Abstract
Black seed oil (BSO) or Nigella sativa contains numerous bioactive components, in which thymoquinone (TQ) becomes a functional key compound and serves as an analytical marker. With its non-polar characteristic, lipid-based delivery systems can be a solution to improve the solubility of BSO. Thus, it is necessary to have a valid basis for TQ quantification, especially in the emulsion as the delivery system of BSO. This study aimed to validate a Gas Chromatography-Flame Ionization Detector (GC-FID) method for quantifying TQ, and subsequently employ the method to evaluate TQ extraction from BSO and BSO-containing emulsion and determine the entrapment efficiency of the emulsion. The method indicated a linearity between 10 and 1280 µg/ml with detection and quantification limits of 13,59 and 41,19 µg/ml, respectively. The extraction of TQ with the sample:methanol ratio of 1:9 and 30-min centrifugation yielded the maximum TQ recovery from BSO and BSO-containing emulsion. The entrapment efficiency in emulsion reached 64,83% obtained with the density-changing centrifugation method. Overall, this validated GC-FID method demonstrated that the selected condition in the methanol extraction could effectively separate TQ from both BSO and its emulsion matrices and support the determination of the entrapment efficiency of the BSO-containing emulsion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential oils extracted from plants and spices bear several biologically active compounds. In recent years, essential oils have emerged as potential candidates for use in agricultural, pharmaceutical, and food applications. Nigella sativa is an annual herbaceous flowering plant from the Ranunculaceae family spread in Southern Europe, Northern Africa, and Southern Asia. The seeds of N. sativa are known as black seeds or black cumin (English) and habbatussawda (Arabic). Black seed oil (BSO) contains many natural components that have remarkable health benefits, namely thymoquinone, thymol, carvacrol, and p-cymene; fatty acids such as linoleic acid and oleic acid [1]; tocopherols; and sterols [2].
Thymoquinone (TQ) is the main bioactive of black seed essential oil. It has the IUPAC name 2-methyl-5-propane-2-yl-cyclohexane-2,5-diene-1, 4-dione phenol, and a molecular formula C10H12O2. Previous studies have shown many functional effects of TQ, including antimicrobials [3], immunomodulatory [4], and antioxidants [5]. Kiralan [6] reports that, although its quantity is less than p-Cymene, TQ remains more stable at 60 and 100 °C. Therefore, TQ is regarded as a functional key component [7] and serves as an analytical marker of commercially available BSO. Several solvents, including supercritical CO2, benzene, hexane, ethanol, and methanol, have successfully been used to extract thymoquinone. However, a methanol extraction has been widely applied to extract the TQ from BSO for analytical purposes [8].
On the other hand, there have been some chromatography-based methods utilized for the determination of TQ concentration, including high-performance thin layer chromatography (HPTLC), high-performance liquid chromatography (HPLC), stable isotope dilution gas chromatography-mass spectroscopy (SID-GC–MS) technique, liquid chromatography coupled with electron spray ionization and tandem time of flight mass spectrometer (LC-ESI-TOF) [9,10,11,12]. Despite their superiority in determining TQ, most of these approaches need expensive apparatus, extensive sample preparation, lengthy analysis durations, and skilled personnel. Furthermore, an essential aspect of conducting a successful analysis utilizing chromatographic methods is preparing the sample, which primarily involves the extraction process. The analyte of interest is separated from the sample matrix during the extraction with an optimal yield and selectivity [13]. Sample preparation is frequently a significant source of error since, for instance, improper extraction can yield flawed separation, and the obtained results may not accurately represent the true value of the desired components. Therefore, it is crucial to select the most appropriate and effective extraction method to accurately quantify the analyte within the matrix while minimizing the extraction of other soluble interferences into the extraction phase.
