Abstract
Aflatoxins are considered a severe hazard, contaminate dietary products, and cause malignant alterations in liver tissues. Fermented milk (FM) is prepared using probiotic lactic acid strains. This investigation aimed to produce an integrated milk beverage, inactivating aflatoxins toxicity and biotransformation. The proximate analysis of the investigated materials and biochemical parameter changes of the in-vivo experiment were determined. Results reflected the extract’s valuable content of polysaccharides and antioxidants. Nine phenolics were identified predominantly with catechin (39.67 ± 1.5 µg/g). FM-fortification is reflected by enhancement in protein (49.5 ± 2.97 g/Kg) and fiber content (1.78 ± 0.54 g/Kg) compared to the FM content. Relative rats’ weight gain improved to 34.29% for the fortified-FM group close to the control; it was recorded at 16.47% for the AFM1 group. Alkaline phosphatase in AFM1 rats was 99.2 ± 1.86 U/L and decreased to 44.2 ± 0.71 U/L in the fortified-FM group (44.2 ± 0.71 U/L) to be close to the control group. Aflatoxin M1 rats exposure reflects tissue alterations and cell damage, which recorded lesser in rats treated by extract and beverage administrations. The beverage’s corrective action relied on two integrated mechanisms, aflatoxin-binding to bacterial and bioactivity interaction of extract substances. This beverage stopped tissue alterations that occurred due to aflatoxins. The result supports the future production of fortified-milk beverages as a bio-shield against aflatoxin toxicity, besides their nutritional and functional properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dairy products have benefits for human health, including nutrition function. It may consume fresh or fermented forms of routine food [1]. The probiotic strains of lactic acid bacteria (LAB) are utilized as a starter to output the fermentation process. Numerous bioactive and organic acids can be produced during the fermentation process, which has been linked to functional effects in the body [2, 3]. Numerous LAB strains adhere to the intestinal tissue [4, 5], which assists their biogenic immunological function. The LAB growth in biosystems reported more effectiveness in prebiotic components, providing essential requirements for enhancing bacterial growth. Some plant extracts and algae can provide nutritional support through their application in the probiotic media growth and act as prebiotic sources. Amphora extract (AE) can support LAB activity and bio functionality. It contains various active phytochemicals and molecules, including phenolic, polysaccharides, vitamins, and antioxidants [6, 7]. The presence of starter strains, which are considered probiotics in the beverage, can enhance their safety due to their activity reported to reduce mycotoxin content [8, 9].
Mycotoxins are a hidden enemy that represents an excellent food hazard, act in shadow, and is not discovered by eye inspection [10, 11]. It causes damage to liver tissues and un-balanced biochemical enzymes in the biological systems [12]. Human diets, particularly dairy products, suffer from aflatoxins (AFs) contamination. It happens directly by aflatoxin M1 (AFM1) or by aflatoxin B1 (AFB1) as indirect contamination [13]. Bioactive molecules were utilized to reduce aflatoxins by bacterial metabolites [14] and phytochemicals of natural extracts [15]. There is a current tendency toward bioactive natural products in various industries, such as pharmaceuticals, cosmeceuticals, and food. This tendency has emphasized expanding marine scientific research, mainly macro and micro-algae. Among other algae molecules, oligo and polysaccharides comprise a biochemical compound with evidence proving to possess significant characteristics, including antioxidant, anti-inflammatory, immune-modulatory ability, antitumor, and cancer preventive [16, 17].
Moreover, other molecules like monounsaturated fatty acids and phenolic compounds represent a source of protective shield against several hazards [18, 19]. The oligosaccharides content of algae characterizes it as a promising bioactive source with a wide implementation as the prebiotic-molecules source. Amphora alga, a better example, contains bioactive compounds, including monounsaturated fatty acids and exopolysaccharides. The synbiotic, formed by prebiotics and probiotics, achieved several benefits in the gastrointestinal tract (GIT), including limiting potential pathogens [20]. Moreover, spirulina and amphora algae were reported to have an antibiotic impact against harmful bacteria in the chicken intestinal tract [21]. The physiological responses and biochemical parameters in treated chickens were enhanced compared to the non-treated ones. Also, amphora alga was implemented in the aquaculture industry against the microbial pathogen of Vibro bacteria instead of the antibiotic application [22]. The result valorizes its effect on aquaculture survival as a natural alternative to chemical antibiotics.
The current study aimed to provide a compatible system of probiotics (starter strains) and prebiotic amphora extract (AE) in a dietary product. Fermented milk (FM) will provide an excellent synbiotic system due to its fortifying with amphora extract. Also, its capacity for mycotoxins detoxification will increase. This system may support the gastrointestinal tract’s immunity against the hazards of aflatoxin contamination. The changes in the liver and kidney tissues due to the aflatoxins effect will be investigated in the presence or absence of the A.E. oral administration.
Materials and methods
Materials
Amphora algae (A. cofeaeformis) biomass was obtained from the Algae Biotechnology Unit, National research, Dokki, Giza, Egypt. Fresh cow milk samples from healthy lactating animals were obtained from a local farm in Giza, Egypt.
Bacterial strains were utilized to demonstrate the anti-pathogenic effect of the algae extract. Bacillus cereus ATCC 10,876, Salmonella typhi ATCC 14,028, Pseudomonas aeruginosa ATCC 9027, Escherichia coli ATTC 8739 were used as bacterial strains cultured and reactivated on tryptic soy agar. Four toxigenic fungi strains of Aspergillus flavus ITEM 698, Aspergillus ochraceus ITEM 5010, Fusarium culmorum KF846, and Fusarium graminearum KF 841 were applied to evaluate the antimycotoxigenic effect. The strains were cultured and reactivated on potato dextrose agar (Difco ™), and the impact was calculated as the inhibition zone diameter of fungal growth.
