Abstract
Changes in quality of fruits and vegetables during processing and storage might impact on the nutritional and economical value of food products. The present study aimed to evaluate the influence of blanching on the content of vitamin C and ellagic acid (EA) as the main bioactive compounds present in Kakadu plum (KP) fruits (Terminalia ferdinandiana) during storage at 40oC to mimic typical temperature when wild harvested. Changes in the profile of fatty acids, malondialdehyde (MDA) production, as a biomarker for lipid peroxidation, and antioxidant properties of KP fruits were evaluated. The results revealed that vitamin C decreased between 25 and 52% over the storage period. Statistically significant differences in the concentration of vitamin C were associated with temperature (p < 0.05) and blanching (p < 0.05), whereas no significant differences in EA during storage were observed. DPPH radical scavenging capacity and total phenolic content of both blanched and control samples decreased by 80% and 35%, respectively, at the end of the storage period compared to day 0. In addition, the change in DPPH activity is significantly correlated (Pearson R2 = 0.829, p ˂ 0.01) with the breakdown of ellagitannins. Furthermore, KP fruit demonstrated excellent antioxidative properties by reducing MDA production. It was concluded that blanching causes significant vitamin C loss whereas neither blanching nor long-term storage at elevated temperature affect the EA content. The results also indicate that the antioxidant compounds present in the KP fruits provided considerable protection against fatty acid oxidation during storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terminalia ferdinandiana Exell fruit, commonly known as Kakadu Plum (KP), fruit extracts demonstrated several health advantages including in vitro anti-inflammatory [1], antioxidant [2], anticancer [3], and antibacterial [4, 5] activities. A relatively high content of specific minerals, vitamins and sugars as well as ETs and EA [2, 6] have also been reported in this fruit. As a results KP fruits and derived products are used in several health and cosmetic enterprises globally [1, 2, 7]. The enterprises supply KP products in diverse forms as frozen fruit puree, freeze-dried powder, beverages, capsules, chutneys, jams and pickles [8,9,10,11] where the change in product quality is inevitable.
According to recent studies, enzymatic and non-enzymatic reactions are some of the most important bioprocesses influencing the quality attributes of food during storage [12,13,14]. Enzymes such as esterase cause the release of fatty acids [15] while polyphenol oxidase and peroxidase catalyse the oxidation of phenolic compounds associated with food browning [16], consequently affecting the overall acceptability of the food. On the other hand, rancidity, either hydrolytic or oxidative is among the nonenzymatic changes [17] which generally effects the marketability and economical value of food. Lipid oxidation is the major biochemical reaction responsible for changes in fruit quality. This process can be accelerated by extreme temperatures via inducing free-radical generation which converts non-reactive unsaturated fatty acids to secondary oxidation products [18]. Particularly malondialdehyde (MDA) is one of the most abundant aldehydes generated during autoxidative degradation of PUFA [17].
Several methods of food processing have been evaluated to preserve the quality attributes of plant-based food products [19, 20]. Blanching is the oldest and simplest technique that has been applied to maintain food quality before drying, frying and canning, and to inactivate quality-deteriorating enzymes [21]. In addition, blanching, normally carried out by boiling water immersion, steam and radiations [12, 22], has shown advantages in stabilizing nutritional quality [21, 23].
Water blanching is the most commercially popular method [21]. The treatment time and temperature of the blanching process depend on the required degree of enzyme inactivation (mostly 90% inactivation) and amount of heat transferred to the samples [23]. For instance, Kim and co-workers [23] blanched Samnamul (Aruncus dioicus var kamtschaticus) in hot water at various temperatures (80, 90 and 98 °C from 15 s to 10 min) and have reported that polyphenol oxidase and peroxidase activities were maximally inhibited by blanching for 30s at 98 °C. Menon et al. [24] and Xanthakis et al. [19] reported the “optimal” polyphenol oxidase and ascorbate oxidase inactivation in cocoa beans and frozen mangoes, respectively, when water blanched at 90 °C for 5 min.
Therefore, the present study was carried out to investigate the influence of water blanching on the vitamin C and ellagic acid (EA) contents, main bioactive compounds in KP fruits and derived products, during storage of KP fruits wild harvested from Kimberley, Western Australia. Furthermore, oxidative rancidity and changes in the fatty acid content were determined to examine if the antioxidant compounds in KP fruit provide oxidative stability during storage.