Since BSO is a non-polar compound, it encounters difficulties being added to food systems in an aqueous or polar environment. Lipid-based delivery systems can enhance the solubility and bioavailability of poorly water-soluble and lipophilic bioactive components delivered orally [14]. Some efforts have been made to deliver TQ into the polar system environment, such as emulsion [15], nanoparticles [16], nanoemulsion [17], and nanostructured lipid carriers [18]. Because of the advantageous health properties of BSO and the valuable physical characteristics exhibited by BSO carrier systems, they have the potential to be employed in the food and cosmetic industries either independently or in conjunction with other vegetable oils [15]. Entrapment efficiency is a crucial parameter for the evaluation of nanocarriers since it describes the systems’ ability to entrap the bioactive components inside the lipid phase [19]. The higher the entrapment efficiency, the more bioactive components can be brought.
Nevertheless, it lacks information regarding the evaluation of TQ extraction from its native matrices and its delivery systems. This study validated a simple and efficient analysis method for TQ quantification using a gas chromatography-flame ionization detector (GC-FID) with diethyl phthalate (DEP) as an internal standard. DEP is an inexpensive synthetic plasticizer commercially available with high purity. The validated GC-FID method was subsequently employed to measure the quantity of TQ in BSO and BSO-containing emulsions. To accomplish this quantification, a methanol extraction technique was selected to separate TQ from both matrices and assessed for its effectiveness. Furthermore, the determination of entrapment efficiency was carried out to support the characterization of TQ delivery systems.
Materials and methods
Materials
TQ reference standard was purchased from TCI (Tokyo, Japan), diethyl phthalate as an internal standard (IS), methanol with gradient grade for liquid chromatography LiChrosolv® Reag. Ph Eur., and trisodium citrate were purchased from Merck (Darmstadt, Germany), Tween 80 was purchased from Roth (Karlsruhe, Germany), rapeseed oil was purchased from Bellasan, and black seed oils were obtained from local producers from Indonesia, Austria, and Bosnia and Herzegovina.
Instrumentation
A Thermo Scientific FOCUS gas chromatography system equipped with a flame ionization detector was employed for method validation. All system parameters are shown in Table 1.
Sample preparation
TQ stock solutions were prepared with the proper amount of TQ dissolved in methanol from 12,80 mg/ml to 0,01 mg/ml by serial dilution. IS solution was prepared using 120 µg/ml DEP in methanol. For calibration, as much as 0,1 ml of TQ stock solutions were diluted to 1 ml with IS solution. The calibration curve was plotted to TQ concentration in stock solution against % area of TQ/% area of DEP. All calibration samples were analyzed in triplicate. For accuracy and precision assessment, serial TQ concentrations of 4, 6, and 8 mg/ml were prepared by spiking TQ into the rapeseed oil. Furthermore, as much as 0,1 ml of TQ-spiked rapeseed oils were diluted to 1 ml with IS solution.
Emulsion preparation
An emulsion was prepared with the composition of BSO as a dispersed phase (2,75 g), Tween 80 as a surfactant (2,75 g), and deionized water as an aqueous phase (44,5 g), with a total emulsion volume was 50 g. Emulsion preparation followed [20] with slight modification, in which an Ultra Turrax® homogenizer (T-25 Basic, Janke and Kunkel IKA, Germany) with an S25KV-25 F model stem was used at a speed of 18,000 rpm for 10 min.
Method validation
A guideline from the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use was used to validate analytical procedures [21]. The GC-FID method was validated in terms of specificity, linearity, the limit of detection (LoD) and quantification (LoQ), accuracy, precision, and robustness.