Methods
Preparation of prebiotic extract
Amphora algae biomass was dried using the hot-air oven (Binder oven, Germany; 40 °C/ 16 h); milled using M.T.I.- Lab Roll mill (M.T.I. Company, U.S.A.). The pressed warm-water extract was prepared (45℃) according to the modified method described by Abdel-Salam et al. (2018). The dried material was extracted using pressing warm distilled water. Afterward, it was mixed homogeneously using a Homogenizer (Cole-Parmer LabGEN 700). The suspension was left (22 °C/ 1 h), filtrated through a muslin cloth (50% cotton/50% polyester), then through Whatman filter paper (No.1), centrifuged (2200 Xg /15 min. /5°C) using a BioSan Centrifuge, (Model LMC-4200R). The cleared extract was packed in amber bottles (1 L) and was refrigerated at 0 °C (Kirsch laboratory refrigerator LABEX 520, ProfiLab24 GmbH, Berlin, Germany). Later, it was lyophilized (− 55 °C/ 24 h) using freeze-drying (Alpha 1–4 LSC basic-Christ Lab freeze dryer, Martin Christ GmbH, Germany).
Determination of the AE characteristics
The extract’s total phenolic (TP) and flavonoid contents were determined, as methodologies described by Shehata et al.[23].The fractions of phenolic and flavonoids were analyzed according to the methodology and measuring condition characterized by Stuper-Szablewska et al.[24]. The analysis was performed utilizing an Acquity H class UPLC system equipped with the Waters Acquity PDA detector (Waters, U.S.A.). Chromatographic separation was accomplished using the Acquity UPLC® BEH C18 column (100 × 2.1 mm, 1.7 μm) (Waters, Ireland).
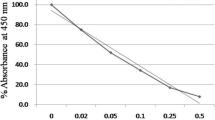
The scavenging activity of samples was estimated by the DPPH radical (1,1-diphenyl-2-picrylhydrazyl) according to the procedure described by Shimada et al. [25]. Briefly, The DPPH was mixed with the AE, the FM, FFM, and standard antioxidant solutions. The mixture was kept darkly (23 °C/30 min), and the absorbance was measured at 517 nm using a spectrophotometer against the blank. A standard curve is calculated using the Trolox solution by the following equation:
Where;
A.A.: antioxidant activity.
As: the absorbance for sample.
Ac: the absorbance for control.
Also, the ABTS-radical scavenging was determined using the 2, 2’-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid assay according to Re et al.[26], with modifications. The ABTS radical solution was prepared by re-acting seven mM ABTS in the presence of 2.5 mM (NH4)2S2O8 at a ratio of 2:1 (v/v). The mixture was kept darkly (25 °C/ 16 h); then was diluted by phosphate buffer saline (PBS; pH 7.4). Each sample’s aliquot (100µL) was mixed with 2.5 mL ABTS solution, and the absorbance change was recorded at a wavelength of 740 nm. The following equation calculates the inhibition percentage:
Where;
A blank: the absorbance for control.
A sample: absorbance value for the sample.
The antioxidant capacity was quantified as micrograms of the Trolox equivalents (µg TE) per milliliter using a standard curve of 6 points.
The functional groups included in the AE lyophilized material were analyzed using absorption spectroscopy in the infrared range (4000 − 400 cm− 1) at 4 cm− 1 resolution [27]. A Bruker IFS48 spectrometer (Bruker, the UK) was used to obtain the FTIR spectra. The FTIR spectrometer was purged to decrease ambient carbon dioxide and water-vapor spectrum contributions. The average of four spectra from different pellets of the same sample was then calculated.
Determination of the antibacterial and antifungal effect
The antimicrobial and antifungal impacts were calculated as an inhibition zone diameter (mm) for applied pathogenic bacteria and toxigenic fungi strains; determined as inhibition zone diameter. The more zone inhibition, the more strain-sensitive for algae extract. The test procedures were done according to the methodology described by Abdel Razek [28]. Each well of testing Petri dishes (6 mm) was filled with 0.5 mg lyophilized extract dissolved in 0.5 mL of dimethyl sulfoxide.
Preparation of fermented milk
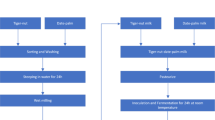
The pasteurized milk was inoculated with the starter cultures of probiotic bacterial strains to produce the FM and FFM beverages. A total volume of 5 L of the FM was prepared using starter cultures containing L. rhamnosus strain ATCC 53,013 and Streptococcus thermophiles ATCC 19,258 (lyophilized strains, Valio Ltd., Helsinki, Finland). Each strain was firstly sub-cultured in a TPP-culture tube containing MRS broth (50 mL; 18 h/37°C). Culture media were centrifuged (3000 g), where the formed pellets were washed with PBS buffer (100 Mmol/L, pH 6.6). Pellets were resolved in 10 mL of pasteurized milk, re-incubated under the same conditions, then injected into a bioreactor containing 5 L of pasteurized milk. Another fermenter was utilized for the FFM production using 2 g lyophilized AE/ L of milk. The temperature, incubation time, and rotation speed of the 7-L BioNet® Bioreactor were set at 38 °C/3 h/ 180 RPM. The final product was packed in 0.3 L sterilized bottles and cooled at refrigeration (2° C).
