising heating temperature (Table 4). Moreover, the formation of each GE also depended on the fatty acid composition of the rapeseed oil used in the cookies, but for 3-MCPDEs, only 3-MCPD linolenic acid esters were detected. These results suggest that heating cookies at 250 °C accelerates GE decomposition, followed by induction of 3-MCPDE formation. In cupcakes, the production of GEs increased at 210 °C compared to 170 °C and was not detected at 250 °C (Table 4). Interestingly, 3-MCPDEs were detected in cookies but not in cupcakes. Cookies are flat and have a large surface area while cupcakes have a smaller area that is directly heated. This difference in heated surface area may influence the production of GEs and 3-MCPDEs. Higher calcination temperatures are thought to cause decomposition rather than formation of 3-MCPDEs [18]. For example, heating pure 3-MCPDE at 180–260 °C resulted in 30–70% degradation in 24 h [19]. Also, during the baking process, heat transfer and water evaporation can interrupt potential reactions leading to the formation of 3-MCPDE [19]. For example, an increase in the rate of moisture loss in cookies with increasing baking temperatures interrupted the hydrolysis reaction, causing degradation rather than formation of 3-MCPDEs [18]. Moreover, the low content of 3-MCPDEs in baked cakes such as cupcakes has been reported to be caused by the latent heat of vaporization, which results in lower temperatures in the center of the baked cake, as supported by the present results.
Comparing the fatty acid composition in the rapeseed oil used in cookies and cakes with the GE composition in heated cookies and cupcakes suggested that the GEs were generated from the corresponding fatty acids found in rapeseed oil. The composition ratios varied depending on heating temperature and confectionery type (Fig. 7). However, the formation of 3MCPDEs did not correspond to the fatty acid composition of rapeseed oil. Oil is hydrolyzed, polymerized, and oxidized to break down triacylglycerol to diacylglycerol (DAG) and monoacylglycerol (MAG), thus facilitating the formation of GE and 3-MCPDE under high temperature conditions [20]. Although GE and 3-MCPDE can be mutually converted, the conversion rate of MCPDE to GE has been reported to be much higher [21]. DAG, MAG, and other polar products (such as free fatty acids) may form acyloxonium ions, which react with chloride ions to form 3-MCPDE [22, 23]. Thus, high temperatures promote hydrolysis of TAG and increase 3-MCPDE content, while also contributing to degradation. In this study, 3-MCPDEs were not detected in the heated rapeseed oil, the 3-MCPDE formation in the heated cookies was probably induced by a reaction between the ingredients in the cookies and the fatty acids or by the changes of the GEs.
Since 3-MCPDE is believed to be the precursor of GE [24], there may be a correlation between the contents of GEs and 3-MCPDEs. Therefore, the correlation between GEs and 3-MCPDEs in foods (meat and cookies) was examined. There was a correlation between the amounts of GEs and 3-MCPDEs in some foods (r = 0.781, p < 0.005) (Table 6; Fig. 7). However, we observed no correlation between the content of GEs and 3-MCPDEs in cookies, suggesting that various influencing factors may be involved in the production of both substances. 3-MCPDEs possess chlorine in their structure and chloride ions are important precursors in their formation [25]. Further, we previously demonstrated that chloride ions and metal catalysts affect the formation of 3-MCPDEs [26]. Thus, the organochlorines in rapeseed oil may act as a chlorine donor during cookie baking. In addition, oily fruits, such as coconut, contain organic or inorganic chlorine compounds that decompose at temperatures > 180 °C, which then release the necessary chloride ions [27]. Since glycidol can form 3-MCPD by a ring-opening reaction with chloride ions [28], the corresponding glycidyl esters are likely to form 3-MCPDE by a similar transformation. In addition to the composition of the raw material, we consider that temperature and moisture content may be involved in the production and degradation of GEs and 3-MCPDEs. The optimal moisture content to produce 3-MCPDEs has been reported as 2–8% at certain temperatures [29]. In contrast, a low content of fat, a potential precursor in the production of GEs and 3-MCPDEs, and high calcination temperature may inhibit their production. Moreover, propolis and quinoa seed extracts have been reported as natural antioxidants in oils during frying, slowing the formation of GEs and 3-MCPDEs [30]. Thus, various ingredients, local heating, surface area, thickness, and thermal conductivity of the food, etc., may influence formation of GEs and 3-MCPDEs. Various raw materials and their composition ratios should be considered to elucidate the mechanisms leading to the formation and decomposition reactions of GEs and 3-MCPDEs.