Evaluation of extraction efficiency
A calibration curve was built to evaluate the extraction efficiency of TQ from BSO. The BSO was spiked with the TQ standard to produce the TQ-added concentrations from 0,5 mg/ml to 4 mg/ml. Extraction was conducted by dissolving the sample to methanol containing IS, mixing with vortex for 2 min, and proceeding with centrifugation using Eppendorf Centrifuge 5424. A two-factorial experiment with two levels was done, including the sample:solvent ratio (1:9 and 1:14) and centrifuge duration (20 and 30 min). Responses were obtained using a simple regression analysis from the calibration curve slope involving the calculated concentration of TQ-spiked BSO as the vertical axis and theoretical TQ concentration as the horizontal axis. The best extraction produced a linear regression curve with a slope close to 1, which meant the recovery was near 100%. Hence, a TQ standard with a 100%-recovery assumption (slope = 1) was made to compare the slope of each of the treatments with a perfect extraction. The best extraction procedure was selected to quantify the TQ from BSO and BSO-containing emulsion. For the TQ quantification in emulsion, the concentration of TQ obtained was converted to the initial TQ in the BSO required to make the emulsion.
Entrapment efficiency
Entrapment efficiency (EE) of TQ-loaded nanoparticles was measured by three different methods: common centrifugation, ethanol-centrifugation, and density-changing centrifugation. The common centrifugation and ethanol-centrifugation methods were modified from the ultracentrifugation method conducted by Rathore [22]. As much as 5 ml emulsion (for common centrifugation), or 1 ml emulsion, which was diluted to 5 ml with ethanol (for ethanol-centrifugation), was centrifuged for 30 min at 12.000 rpm, followed by filtration through Whatman filter paper of 0,45 μm size filter. On the other hand, the density-changing centrifugation method followed [23] with slight modifications in the speed and temperature of centrifugation. The prepared emulsion was mixed with trisodium citrate (TC) solution until the concentration reached 10% (m/v). Afterward, the mixtures were centrifuged at room temperature at 12.000 rpm for 30 min to separate free drugs from the emulsion. After centrifugation, the TQ-loaded nanoparticles floated to the uppermost layer, and the free TQ precipitated to the bottom or remained in the middle liquid layer. Subsequently, the middle liquid layer was extracted carefully by a syringe and mixed with IS-containing methanol. The amount of TQ present from each method was then analyzed using the validated GC-FID method. %EE of TQ was calculated using Eq. 1.
wherein, T is the total amount of drug present both in the supernatant and sediment, and C is the amount of drug detected only in the supernatant.
Data analysis
The test results were graphed and statistically analyzed using Statgraphics Centurion 19 and Microsoft® Excel® LTSC MSO. All the sample tests were taken triplicate and expressed as mean ± standard deviation. The significant level of the extraction scopes from the calibration curve was calculated by ANOVA, at P < 0.05 being considered a statistically significant difference.
Results and discussion
Specificity and linearity
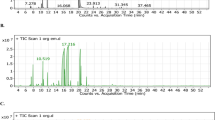
After examining the peak purities of reference TQ and IS solution with GC-FID, both TQ and IS exhibited excellent separation, and no interfering peaks were found. Figure 1(A) and 1(B) show the chromatogram of the TQ reference dissolved in IS solution and the BSO sample in IS solution, respectively.
Furthermore, a linear relationship was obtained across the studied concentration range of 10-1280 µg/ml (Fig. 2). The equation of the calibration curve graphic was utilized to generate the calculation formula in accuracy and precision testing to quantify TQ in the rapeseed oil samples, which:
Limit of detection and limit of quantification
The limit of detection (LoD) and limit of quantification (LoQ) are used to evaluate the sensitivity of a method. LoD refers to the smallest amount of analyte in a sample that can be detected but not necessarily quantified under the conditions of the experiment. LoQ, on the other hand, indicates the smallest amount of an analyte that can be quantitatively determined with sufficient precision and accuracy. LoD and LoQ were calculated based on the standard deviation of the response (σ) and the slope (S) of the linearity curve (Fig. 2), in which LoD = 3.3σ/S and LoQ = 10σ/S [21]. LoD and LoQ of this GC-FID method were determined as 13,59 µg/mL and 41,19 µg/mL, respectively.
Accuracy and precision
The accuracy of an analytical procedure is the closeness of test results obtained by that procedure to the true value. Response of the three levels (67%, 100%, and 133%) of TQ samples spiked in rapeseed oil in triplicate yielded a mean recovery of 99,60% with an RSD (n = 3) of 0,92% (Table 2).