Determination of the features of the investigated materials
The pH value of the FM, FFM, and A.E. solution was determined using JANEWAY portable device (3510 Bench pH Meter, the UK). The titratable acidity was determined by titrating homogenized FM (10 mL) with (0.10 N) NaOH (Merck, Munchen, Germany) to the phenolphthalein endpoint. The titratable acidity of FM and FFM are expressed as a lactic acid percentage with a methodology described by Saljooghi et al. [29]. While the viscosity was determined using a digital rotary Viscometer (model C.A.P. 2000 Brookfield; the U.K.), with spindle probe number 2 at 30 RPM. The samples were kept at 4 ± 0.5 ºC and were immediately handed homogenized before the analysis [30].
Animals’ experiment
Male albino rats weighing 180.7 ± 2.15 (Mean ± SD) were procured from the National Research Centre’s Animal House in Cairo, Egypt. The rats were housed in the controlled housing unit, maintained at a stable temperature and humidity levels. The rats were fed a baseline meal during the trial according to the American Institute of Nutrition Rodent Diets (AIN-93) recommendations and given free access to water [31]. The balanced basal diet fed to the experiment rats included 21.6% casein-supplemented protein, 15% corn oil, 58.4% maize starch, 4% salt combination, and 1% vitamin mixture. The oil-soluble vitamins were given to rats separately from their meal on a weekly basis.
The experiment design
The experimental rats were treated in conformity according to the Ethical Committee of the national research centre (NRC), Dokki, Egypt, and according to the instruction of the European Union Directive for animal use in scientific research [32] during the experiment (35 days). The AFM1 was dissolved in phosphate buffer saline (pH = 7.3) before administrating to rats as a body-fluid-like solution. Rats were randomly allotted into seven groups (n = 7 rats/group); all groups were fed on a basal diet besides treatments. The aflatoxin dose was calculated according to the body weight (BW).
Group I
Rats fed a basal diet; none treated group (C-).
Group II
Rats were administrated by AFM1 (80 µg/kg BW; C + group).
Group III
Rats orally administered with 2 mL FM.
Group IV
Rats administered with 20 mg A.E./kg BW.
Group V
Rats administered with the FFM (2mL FM contained 20 mg A.E./ kg BW).
Group VI
Rats were administered AFM1 (80 µg/kg BW) followed by 20 mg AE/ kg BW.
Group VII
Rats were administered with AFM1 (80 µg/kg BW), followed by 2mL FFM.
Blood samples for serum were collected by retro-orbital puncture using blood capillary tubes. Blood samples were centrifuged at 1200 Xg/ 5 min/ 5 °C. The Sera were harvested and were transferred carefully into clean tubes for analysis. At the end of the experiment period, the rats were fasting overnight and, anesthetized with an anesthesia solution, sacrificed.
Red blood cells (RBCs), Hemoglobin (HG), hematocrit (Hct), Platelet count (Plt), and Total iron-binding capacity (TICB) were all included in the analysis of the hematological parameters, which were determined by an automated blood analyzer [33]. In separated sera, urea and creatinine, as kidney function indicators, were determined by the methodology of Larsen [34]. Total cholesterol was measured due to Watson’s procedure [35]. At the same time, the triglycerides were evaluated by McGowan et al. [36]. Aspartate transaminase (AST) and alanine transaminase (ALT) activities were determined by the method described by Reitman and Frankel [37]. The malondialdehyde (MDA) activity also was determined [38], catalase activity [39], superoxide dismutase (SOD) activity [40], and finally, the glutathione-S-transferase (GST) activity [41].
After blood sampling, rats were dissected, where the liver, kidneys, and pancreas were separated from each rat, weighed, and immersed immediately in a 10% formalin solution for histopathological examination. The current study was performed according to the Ethics Committee, National Research Centre, Cairo, Egypt, and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85 − 23, revised 1985).
Histopathological examination
The liver and renal samples were fixed in saline for 24 h. The samples were cleaned with xylene (10%), dehydrated with ascending concentrations of aqueous ethanol grades, and embedded in paraffin wax (melting point 55–60 °C). The sections were cut using a Leica microtome (5 μm), then de-paraffined in xylene, rehydrated, and washed with a decreasing series of aqueous ethanol. Hematoxylin and eosin-stained slides were mounted into D.P.X., covered, and scanned with a camera (Leica, Germany) attached to a computer under a light microscope [42].
Statistical analysis
The SPSS software (version 16) was utilized for analyzing the results statistically. One-way ANOVA analyzed the obtained data at a significance level of P = 0.05.
Results
The proximate analysis, bioactivity, and mineral contents of applied materials
Amphora chemical composition analysis reflects a valuable content of protein and crude fibers, which support the F.M. by the fortification process. The results manifested the raising of the FFM’s protein, carbohydrate, fiber, and fat content compared to the F.M. (Table 1). According to the analysis results, high phenolic and flavonoid content was determined in the A.E., which assists its antioxidant potency. The antioxidant activity determined by the assays recorded more power of the analyzed extract of amphora than the FM (Table 1). The richness of these molecules in amphora extract supports its bioactivity by its existence in a biological system. High ash content recorded in amphora has macro-elements (Ca, Mg, K, P) besides microelements (Fe, Zn). The phenolic fractions are recorded by nine molecules, where the dominant is the catechin (39.67 ± 1.5 µg/g), followed by the P-coumaric (37.73 ± 1.82 µg/g). Gallic, protocatechuic, caffeic, cinnamic, chlorogenic, P-hydroxybenzoic, and Kaempferol are other phenolic chemicals in the extract. The data represented by the proximate analysis reflect tiny changes that may not be clear for the nutritional values, but their in-vivo impacts could be more effective. The FTIR chart of the AE extract reflects the wealth content of the active groups, such as the O-H stretching, N-H stretching, N = C = O stretching, S-CΞN stretching, N = C = S C = O, and C-O stretching. These addressed the intramolecular bonds with alcohols, amines, thiocyanate, isothiocyanate, carboxylic acid, conjugated bonds, and acid halide conjugated bonds. These active groups can explain behave of extract bioactivity.