Materials and methods
Plant materials and storage experiment
The KP fruits were wild harvested in January 2020 from Bidyadanga (located 180 km south of Broome, Kimberley, Western Australia). The samples were transported to the laboratory under refrigerated conditions and then immediately stored at -80 °C for further analysis. A preliminary blanching study was conducted with total of 60 randomised fruits (20 fruits per blanching temperatures of 80 °C, 90 and 100 °C) for 5 min using a pre-heating water bath (TW20, John Morris Scientific LTD, Germany) in order to select the optimum blanching temperature. The 5 min duration was based on previous studies [19, 24] while 45 days storage was time length selected due to the fact that free radicals could cause damage to lipids with increased storage time [25].
A total of 800 fruits were randomly selected for the storage study, in which 400 fruits were subjected to blanching treatment at 90 °C for 5 min as given below (Fig. 1). The remaining fruits were control samples (no heat treatment). Both the blanched and control fruit samples were immediately frozen at -80 °C and freeze-dried under vacuum at -50 oC for 72 h, using a Scanvac Coolsafe superior freeze drier (Labgear, Brisbane, QLD, Australia). Half of the freeze-dried fruits with seeds (n = 200) in each sample group (control or blanched) were finely ground into a homogenous powder using a Retsch MM301 cryomill (Retsch GmbH, Haan, Germany). Approximately, 20–30 whole fruits and 20–25 g of freeze-dried powder from each sample group were packed in individual vacuum pouches (150 mm x 200 mm x 70 μm; Length x Height x Thickness) (Pac Plus, QLD, Australia). The pouches were sealed and the air in the pouch was completely removed using a vacuum seal C 500 Multivac Chamber machine (Multivac Sepp Haggenmüller GmbH & Co. KG, Germany). Figure 1 illustrates the flow-chart for processing the Kakadu plum fruit for the storage trial and the experimental design.
The pouches containing either the whole fruit or fruit powder samples were stored at 40 °C for 45 days. An elevated storage temperature at 40 °C was used to mimic the hot climate conditions of Northern Australia. Duplicate samples were collected at time zero before packaging (Day 0), on the 27th day (Day 27) and 45th day (Day 45) for further analysis. A total of 105 samples were collected in duplicate including heat treatment (control and blanching), powder and whole fruit, different storage times (Day 0, 27th & 45th ). Once sampled, the whole fruit stored in pouches were finely ground with the seeds using ball milling as with the powder samples prior to the determination of vitamin C, EA, TPC, DPPH radical scavenging capacity, and Thiobarbituric Acid Reactive Substances’ (TBARS).
Total phenolic content (TPC) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity
Approximately 100 mg (in triplicate) of samples was mixed with 5 ml aqueous acidified ethanol (80% ethanol, 19.8% water, 0.2% HCl) (AAE), vortexed and sonicated for 10 min. The mixture was centrifuged (3900 rpm, 5 min at 20 °C; Eppendorf Centrifuge 5810 R, Hamburg Germany) and the supernatant was collected. The extraction was repeated three times and the supernatants were combined and subjected to the TPC assay using the Folin-Ciocalteu reagent and DPPH radical scavenging capacity assay according to Moore & Yu [26] as described previously [27] without modification.
Analysis of ellagic acid (EA) and vitamin C
The extraction and analysis of EA and vitamin C were carried out using UHPLC-PDA as described previously [27] without modification.
Thiobarbituric acid reactive substances (TBARS) assay
The fatty acid oxidative product, MDA, was extracted using 80% aqueous ethanol based on Du & Bramlage [28] and Hodges et al. [29]. The analysis of TBARS was adopted from DeLong et al. [30] and Hodges and his colleagues [29]. Briefly, about 0.5 g of the samples were extracted with 10ml of 80% ethanol containing 0.01% (w/v) BHT, followed by centrifugation (3000 g, 10 min), and supernatant was collected. The extracts, water, and reagent solutions with TBA (+ TBA) or without TBA (-TBA) were mixed at the ratio of 1:4:5 (v/v/v), then incubated at 95 °C (25 min) and centrifuged at 3000 g for 10 min. The supernatant was collected for measurement of absorbance at 440, 532, and 600 nm, using a spectrophotometer (Thermo Fisher Scientific GeneSys 20, Victoria, Australia). TBARS values were reported as nanomole (nmol) malonaldehyde (MDA) equivalent /g DW ml [31]:
[(Abs532+ TBA-Abs600+ TBA) - (Abs532− TBA-Abs600− TBA)] = A (1).
[(Abs440+ TBA-Abs600+ TBA) *0.0571] = B (2).
MDA equivalents (nmol*ml− 1) = (A-B)/157,000 × 106 (3).
Where, Abs is absorbance.