Conclusion
In this study, we measured the amount of GEs and 3-MCPDEs produced when heating ground meat (beef, pork, and chicken) patties, cookies, and cupcakes under various conditions using a temperature-controlled device. The results demonstrated that the formation of GE and 3-MCPDE in foods are affected by food ingredients, cooking device used, and heating time, as well as the heating temperature. There are two limitations of this study. The first is that it is unclear whether GE or 3-MCPDE was meant to be produced or suppressed according to the heating conditions and the type of food. The second is that the evaluation was performed only on cookies and cupcakes prepared with one raw material composition. To address these two limitations, future research is needed to elucidate the trends in the formation and decomposition reactions of GEs and 3-MCPDEs in foods using a variety of raw materials and different composition ratios.
Data availability
The data presented in this work are available in the article.
References
R.H. Stadler, I. Blank, N. Varga, F. Robert, J. Hau, P.A. Guy, M.C. Robert, S. Riediker, Nature 419, 449 (2002). https://doi.org/10.1038/419449a
N. Muttucumaru, S.J. Powers, J.S. Elmore, A. Briddon, D.S. Mottram, N.G. Halford, Ann. Appl. Biol. 164, 286 (2014). https://doi.org/10.1111/aab.12101
Z. Yao, J. Li, B. Wu, X. Hao, Y. Yin, X. Jiang, Environ. Sci. Pollut. Res. 22, 16110 (2015). https://doi.org/10.1007/s11356-015-4837-4
K. Puangsombat, P. Gadgil, T.A. Houser, M.C. Hunt, J.S. Smith, Meat Sci. 90, 739 (2012). https://doi.org/10.1016/j.meatsci.2011.11.005
X. Sun, J. Tang, J. Wang, B.A. Rasco, K. Lai, Y. Huang, Food Chem. 172, 802 (2015). https://doi.org/10.1016/j.foodchem.2014.09.129
BfR, Initial evaluation of the assessment of levels of glycidol fatty acid esters detected in refined vegetable fats - BfR Opinion No. 007/2009 (2009)
K. Hrncirik, G. van Duijn, Eur. J. Lipid Sci. Technol. 113, 374 (2011). https://doi.org/10.1002/ejlt.201000317
S. Robjohns, R. Marshall, M. Fellows, G. Kowalczyk, Mutagenesis 18, 401 (2003). https://doi.org/10.1093/mutage/geg017
J. Sun, S. Bai, W. Bai, F. Zou, L. Zhang, Z. Su, Q. Zhang, S. Ou, Y. Huang, J. Agric. Food Chem. 41, 9955 (2013). https://doi.org/10.1021/jf400809r
F. Pudel, P. Benecke, P. Fehling, A. Freudenstein, B. Matthäus, A. Schwaf, Eur. J. Lipid Sci. Technol. 113, 368 (2011). https://doi.org/10.1002/ejlt.201000460
J. Kuhlmann, J. Eur. J. Lipid Sci. Technol. 113, 335 (2011). https://doi.org/10.1002/ejlt.201000313
Y.H. Wong, H. Muhamad, F. Abas, O.M. Lai, K.L. Nyam, C.P. Tan, Food Chem. 219, 126 (2017). https://doi.org/10.1016/j.foodchem.2016.09.130
Y.H. Wong, K.M. Goh, K.L. Nyam, L.Z. Cheong, Y. Wang, I.A. Nehdi, L. Mansour, C.P. Tan, Sci. Rep. 10, 1 (2020). https://doi.org/10.1038/s41598-020-72118-z
C. Crews, P. Brereton, A. Davies, Food Addit. Contam. 18, 271 (2001). https://doi.org/10.1080/02652030120064