The antimicrobial impact of the A.E. was evaluated against bacterial and fungal strains. The extract inhibition represents more antifungal than antibacterial against Fusarium fungi (Fig. 1). Applied strains of fungi are classified as toxigenic fungi that can produce mycotoxins. The inhibition effect of the fungal growth conjugates to the amount of toxins secreted in the media.
Characterization of the FM and FFM
In comparing the FM and FFM pH values, the results reflected a slight increment in the FFM pH value. The pH value of the FM is 4.61, while it is recorded at 4.12 for the FFM. The titratable acidity of the F.M. was measured at 1.05%, and it is recorded at 1.2% for the FFM. The data outlined in Fig 2 represented the viscosity values of the FM and FFM during the storage time. The viscosity values of the FFM samples showed fewer values than the FM samples. The viscosity values rising for the FFM during the storage were recorded in fewer values in contrast to the viscosity in the FM samples.
Determination of the in vivo impacts of the AE and FFM
The impact of AE and FFM inside the living system was examined using experimental rats. Firstly, the effect on the feed intake, body weight gain, relative liver weight, and relative weight gain was determined for the treated and non-treated rats with the AFM1-oral administration. The results presented in Table 2 recorded significant changes in these parameters regarding the AFM1-rats group. This group’s liver weight is linked to the prospective tumors formed due to aflatoxin. Meanwhile, the treatment of AE and FM was reflected in non-significant changes in the investigated parameters. The A.E., FM, or their mix, utilized to cure AFM1 toxicity in rats, ameliorates the normality recording of these parameters, particularly for the relative liver weight. The amelioration impact was less evident in the AFM1-rats group, where only fermented milk was present.
The complete blood picture for treated rats was recorded in Table 3. The results reflected positive changes using the AE, FM, and FFM for the administrated AFM1-rats group. The AFM1 showed significant differences for evaluated parameters of platelets, hemoglobin, hematocrits, iron capacity, and red and white blood cells. The impact of the AE was non-significant for hemoglobin and red or white blood cells, both if it was applied lonely or with the FM. The administration of the FFM showed an ability to cure the inflammation indicators resulting from the oral administration of AFM1 (as a decreased number of white blood cells).
Regarding the serum enzymes, liver and kidney functions were estimated through the changes in the ALT, AST, urea, and creatinine indicators (Table 4). The rat groups that the AE or FM administrated possessed a safe impact on the liver and kidney function parameters. While in the AFM1-rats, fundamental changes were recorded in the liver and kidney parameters due to the treatment. These changes were eased into the normal, where the FM, AE, and FFM treated the AFM1-rats. The amelioration is arranged ascendingly in their powerful impact as the FM < AE < FFM.
These administrations (AE or FM) improved the AFM1-treated group’s recorded levels to close to the control. The tissue enzymes of glutathione transferase, catalase, super oxidase dismutase, and malondialdehyde were determined (Fig. 3).The value changes in treated rat groups reveal their response degree for the toxicity and the remedy. Mainly, the presence of AFM1 in rat tissue negatively affected the level of these enzymes. Non-significant differences were recorded for the AE and FM groups compared to the control.
Histopathological investigations of treated rat-groups
The liver tissues of the treated groups were investigated compared to the tissue in the control group. The tissues of the control group showed a hepatic lobules’ naturalistic architecture. The principal veins fall throughout the center of lobules, surrounded by hepatocytes. The hepatic sinusoids were also seen between strands of hepatocytes (Fig. 4-A). For the rats’ group treated by the AFM1-administration, the tissues showed congested central veins and centrilobular necrosis of the hepatocytes associated with inflammatory cell infiltration (Fig. 4-B). Besides, a portal-lymphocytic infiltration was recorded, and acute tubular necrosis with slight cirrhosis by a clear vision and dilated and congested blood sinusoids were noticed clearly. Moreover, the tissues appeared to have congestion for the portal vessels with necrosis of the hepatocytes, which surrounded the portal areas.