Fatty acid analysis
The lipids were extracted in a process according to Srivarathan et al. [32] with slight modifications. Briefly, approximately 200 mg of the powdered sample (in triplicate) were mixed with methanol in glass test tubes and vortexed for 5 min. The tubes were sonicated for 15 min at room temperature (RT). Chloroform (CHCl3) and Milli Q water were added to the tubes at the ratio of 2:1:1 (methanol, chloroform, water; v/v/v), followed by centrifugation at 800 rpm for 5 min at RT (Eppendorf Centrifuge 5804, Eppendorf, Hamburg, Germany) and the lower layer was collected. The sample in the tubes was re-extracted with chloroform and methanol (1:1, v/v), centrifuged (800 rpm for 5 min) and the supernatant was collected and combined with the previous collected solution. Finally, the combined solution was evaporated at 45 °C under nitrogen flow using a Ratek dry heat blocker (DBH20D, Ratek Instruments Pty Ltd., Melbourne, VIC, Australia) and reconstituted in chloroform (10 mg/mL) before derivatization to fatty acid methyl esters (FAME) as previously described by Srivarathan et al. [32]. FAME analysis was conducted using A Thermo ISQ-7000™ Single quadrupole GC-MS system (Thermo Scientific, Brisbane, QLD, Australia) equipped with an Agilent DB-23 fused silica capillary column (60 m × 0.25 mm diameter; i.d, 0.15 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). A Supelco 37-component FAME mix standard (Sigma-Aldrich, NSW, Australia) was used for compound identification and quantification.
Statistical analysis
Data are expressed as mean ± SEM (standard error of mean). Differences between the samples were determined using a one-way analysis of variance (ANOVA) followed by Tukey post hoc test using IBM SPSS Statistics 25 (SPSS Inc. Chicago, IL, USA). Further correlations were obtained by Pearson correlation coefficient in bivariate correlations. p < 0.05 was considered as significant different.
Results and discussion
Changes in bioactive compounds and antioxidant capacity during storage
Vitamin C
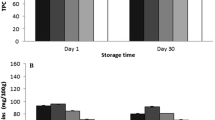
Results of a preliminary blanching experiment displayed in Fig. 2 showed that KP fruit samples blanched at 90oC retained significantly (p˂0.05) more vitamin C (7103.3 ± 56.8 mg/100 g DW) than that at 80oC (5489.4 ± 211.8 mg/100 g DW) and 100oC (5365.9 ± 42.7 mg/100 g DW). Therefore, blanching KP fruit at 90oC was selected as the heat treatment condition for the present study.
Vitamin C content was reduced by approximately 25–52% over the storage period of 45 days (Table 1). Statistically significant (p < 0.05) effect of blanching treatment was observed on the vitamin C loss, accelerated by the storage condition. Similarly, Sommano et al. [33] found a higher loss of vitamin C in KP fruit product caused by heating and high temperature and longer holding times. The almost two-fold higher vitamin C loss (50%) in the blanched samples compared to the control samples (25% loss) might be due to the plant tissue disruption during thermal treatment, which enabled the fruit to be exposed to oxidative reactions during storage at high temperature (40oC) [12, 19, 21, 34]. In agreement with our findings, Ullah and co-workers [35] also reported a reduction of vitamin C in Azadirachta indica leaves by 59 and 72% after storage for 1 and 2 months at 50oC, respectively. Similar trends in the reduction of vitamin C were also observed in strawberry fruit stored at high temperatures (40–45 0 C) compared with fruit stored at refrigerated conditions (4–6 0 C) [36].
The L-ascorbic acid (LAA) content of the studied KP fruit at the beginning of storage varied from 6 to 8% dry weight of whole fruits which is lower than that reported in previous studies of 18% [2]. However, it is still considerably higher than the LAA content found in common dietary sources of vitamin C such as orange, grapefruit, lemon, lime, kaffir lime and musk lime [34].
Ellagic acid (EA) content
The change in EA (both free and total) during storage is shown in Table 2. The difference in FEA between the three sampling times (day 0, day 27 and day 45) as well as the sample pre-treatment (blanched vs. control) was not significant (p > 0.05). A similar result of relative EA stability have been reported in previous studies [6, 37] which might be due to either the chemical thermostability of EA (melting point of 450 °C and a boiling point of 796.5 °C) [38] or the ability of endogenous ascorbic acid on preventing the oxidation of EA [6]. However, a significant (p˂0.05) difference in TEA between day 0 and the final sampling day (day 45) were observed (Table 2). This might be caused by ETs degradation/ polymerization/structural changes presumably linked to the storage temperature (40oC) or involvement in scavenging free radicals [39, 40]. Yu et al. [41] also found that punicalagin released from pomegranate leaf infusion, one of the main ETs in KP fruit [27], underwent degradation after storing at 20-25oC for one day.