S.S. Syed Putra, W.J. Basirun, A.A. Elgharbawy, M. Hayyan, W. Al Abdulmonem, A.S. Aljohani, A. Hayyan, J. Food Meas. Charact. (2023). https://doi.org/10.1007/s11694-023-01883-y
R. Inagaki, C. Hirai, Y. Shimamura, S. Masuda, J. Food Process. Technol. 7, 557 (2016). https://doi.org/10.4172/2157-7110.1000557
[AOCS/JOCS] American Oil Chemists’ Society/Japan Oil Chemists’ Society, Joint AOCS/JOCS Official Method Cd-28 (2012)
K.M. Goh, Y. Wong, F. Abas, O. Lai, L. Cheong, Y. Wang, Y. Wang, C. Tan, LWT. 116, 108553 (2019). https://doi.org/10.1016/j.crfs.2021.07.002
A. Ermacora, K. Hrncirik, Food Chem. 150, 158 (2014). https://doi.org/10.1016/j.foodchem.2013.10.063
C.G. Hamlet, P.A. Sadd, D.A. Gray, Food Res. Technol. 216, 122 (2003). https://doi.org/10.1007/s00217-002-0621-z
S. Wang, G. Liu, W. Cheng, Food Res. Int. 140, 109879 (2021). https://doi.org/10.1016/j.foodres.2020.109879
P.D. Collier, D.D.O. Cromie, A.P. Davies, J. Am. Oil Chem. Soc. 68, 785 (1991). https://doi.org/10.1007/BF02662173
B. Gao, Y. Li, G. Huang, L. Yu, Annu. Rev. Food Sci. Technol. 10, 259 (2019). https://doi.org/10.1146/annurev-food-032818-121245
R. El Ramy, M. Ould Elhkim, S. Lezmi, J.M. Poul, Food Chem. Toxicol. 45, 41 (2007). https://doi.org/10.1016/j.fct.2006.07.014
R. Weißhaar, Eur. J. Lipid Sci. Technol. 110, 671 (2008). https://doi.org/10.1002/ejlt.200800154
R. Inagaki, F. Ito, Y. Shimamura, S. Masuda, Food Addit. Contam. A 36, 236 (2019). https://doi.org/10.1080/19440049.2018.1562231
K. Nagy, L. Sandoz, B.D. Craft, F. Destaillats, Food Addit. Contam. A 28, 1492 (2011). https://doi.org/10.1080/19440049.2011.618467
A.K.K. Rahn, V.A. Yaylayan, Eur. J. Lipid Sci. Technol. 113, 323 (2011). https://doi.org/10.1002/ejlt.201000310
C.G. Hamlet, P.A. Sadd, D.A. Gray, J. Agric. Food Chem. 52, 2059 (2004). https://doi.org/10.1021/jf035077w
M.M. Ceylan, A. Baştürk, J. Food Meas. Charact. 17, 33 (2023). https://doi.org/10.1007/s11694-022-01801-8
Funding
This work was supported by JSPS KAKENHI, Grant Number 20k05894 and a grant for specially promoted research of the University of Shizuoka.
Author information
Authors and Affiliations
Contributions
Conceptualization: SM, Methodology: SM, HH; Formal analysis and investigation: YS, MM, SS, RI; Writing—original draft preparation: YS; Writing—review and editing: SM, HH; Funding acquisition: SM, HH; Supervision: SM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Although H.H. is an employee of Kao Corporation, which provided funding for this work, this study was conducted for non-profit purposes. No inappropriate data have been created based on the existence of a financial relationship with a company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimamura, Y., Miyazaki, M., Sawaki, S. et al. Formation of glycidol fatty acid esters and 3-monochloro-1,2-propanediol fatty acid esters in heated foods. Food Measure 18, 2268–2279 (2024). https://doi.org/10.1007/s11694-023-02301-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02301-z