A) Basic control, where the natural architecture of the hepatic lobule is shown in rat liver; B) positive control, where the rat has damaged cell wall, large nucleus, and hepatocytes with centrilobular necrosis; C) liver of the treated rat was given only fermented milk (FM) shows the normal architecture of the liver tissue; D) liver of the treated rat was given only the extract (20 mg/Kg BW) shows the central vein is surrounded by a normal hepatic lobule and is encircled by hepatocyte cords; E) liver of treated rat was given fortified fermented milk, shows a normal architecture of hepatic lobule, and the central vein lies at the down-right corner; F) liver of treated rat was given the extract (20 mg/Kg BW) after exposure to a dose of 80 µg aflatoxin M1/ Kg BW, where the the hepatic lobule architecture showed a little bit changes, and some nuclus were free and immigrated to the central vein; G) liver of treated rat was given fortified fermented milk after exposure to a dose of 80 µg aflatoxin M1/ Kg BW, where the tissue shows the architecture of hepatic lobule appeared more or less like normal
The rats-tissue of the FM group manifested naturalistic architecture for the hepatic lobules (Fig. 4-C). Otherwise, the sections in a few AE-rats tissues were shown a slight disturbance in a typical architecture of hepatic lobule hydropic degeneration and micro and macro-vesicular changes in the hepatocytes (Fig. 4-D). For rats given the AE plus AFM1 administration, the liver sections displayed a hepatic lobule architecture nearly normal (Fig. 4-E). Micro and macro vacuoles of fatty change were shown in a few rats, besides a tenuous occurrence for the hepatocytes-necrosis foci and congested blood sinusoids. The FM-AFM1 rats’ group displayed inconsiderably disturbance for the hepatic lobule hydropic degeneration neural architecture and micro and macro-vesicular changes in the hepatocytes. Besides, scarcely dilated and congested blood sinusoids were observed (Fig. 4-F). Finally, the FFM-AFM1 rats-group inspection of the tissues manifested the architecture of the hepatic lobule that appeared approximately as in control (Fig. 4-G). The neural architecture of hepatic lobules was associated with no changes for the micro and macro-vesicular in the hepatocytes.
The modern approaches to food processing look forward to integrated nutritional food products. In the synbiotic system, beneficial strains of bacteria were growing in the presence of some growth catalysts. This system provides several health benefits, particularly against inflammations, tumors, and diseases [43]. Algae are considered the best source of prebiotics with a low-cost value. The growth rate of algae is relatively fast and could be considered a significant source of bioactive compounds, mainly phenolic compounds and pigments.
Recent attention has been paid to the protective effects of natural antioxidants toward chemically induced oxidative stress, mainly if free radicals form. The present study represents the prebiotic source (AE) with real valuable phenolics and antioxidants, besides its antimicrobial and antifungal impacts. The manufactured FM possessed health benefits as an outlet of its content of the two probiotic strains. Producing FM was ameliorated by fortifying the AE sourced from the algae biomass. The AFM1 was previously reported to have a malignant impact on the tissue organs and destroyed the metabolic balance in life systems [13]. The AFM1 occurs directly in milk products as a metabolite of another mycotoxin. Thus, it can be presented in dairy diets directly or indirectly through cross-contamination.
Several strains of lactic acid bacteria were reported to detoxify the aflatoxin and ochratoxin contamination. An extended contact between bacterial strain and mycotoxins positively affected the detoxification ratio [44]. The existence of probiotic strains within prebiotic media was known to increase mycotoxins’ detoxification activity [45, 46]. Various mechanisms, including cell-binding complexation, enzyme transformation, and LAB-metabolites degradation, explained the mycotoxin detoxification by the LAB. This degradation could be linked to organic acids, phenolic compounds, fatty acids, bacterial antibiotics, and bioactive peptides [47]. As a fermented milk type, yogurt was recently reported to reduce aflatoxin contamination from manufactured milk [48].
Furthermore, the extract contents of bioactive molecules also support bacterial detoxification potency. The phenolic and antioxidant phenolics suppressed the aflatoxin mutagenesis by concurrent administration to the cell [49, 50]. Several phenolics were registered to correct the alteration resulting from mycotoxin toxicity in the experimental animals [51,52,53]. The FFM components contained phenolic (from AE-fortification) and probiotic strains (as a starter) compounds that improved aflatoxin breakdown (by binding and degradation mechanisms). The strain of L. rhamnosus could reduce aflatoxin toxicity through the binding mechanism [54]. Aflatoxins within the FFM will be trapped, resulting in limited toxin bioavailability. In this sense, completing these combined processes will result in the inactivation of aflatoxin toxicity.
The results can be attributed to amphora extract’s beneficial impact on fermented strain functioning, revealing the FFM effectiveness against aflatoxin toxicity. Prebiotics encapsulating beneficial bacterial strains can play this role and increase bacterial efficiency [55]. Again, the presence of bioactive prebiotics in bacterial media supports strain functionality and survival. Implementing amphora, as a prebiotic source, into fermented milk can support the starter strains with various beneficial minor components [56]. These minor components will play numerous roles, including supporting starter survival, enhancing cell wall binding, and protecting bacterial cells [57].
The in-vivo impacts of the AE, FM, and FFM were studied on rat tissues, where the results represent safety properties for their consumption. Moreover, their administrations in toxic rat groups treated by the AFM1 reflect an amelioration against the negative effect of the toxin on tissues. This effect could be explained by the influences of probiotic bacteria [48, 53], besides bioactive components that included antioxidants [58] and phenolic compounds. The impact of phenolics was demonstrated through their capability to suppress the adduct formation between the DNA. and aflatoxin [59]. The AE bioactives emphasized through the enhancement shown in tissues. The influence is displayed principally as necrosis’s disappearance in tissues and reset of the neutral architecture for the hepatic liver lobule. The recorded bioactive components were more varied in the FFM than in the AE or FM treatment. This variation is linked to the content of probiotic bacteria and prebiotic extract. Also, this link demonstrates the FFM amelioration impact against the AFM1 toxicity compared to the individual administration of the AE or the FM. These results may support the hypothesis of improving the bacteria’s ability to reduce mycotoxins when presented alongside the prebiotics. This impact can occur through their consumption in fortified fermented milk or their oral administration, as they play a vital role in the in-Vivo reduction of mycotoxins.