Meanwhile, the precision of an analytical procedure measures how consistent the test results are when the procedure is regularly performed on multiple samplings of a homogenous sample. The precision of the method in this study was assessed in terms of repeatability and intermediate precision. Repeatability pertains to utilizing the analytical procedure within a laboratory over a brief timeframe, involving the same analyst and equipment. Intermediate precision, on the other hand, refers to the within-laboratory variation observed on different days, with different analysts, or using different equipment within the same laboratory [21]. Table 3 illustrates the repeatability and intermediate precision of the GC-FID method employed in this study.
Robustness
The method’s robustness was examined by slightly adjusting the operational parameters, which were flow rate and split ratio, one at a time. Table 4 provides a summary of the variance in peak area percentages.
The recovery discrepancies of the TQ identified in the spiked rapeseed oil utilizing each of the adjusted GC conditions of flow rate and split ratio, as well as the procedural GC settings, were within 3% of those found for the procedural conditions. The observed variations in retention time for TQ and IS were also minimal. These findings indicated that even with minor intentional changes to the chromatographic conditions, there was no significant impact on the area and retention time. The method demonstrated robustness, as indicated by the low % RSD values (less than 3).
Evaluation of thymoquinone extraction efficiency from black seed oil
Among the various bioactive compounds found in BSO, TQ is known to be highly abundant [Hameed, Rathore], making it a suitable analytical marker for the oil. In previous studies, different solvents have been employed to extract TQ [8], with Chung et al. [24] reported that methanol performed better TQ extraction ability than hexane. In this case, methanol was selected as the extraction solvent. The validated GC-FID method was applied to examine the effect of the centrifugation duration and the amount of solvent in TQ extraction from BSO, which is summarized in Fig. 3; Table 5.
Different concentrations of TQ spiked in BSO were applied to observe whether the extraction calibration curve exhibited a linear relationship. The results indicated that the calibration curves of different amounts of solvent and duration were linear, as demonstrated by the high coefficient determination. However, when comparing the slopes, there were only two treatments, which were a BSO:methanol ratio of 1:9 for 30-min centrifugation and a BSO:methanol ratio of 1:14 for 30-min centrifugation, showing no significant difference (P < 0,05) from TQ standard extraction with a perfect recovery assumption (slope = 1). The extraction yield is proportional to the time the solute and solvent were in contact with each other. The longer the contact time, the greater the mass transfer in the system. However, TQ compounds in the extracts might oxidize or degrade when exposed to heat, air, and light during extraction [25]. Considering the implementation of green extraction that requires the least solvent involved as well as the cost for the solvents, extraction using BSO:methanol ratio 1:9 for 30-min centrifugation was selected for TQ extraction conditions from BSO and BSO-containing emulsion.
Quantification of thymoquinone from commercial black seed oils
Table 6 shows the TQ quantities of cold-pressed extracted BSO obtained from local producers in Indonesia, Austria, and Bosnia and Herzegovina, analyzed using the validated GC-FID method with the specified extraction setting.
The concentrations of TQ in the BSO samples ranged from 1,34 to 14,88 mg/ml. Demirbolat et al. [26] reported that cold-pressed BSO contains 5,49 − 9,63 mg/ml, while TQ content in the commercial BSO products in Malaysia is reported by Alkhatib et al. [27] ranging from 0.07 to 1.88% (w/w). According to previous studies, BSO with cold-pressed extraction contains TQ at a lower level than BSO with supercritical CO2 extraction, which might be caused by the extraction performance and the possibility of TQ losses during the extraction [10, 26, 28].
Evaluation of thymoquinone extraction efficiency from black seed oil-containing emulsion
Methanol extraction of TQ from BSO-containing emulsion was set with emulsion:methanol ratio 1:9 and 30-min centrifugation, checked with the validated GC-FID method. Figure 4; Table 7 display the calibration curve of TQ in serial concentrations from the 5,5% BSO-containing emulsion and the evaluation of the extraction slope, respectively.