Conclusion
Aflatoxins are a class of pre-carcinogens that cause various harm to living tissues. The synbiotic system of probiotics and prebiotics provides several health advantages when the FM is fortified with amphora extract and can reduce aflatoxin toxicity. The AE was high in bioactive compounds that serve as starting fermentation prebiotics. The manufactured FFM is high in phenolics, antioxidants, and flavonoids. The physiological features of the FFM were found to be non-significantly different from the control. The results regarding in-Vetro amphora bioactivity reflect the valorized antimicrobial impact of the extract. In the rat trial, FFM was more effective than AE and FM in reducing the carcinogenic effects of AFM1. The FFM administration to AFM-rats ameliorates several parameters, including body weight gain and complete blood picture, and fixes the changes recorded in the serum parameters. Also, tissue enzymes of treated AFM-rats were turned close to the control values after being administrated by the FFM. The FFM’s protective benefits were observed in healing the alterations in tissues caused by aflatoxin poisoning, restoring the natural levels of estimated tissue enzymes, and enhancing the devastated tissues’ ability to regenerate.
References
J. Buttriss, Nutritional properties of fermented milk products. Int. J. Dairy. Technol. 50(1), 21–27 (1997). https://doi.org/10.1111/j.1471-0307.1997.tb01731.x
C. Stanton, R.P. Ross, G.F. Fitzgerald, D.V. Sinderen, Fermented functional foods based on probiotics and their biogenic metabolites. Cur. Opi. Biotechnol., 2005. 16(2): 198–203. https://doi.org/10.1016/j.copbio.2005.02.008
J.P. Tamang, D.-H. Shin, S.-J. Jung, S.-W. Chae, Functional Properties of Microorganisms in Fermented Foods. Frontiers microbiol., 2016. 7: p. 578–578. https://doi.org/10.3389/fmicb.2016.00578
S. Chomwong, W. Charoensapsri, P. Amparyup, A. Tassanakajon, Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannamei. Dev. Comp. Immunol. 89, 54–65 (2018). https://doi.org/10.1016/j.dci.2018.08.002
M. Juntunen, P.V. Kirjavainen, A.C. Ouwehand, S.J. Salminen, E. Isolauri, Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin. Diagn. Lab. Immunol. 8(2), 293–296 (2001). https://doi.org/10.1128/CDLI.8.2.293-296.2001
de J. Raposo, M.F.,A.M. de Morais, R.S. de Morais, Emergent sources of Prebiotics: seaweeds and Microalgae. Mar. Drugs 14(2), 27 (2016). https://doi.org/10.3390/md14020027
L. O’Sullivan, B. Murphy, P. McLoughlin, P. Duggan, P.G. Lawlor, H. Hughes, G.E. Gardiner, Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs, 2010. 8(7): 2038–2064. https://doi.org/10.3390/md8072038
F.A. Sadiq, B. Yan, F. Tian, J. Zhao, H. Zhang, W. Chen, Lactic acid Bacteria as antifungal and anti-mycotoxigenic agents: a Comprehensive Review. Compre Rev. Food Sci. Food Safety 18(5), 1403–1436 (2019). https://doi.org/10.1111/1541-4337.12481
S. Ahlberg, P. Kärki, M. Kolmonen, H. Korhonen, V. Joutsjoki, Aflatoxin M1 binding by lactic acid bacteria in milk. World Mycotoxin Journal 12(4), 379–386 (2019). https://doi.org/10.3920/WMJ2019.2461
A.G. Abdel-Razek, A.N. Badr, M.G. Shehata, Characterization of olive oil By-products: antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Ann. Res. Rev. Biol. 14(5), 1–14 (2017). https://doi.org/10.9734/ARRB/2017/35065
A.N. Badr, A.F. Logrieco, H.A. Amra, T. Hussein, Ochratoxin a occurrence on egyptian wheat during seasons (2009–2014). Asian J. Scientific Res. 10(3), 178–185 (2017). https://doi.org/10.3923/ajsr.2017.178.185
M.S. Shahat, A.N. Badr, A.I. Hegaziy, S. Ramzy, M.A. Samie, Reducing the histopathological and biochemical toxicity of aflatoxins contaminated soybean using ozone treatment. Ann. Res. Rev. Biol., 2017. 15(3). https://doi.org/10.9734/ARRB/2017/35075
S. Marchese, A. Polo, A. Ariano, S. Velotto, S. Costantini, L. Severino, Aflatoxin B1 and M1: Biological properties and their involvement in cancer development. Toxins 10(6), 214 (2018). https://doi.org/10.3390/toxins10060214
M.G. Shehata, A.N. Badr, S.A. El Sohaimy, D. Asker, T.S. Awad, Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Annals Agri. Sci. 64(1), 71–78 (2019). https://doi.org/10.1016/j.aoas.2019.05.002
R. Makhuvele, K. Naidu, S. Gbashi, V.C. Thipe, O.A. Adebo, P.B. Njobeh, The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon, 2020. 6(10): p. e05291. https://doi.org/10.1016/j.heliyon.2020.e05291
H. Tan, W. Chen, Q. Liu, G. Yang, K. Li, Pectin oligosaccharides ameliorate Colon cancer by regulating oxidative stress- and inflammation-activated signaling pathways. Frontiers in immunology, 2018. 9: p. 1504–1504. https://doi.org/10.3389/fimmu.2018.01504
S.K. Singdevsachan, P. Auroshree, J. Mishra, B. Baliyarsingh, K. Tayung, H. Thatoi, Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: a review. Bioactive Carbohydr. Diet. Fibre 7(1), 1–14 (2016). https://doi.org/10.1016/j.bcdf.2015.11.001
A.G. Abdel-Razek, A.N. Badr, T.M. El-Messery, M.M. El-Said, A.M.S. Hussein, Micro-nano encapsulation of black seed oil ameliorate its characteristics and its mycotoxin inhibition. Bioscience Res. 15(3), 2591–2601 (2018)
A.G. Abdel-Razek, M.G. Shehata, A.N. Badr, K. Gromadzka, L. Stępień, The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J Chem 62, 47–61 (2019). https://doi.org/10.21608/EJCHEM.2019.15895.1967
K. Śliżewska, P. Markowiak-Kopeć, A. Żbikowski, P. Szeleszczuk, The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 10(1), 4281 (2020). https://doi.org/10.1038/s41598-020-61256-z
E.A. Alwaleed, M. El-Sheekh, M.M. Abdel-Daim, H. Saber, Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens. Environ. Sci. Pollut Res. Int. 28(2), 1801–1811 (2021 Jan). https://doi.org/10.1007/s11356-020-10597-3. Epub 2020 Aug 28. PMID: 32857306
N.A. Rosland, N. Ikhsan, C.C. Min et al., Influence of Symbiotic Probiont strains on the growth of Amphora and Chlorella and its potential protections against Vibrio spp. in Artemia. Curr. Microbiol. 78, 3901–3912 (2021). https://doi.org/10.1007/s00284-021-02642-2
M.G. Shehata, F.T. Ahmad, A.N. Badr, S.H. Masry, S.A. El-Sohaimy, Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Annals of Agricultural Sciences 65(2), 209–217 (2020). https://doi.org/10.1016/j.aoas.2020.12.001
K. Stuper-Szablewska, D. Kurasiak-Popowska, J. Nawracała, J. Perkowski, Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol. 33(10), 949–962 (2017). https://doi.org/10.1080/02757540.2017.1381689
K. Shimada, K. Fujikawa, K. Yahara, T.J.J.o.a. Nakamura, and f. chemistry, antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. 1992. 40(6): p. 945–948
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med., 1999. 26(9): 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
N.M. Vicentini, N. Dupuy, M. Leitzelman, M.P. Cereda, P.J.A. Sobral, Prediction of Cassava Starch Edible Film Properties by Chemometric Analysis of Infrared Spectra. Spectrosc. Lett. 38(6), 749–767 (2005). https://doi.org/10.1080/00387010500316080
A.G. Abdel-Razek, M.G. Shehata, A.N. Badr, K. Gromadzka, L. Stępień, The effect of chemical composition of wild Opuntia ficus indica byproducts on its nutritional quality, antioxidant and antifungal efficacy. Egypt. J. Chem. 62, 47–61 (2019). https://doi.org/10.21608/EJCHEM.2019.15895.1967
S. Saljooghi, L. Mansouri-Najand, H. Ebrahimnejad, F. Doostan, N. Askari, Microbiological, biochemical and organoleptic properties of fermented-probiotic drink produced from camel milk. Veterinary Res. Forum 8(4), 313–317 (2017)
M.B.d.Md Almeida, J.A.G.d. Almeida, M.E.L. Moreira, F.R. Novak, Adequacy of human milk viscosity to respond to infants with dysphagia: experimental study. J. Appl. Oral Sci. 19(6), 554–559 (2011). https://doi.org/10.1590/S1678-77572011000600003
P. Reeves, F. Nielson, G. Fahmy, Reports of the American Institute of Nutrition, adhoc wiling committee on reformulation of the A.I.N. 93. Rodent Diet. J. Nutri 123, 1939–1951 (1993)
C. Directive, Directive 2010/75/E.U. of the European Parliament and of the Council. Off J. Eur. Union L 334, 17–119 (2010)
D.G. Garrett, H.C. Lee, S.-C. Wong, B. Roemer, S.M. Edmondson Jr., Method for determination of nucleated red blood cells and leukocytes in a whole blood sample in an automated hematology analyzer. 2010, Google Patents
K. Larsen, Creatinine assay by a reaction-kinetic approach. Clin. Chem. Acta 41, 209–217 (1972). https://doi.org/10.1016/0009-8981(72)90513-x
D. Watson, A simple method for the determination of serum cholesterol. Clin. Chim. Acta 5(5), 637–643 (1960). https://doi.org/10.1016/0009-8981(60)90004-8
M. McGowan, J. Artiss, D. Strandbergh, B. Zak, A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin. Chem. 29(3), 538–542 (1983)
S. Reitman, S. Frankel, Determination of glutamate-pyruvate transaminase (ALT) and aspartate aminotransfrase (AST). Clin. Pathol. 28, 56 (1957)
H. Ohkawa, W. Ohishi, K. Yagi, Determination of lipid peroxidation by M.D.A. Anal Biochem, 1979. 95: p. 351–358
R.F. Beers, I.W. Sizer, A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. chem. 195(1), 133–140 (1952)
Y. Sun, L. Oberley, Y. Li, A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34(3), 497–500 (1988)
W. Habig, M. Pabst, G. Fleischner, Z. Gatmaitan, I. Arias, W. Jakoby, The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proceedings of the National Academy of Sciences, 1974. 71(10): p. 3879–3882
J. Bancroft, A. Stevens, Theory and practice of histological techniques. 1996. Vol. 4. 1996, New York: Churchill Livingstone
K.R. Pandey, S.R. Naik, B.V. Vakil, Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 52(12), 7577–7587 (2015). https://doi.org/10.1007/s13197-015-1921-1