TQ has limited solubility in aqueous systems [29, 30]; hence, it is entrapped in the emulsion’s oil phase. However, an emulsion system with poor entrapment efficiency, which may be caused by droplet particle size, will have some free TQ and nanocarriers of TQ in the aqueous phase [19]. Therefore, the extraction of TQ from the BSO-containing emulsion will require the solvent’s ability to break the emulsion and extract TQ from the oil and water phases afterward.
The calibration curve of TQ in BSO-containing emulsion indicated a linear relationship with a high coefficient of determination. The selected extraction condition also yielded a slope that was significantly different from the TQ standard with a 100%-recovery assumption (P < 0,05). However, it was still considered to yield a lofty recovery that reached 97,36%. It indicated that the selected methanol extraction process could be applied not only in BSO but also in BSO-containing emulsion, with regards to the high recovery of TQ. The addition of alcohol with low-molecular-weight, such as methanol, in a high concentration can lead to destabilization of the emulsion due to its ability to interfere with the hydrogen bonding of the aqueous phase and the surfactants, lower the surface tension between the surfactant’s molecules, as well as increase degree of disassociation of the anionic surfactants that will develop separation behavior of the emulsion [31,32,33]. In addition, methanol is an excellent solvent for extracting the terpenoids, such as TQ [34, 35], and it will effectively take out the TQ in both the oil and water phase. Muzolf-Panek and Gliszczyńska-Świgło [36] reported that extracting Nigella sativa seeds using methanol with optimized conditions yielded the highest phenolic content and antioxidant activity with regards to the extracted TQ. In other studies [37], acetonitrile was employed as a different solvent to break down TQ-nanoliposomes prior to quantifying the TQ amount. The introduction of acetonitrile molecules increased the variability of lipid membranes, ultimately causing destabilization within the liposomes [38].
Determination of entrapment efficiency of the emulsion
EE is one of the quality parameters for nanocarrier systems. The entrapment efficiency can be calculated using the direct or indirect method. The indirect method estimated the unentrapped or free amount in the supernatant after centrifugation. On the contrary, in the direct method, nanocarriers were solubilized in a suitable solvent and analyzed after filtration and suitable dilution to estimate entrapment efficiency by a suitable technique [39, 40]. In this work, standard centrifugation was performed with three different treatments. In the first method, only centrifugation was applied; in the second method, ethanol was utilized to break the emulsion; while in the third method, trisodium citrate was added to increase the density of the aqueous phase. Table 8 displays the determination of EE following the determination of TQ concentration in the part of the emulsion phases.
An emulsion containing 5,5% BSO produced using the Ultra Turrax® method had 61,70%; 5,28%; and 64,83% entrapment efficiency, which meant that the remaining 38,30%; 94,72%; and 35,17% TQ were unentrapped by the oil phase and laid in the liquid phase, determined using a common centrifuge, centrifugation-ethanol, and density-changing centrifugation method, respectively. EE is greatly affected by composition adding order, essential oil type, concentration, drying process type, extraction solvent type, and particle size of the dispersed phase [41, 42]. Standard centrifugation separates free drugs from particulates by using the differences in weight, density, or viscosity [43, 44]. Centrifugation is also recognized as a viable technique for extracting chia mucilage from its seeds [45]. However, the density of nanoparticles is very close to the density of the aqueous medium, resulting in inadequate separation and a waste of time [19, 46]. Nanoparticles also exhibit varied motion patterns in distinct separation setups. Fundamentally, the manner in which particles move within liquid environments is influenced not only by centrifugal force but also by factors such as particle density, size, and shape, as well as the density and viscosity of the liquid medium. Elements like gravity and intermolecular force can be disregarded in this context [47]. Adding TC will sharply increase the density of the aqueous phase; hence, the particulates turn out to be significantly lighter than the liquid. In this condition, the particulates and free drugs would experience distinct outcomes after the centrifugation: the lighter particulates floated to the topside of the centrifuge tube, while the free drugs remained in the liquid or precipitated in the bottom [22]. As observed, TQ content in the supernatant fraction was lower when processed with density-changing centrifugation than the common centrifugation. Additionally, Li et al. [48] succeeded in optimizing a density-changing ultracentrifugation system with the introduction of cyclohexane/carbon tetrachloride (CCl4) and found the best separation parameters for the polydispersed cadmium stearate nanoparticles at 51,012 rpm of centrifugal rotational speed, 1.65 h of centrifugal time within a 5∼60% density range. On the contrary, the small EE obtained from the ethanol-centrifugation method could be caused by the ability of ethanol to extract TQ from both matrices in the emulsion. Iqbal et al. [49] reported that ethanol could serve as a suitable extractor for TQ from the seeds of N. sativa. Thus, the number might represent the total TQ in the emulsion instead of the supernatant fraction. Consequently, based on the finding of this study, this ethanol-centrifugation method was not applicable to determine the EE in BSO-containing emulsion.
Conclusions
A validated GC-FID method successfully evaluated that the methanol extraction could be applied to separate TQ from BSO and its emulsion, using a combination of sample:methanol ratio of 1:9 and 30 min centrifugation to yield maximum TQ recovery. Thus, the findings obtained in this study can function as a valid basis for further TQ quantification, especially in the delivery systems of BSO. The entrapment efficiency of the BSO-containing emulsion was 64,83%, obtained with density-changing centrifugation.
References
A.H. Rizvi, M.M.A.A. Khan, G. Saxena, A.A. Naqvi, J. Biol. Chem. Res. 29(1), 44–51 (2012)
B. Matthaus, M. Özcan, M. Czech, J. Food Sci. 29(2), 145–150 (2012)
S.A.M. Mahgoub, A. Osman, M.F. Ramadan, J. Food Meas. Charact. (2017). https://doi.org/10.1007/s11694-017-9587-1
M.L. Salem, Int. Immunopharmacol. (2005). https://doi.org/10.1016/j.intimp.2005.06.008
M. Soleimanifar, R. Niazmand, S.M. Jafari, J. Food Meas. Charact. (2019). https://doi.org/10.1007/s11694-018-9953-7
M. Kiralan, Int. J. Food Prop. (2014). https://doi.org/10.1080/10942912.2012.723231
S. Hameed, A. Imran, M. Nisa, M.S. Arshad, F. Saeed, M.U. Arshad, M.A. Khan, M.A. Int. J. Food Prop. (2019). https://doi.org/10.1080/10942912.2019.1599390
A. Yalçin, M. Gönen, in Chemical Science and Engineering Technology, 1st edn, ed. By D. Balköse, A. C. F. Ribeiro, A. K. Haghi, S. C. Ameta, T. Chakraborty (Apple Academic Press, Boca Raton, 2019), p. 133
R.M. Velho-Pereira, C.R. Barhate, S.R. Kulkarni, A. G. Jagtap. Phytochem. Anal. (2011). https://doi.org/10.1002/jssc.202200991
H. Lutterodt, M. Luther, M. Slavin, J. Yin, J. Parry, J. Gao, L Yu. LWT. (2010). https://doi.org/10.1016/j.lwt.2010.04.009
O.R. Johnson-Ajinwo, W.W. Li, J. Agric. Food Chem. (2014). https://doi.org/10.1021/jf500357x
M.A. Farag, H.A. Gad, A.G. Heiss, L. A. Wessjohann. Food Chem. (2014). https://doi.org/10.1016/j.foodchem.2013.11.032
F. Falaki, in Gas Chromatography-Derivatization, Sample Preparation, Application Sample Preparation Techniques for Gas Chromatography, ed. By P. Kusch (IntechOpen, 2019), p.1–26, https://doi.org/10.5772/intechopen.84259
C.M. O’Driscoll, B.T. Griffin, Adv. Drug Deliv Rev. (2008). https://doi.org/10.1016/j.addr.2007.10.012
M. Varlı, R. Solak, S. Turan, M.F. Ramadan, J. Food Meas. Charact. (2021). https://doi.org/10.1007/s11694-021-00900-2
I. Rahat, S. S. Imam. Drug Deliv. (2021). https://doi.org/10.1080/10717544.2021.1927245
M.F. Radwan, M.A. El-Moselhy, W.M. Alarif, M. Orif, N.K. Alruwaili, N. A. Alhakamy. Dose-Response. (2021). https://doi.org/10.1177/15593258211013655
F.F. Qizilbash, M.U. Ashhar, A. Zafar, Z. Qamar, J. Annu, S. Ali, M.M. Baboota, S. Ghoneim, A. Alshehri, Ali, Pharmaceutics (2022) https://doi.org/10.3390/pharmaceutics14030656