M.T. Fouad, M. El-Shenawy, T.A. El-Desouky, Efficiency of selected lactic acid bacteria isolated from some dairy products on aflatoxin B1and Ochratoxin A. J. Pure Appl. Microbiol. 15(1), 312–319 (2021). https://doi.org/10.22207/JPAM.15.1.24
R.A.M. Vasconcelos, D.L. Kalschne, K.F. Wochner, M.C.C. Moreira, T.A. Becker-Algeri, A.I. Centenaro, E. Colla, P.C.A. Rodrigues, D.A. Drunkler, Feasibility of L. plantarum and prebiotics on aflatoxin B1 detoxification in cow milk. Food Sci. Technol. 41, 627–632 (2020). https://doi.org/10.1590/fst.34120
K.F. Wochner, M.C.C. Moreira, D.L. Kalschne, E. Colla, D.A. Drunkler, Detoxification of aflatoxin B1 and M1 by Lactobacillus acidophilus and prebiotics in whole cow’s milk. J. Food Saf., 2019. 39(5). https://doi.org/10.1111/jfs.12670
B.J. Muhialdin, N. Saari, A.S.M. Hussin, Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules, 2020. 25(11). https://doi.org/10.3390/molecules25112655
F. Mosallaie, H. Jooyandeh, M. Hojjati, A. Fazlara, Biological reduction of aflatoxin B1 in yogurt by probiotic strains of Lactobacillus acidophilus and Lactobacillus rhamnosus. Food Sci. Biotechnol. 29(6), 793–803 (2020). https://doi.org/10.1007/s10068-019-00722-5
R.H.C. San, R.I.M. Chan, Inhibitory effect of phenolic compounds on aflatoxin B1 metabolism and induced mutagenesis. Mutat. Research/Fundamental Mol. Mech. Mutagen., 1987. 177(2): 229–239. https://doi.org/10.1016/0027-5107(87)90005-4
L.A. Shelef, B. Chin, Effect of phenolic antioxidants on the mutagenicity of aflatoxin B1. Appl. Environ. Microbiol. 40(6), 1039 (1980)
K. Langeswaran, R. Revathy, S.G. Kumar, S. Vijayaprakash, M.P. Balasubramanian, Kaempferol ameliorates aflatoxin B1 (AFB1) induced hepatocellular carcinoma through modifying metabolizing enzymes, membrane bound ATPases and mitochondrial T.C.A. cycle enzymes. Asian Pacific Journal of Tropical Biomedicine, 2012. 2(3, Supplement): p. S1653-S1659. https://doi.org/10.1016/S2221-1691(12)60471-7
Z. Abbas, R. Blank, S. Wein, S. Wolffram, Effect of quercetin on the toxicokinetics of ochratoxin A in rats. Food Addit Contam Part A Chem Anal Control Expo Risk assess, 2013. 30(5): p. 861–6. https://doi.org/10.1080/19440049.2013.793823
M. Afzaal, A.U. Khan, F. Saeed, A. Ahmed, M.H. Ahmad, A.A. Maan, T. Tufail, F.M. Anjum, Hussain, S. Functional exploration of free and encapsulated probiotic bacteria in yogurt and simulated gastrointestinal conditions. Food Sci. Nutr. 7, 3931–3940 (2019). https://doi.org/10.1002/fsn3.1254
J.C. Assaf, S. Nahle, A. Chokr, N. Louka, A. Atoui, and A. El Khoury, assorted methods for decontamination of aflatoxin M1 in milk using Microbial Adsorbents. Toxins 11(6), 304 (2019). https://doi.org/10.3390/toxins11060304
L. Escriva, M. Jose Ruiz, G. Font, L. Manyes, Effects of quercetin against mycotoxin induced cytotoxicity: a mini-review. Curr. Nutr. Food Sci. Biotechnol., 2017. 13(4): 240–246. https://doi.org/10.2174/1573401313666170725112637
F. Bovo, L.T. Franco, R.E. Rosim, and C.A.F.d. Oliveira, ability of Lactobacillus rhamnosus strain cultured in milk whey based medium to bind aflatoxin B1. Food Sci. Technol. 34(3), 566–570 (2014). https://doi.org/10.1590/1678-457x.6373
E. Alouda, H. El-Garhy, ‘molecular and microscopic identification of amphora sp. Isolated from an aquatic source in Egypt’. Annals of Agricultural Science, Moshtohor 58(1), 53–60 (2020). https://doi.org/10.21608/assjm.2020.108481
A.V. Vipin, R.K. Raksha, N.K. Kurrey, K.A. Appaiah, G. Venkateswaran, Protective effects of phenolics rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 91, 415–424 (2017). https://doi.org/10.1016/j.biopha.2017.04.107
K.W. Thomas, E.A. Patricia, T.A. Michael, B. Ernest, J.D. Groopman, Modification of aflatoxin B1 binding to D.N.A. in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis 6(5), 759–763 (1985). https://doi.org/10.1093/carcin/6.5.759
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ahmed M. Abdel-Salam: conceptualization, formal analysis, methodology, validation, visualization; roles/writing-original draft. Ahmed H. Zaghlol: Data curation, methodology, Project administration, and resources. Ahmed Noah Badr: Conceptualization, Formal analysis, Investigation, Methodology, validation, Supervision, Writing - review & editing. Abdel-Razik H. Farag: formal analysis, investigation, project administration, software, supervision, Visualization, Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest in the data represented in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badr, A.N., Abdel-Salam, A.M., Zaghloul, A.H. et al. Fortified milk-beverage with amphora algae and its functionality for aflatoxin inactivation in rats. Food Measure 17, 2340–2352 (2023). https://doi.org/10.1007/s11694-022-01778-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01778-4