Y. Lv, H. He, J. Qi, Y. Lu, W. Zhao, X. Dong, W. Wu, W Int. J. Pharm. (2018). https://doi.org/10.1016/j.ijpharm.2018.06.025
M.A. Cabrera-Trujillo, A. Filomena-Ambrosio, M.X. Quintanilla-Carvajal, L.I. Sotelo-Díaz, Int. J. Gastron Food Sci. (2018). https://doi.org/10.1016/j.ijgfs.2018.06.002
International Council of Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Q2 (R1); Validation of Analytical Procedures: Text and methodology. International Conference on Harmonization, Geneva, Switzerland, (2005)
C. Rathore, N.K. Upadhyay, A. Sharma, U.R. Lal, K. Raza, P. Negi, J. Drug Deliv Sci. Technol. (2019). https://doi.org/10.1016/j.jddst.2019.04.041
L. Han, H. Zhan, X. Sun, Z.R. Zhang, L. Deng, AAPS J. (2019). https://doi.org/10.1208/s12248-019-0306-1
K.X. Chung, P.L.Y. Wie, G.A. Akowuah, Indian J. Nat. Prod. Resour. (2023). https://doi.org/10.56042/ijnpr.v14i1.1134
S. Machmudah, Y. Shiramizu, M. Goto, M. Sasaki, T. Hirose. Sep. Sci. Technol. (2005). https://doi.org/10.1081/SS-200053005
I. Demirbolat, M. Kartal, Ã. Karık., J. Res. Pharm. (2019). https://doi.org/10.12991/JRP.2019.157
H. Alkhatib, S.M. Mawazi, S.M.A. Al-Mahmood, A. Zaiter, A.A. Doolaanea, J. Pharm. Bioallied Sci. (2020). https://doi.org/10.4103/jpbs.JPBS_208_20
R. Salea, E. Widjojokusumo, A.W. Hartanti, B. Veriansyah, R.R. Tjandrawinata, Biochem. Compd. (2013). https://doi.org/10.7243/2052-9341-1-1
J.M. Salmani, S. Asghar, H. Lv, J. Zhou Molecules. (2014). https://doi.org/10.3390/molecules19055925
A. Ahmad, M. Raish, K.M. Alkharfy, I.A. Alsarra, A. Khan, A. Ahad, B.L. Jan, F. Shakeel. J. Mol. Liq. (2018). https://doi.org/10.1016/j.molliq.2018.10.104
E. Dickinson, M. Golding, J. Colloid Interface Sci. (1998). https://doi.org/10.1006/jcis.1997.5221
U. Vainio, R.A. Lauten, S. Haas, K. Svedström, L.S. Veiga, A. Hoell, R. Serimaa. Langmuir. (2012). https://doi.org/10.1021/la204479d
J. Ruwoldt, G. Øye., ACS Omega. (2020). https://doi.org/10.1021/acsomega.0c04650
S.J. Mohammed, H.H.H. Amin, S.B. Aziz, A.M. Sha, S. Hassan, J.M.A. Aziz, H.S. Rahman, Evid. Based Complement. Alternat Med. (2019). https://doi.org/10.1155/2019/6515671
N. Habib, S. Choudhry, Evid. Based Complement. Alternat Med. (2021). https://doi.org/10.1155/2021/6645680
M. Muzolf-Panek, A. Gliszczyńska-Świgło, J. Food Meas. Charact. (2022). https://doi.org/10.1007/s11694-022-01575-z
F. Odeh, S.I. Ismail, R. Abu-Dahab, I.S. Mahmoud, A. Al-Bawab. Drug Deliv. (2018). https://doi.org/10.3109/10717544.2012.727500
K. Yoshida, R. Mitsumori, K. Horii, A. Takashima, I. Nishio. Colloids Interfaces. (2018). https://doi.org/10.3390/colloids2010006
F.D. Cui, K. Shi, L. Zhang, A.J. Tao, Y J. Kawashima J. Control. (2006). https://doi.org/10.1016/j.jconrel.2006.05.013
V.L. Gaikwad, P.B. Choudhari, N.M. Bhatia, M.S. Bhatia, Nanomaterials for Drug Delivery and Therapy, ed. by A.M. By, Grumezescu (Elsevier, 2019), pp. 33–58. https://doi.org/10.1016/B978-0-12-816505-8.00016-3
K.S. Shalaby, M.E. Soliman, L. Casettari, G. Bonacucina, M. Cespi, G.F. Palmieri, O.A. Sammour, A. A. El Shamy. Int. J. Nanomed. (2014). https://doi.org/10.2147/IJN.S68737
A.C.R. Neto, A.B.O. da Rocha, M. Maraschin, R.M. di Piero, E. Almenar. Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2017.10.029
X. Sun, S.M. Tabakman, W.S. Seo, L. Zhang, G. Zhang, S. Sherlock, L. Bai, H. Dai. Angew. Chem. Int. Ed. Eng. (2009). https://doi.org/10.1002/anie.200805047
P. Qiu, C. Mao, Adv. Mater. (2011). https://doi.org/10.1002/adma.201102636
R.S. Zamora, A. Baldelli, A. Pratap-Singh, Food Res. Int. (2023). https://doi.org/10.1016/j.foodres.2023.112466
N. Ohnishi, S. Tanaka, K. Tahara, H. Takeuchi, Int. J. Pharm. (2015). https://doi.org/10.1016/j.ijpharm.2014.12.056
Q. Zhang, X. Sun, Nanoseparation Using Density Gradient Ultracentrifugation, ed. by X. By, L. Sun, Y. Luo, P. Kuang, Li (Springer, 2018), pp. 37–58. https://doi.org/10.1007/978-981-10-5190-6_3
P. Li, J. Huang, L. Luo, Y. Kuang, X. Sun. Anal. Chem. (2016). https://doi.org/10.1021/acs.analchem.6b01092
M.S. Iqbal, A. Ahmad, B. Pandey, Physiol. Mol. Biol. Plants. (2018). https://doi.org/10.1007/s12298-018-0593-5
Acknowledgements
The authors would like to acknowledge the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia and Österreichs Agentur für Bildung und Internationalisierung (OeAD) for supporting the study through the Indonesia-Austria Scholarship Program (No. 1488/E4/KD.02.01/2021). Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU) as well as technical assistance provided by Iris Biedermann and Nicole Schamberger are duly appreciated.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Contributions
Aulia Ardhi: conceptualization, GC-FID methodology and validation, formal analysis and investigation, writing-original draft preparation and editing. Matthias Schreiner: conceptualization, resources, method evaluation, writing-review and editing, and supervision.
Corresponding author
Ethics declarations
Compliance with ethical standards
This publication does not contain any human or animal experimental data and features only experiments without human participants or animal subjects.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ardhi, A., Schreiner, M. Evaluation of extraction and entrapment efficiency of black seed oil-containing emulsion as a delivery system for thymoquinone. Food Measure 18, 393–401 (2024). https://doi.org/10.1007/s11694-023-02185-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02185-z