Furthermore, the samples analysed in this study were vacuum sealed prior to storage. Vacuum packaging has been successfully used to prevent quality loss associated with moisture absorption and reactive oxygen metabolism during storage [42, 43]. This is probably the reason for the high amount of TEA (1800 to 2100 mg/100 g DW; Table 2) retained in the sample, comparable with previous frozen storage studies [6, 37, 44] and slow viamin C degradation because of the stress protective effect of vacuum packaging. In addition, the vitamin C, EA and ETs in KP fruits are located predominantly in the fruit flesh [2, 45] and therefore comparable to the previous results reported for the KP lyophilized fruit flesh [2, 6].
Antioxidant capacity
Significant (p < 0.05) differences in TPC and DPPH radical scavenging capacity between the storage days are shown in Table 3. However, sample pre-treatment did not affect the antioxidant capacity as both TPC and DPPH values were statistically not different (p > 0.05) between the blanched and the control samples. The DPPH radical scavenging capacity decreased significantly of almost 80% at the end of the storage period, whereas the TPC decreased only about 35% between the first and the last day of storage. A positive correlation (Pearson R2 = 0.829, p˂0.01) between the decrease in DPPH radical scavenging capacity and ETs was observed. ETs can prevent free radical damage via the hydrogen donation process [46] which might contribute to the variation of TPC and DPPH values during storage (Table 3). Previously reported studies [47, 48] support our findings of decreasing TPC and DPPH radical scavenging capacity during storage, showing decreased TPC (37–45%) and DPPH (70%) values in grapefruits, eggplant and cucumbers stored at 25-30oC for 4–10 days.
Development of rancidity during storage
Changes in fatty acids
The results presented in Fig. 3; Table 4 show the changes in total fatty acid content and fatty acid profile of the KP samples analysed. A similar total fatty acid content was observed between the samples at the beginning (1.8-2.4 mg/g DW) and at the end of the storage (2-3 mg/g DW) (Fig. 3). The main fatty acids in the samples are PUFA - linoleic acid (C18:2), monounsaturated fatty acid (MUFA) - oleic acid (C18:1), and saturated fatty acids (SFA) - palmitic acid and stearic acid. Linoleic acid (C18:2) was found at the proportion of 43–49% of the total fatty acid content at day 0 and remained at almost the same level until day 45 (43–51%). The linoleic acid, oleic acid (C18:1) (16–19% of total fatty acid content at Day 0 vs. 15–19% at day 45), palmitic acid (C16:0) (14–18% Day 0, 13–16% Day 45) and stearic acid (C18:0) (6–9% Day 0 and Day 45) proportion were also unchanged (p > 0.05) during storage. Other fatty acids that were found at relatively low proportion in KP fruit include the SFA myristic acid (C14:0), the MUFA eicosenoic acid (C20:1(n-9)) and the PUFA α-linolenic acid (C18:3(n-3)).
Total fatty acid methyl esters (FAME) concentration (mg/g DW) of KP fruit at the beginning and end of the storage trial. Values are expressed as mean ± SEM, n = 6. DW: dry weight of the fruits including the stone; a, b indicates significant differences between the samples at the beginning of the storage; x, y represents significant differences between the samples at the end of the storage trial; *, ** represents significant difference in each sample at the beginning (Day 0) and end of the storage (Day 45); the mean difference on each storage day is determined using One-Way ANOVA followed by Tukey’s Post Hoc analysis; difference in the fatty acid content of sample between days is determined using Chi-Square tests
Previous studies have indicated that the unsaturated fatty acids (UFA) are easily oxidized in many fruits during storage [17, 49]. Although the UFA constitute more than 65% of the total fatty acids in each sample (Table 4), the fatty acid content of KP did not significantly (p > 0.05) change over the storage period. These results are similar to those reported for extra virgin olive oil after 36 months of storage at 15, 22 and 37 °C [50]. It has been demonstrated that storing vegetable oil mixed with gallic acid, ellagic acid and quercetin at 50 °C decreased the oxidation process [51]. Therefore, the antioxidant compounds in KP fruit, especially ETs, EA and vitamin C, are most likely responsible for the observed “antioxidant defence” during storage. These antioxidants might also be responsible for quenching free radicals and breaking the auto-oxidative chain reaction initiated during storage [52,53,54,55].
Akter and collaborators [8] reported the fatty acid content of KP fruit kernels and found linoleic acid as the major constituent, followed by oleic acid, palmitic acid and stearic acid. These results agree with the findings in the present study. Results of this study are also in accordance with previous reports regarding the fatty acid profile of the seeds of different Terminalia species [56,57,58,59] and commonly reported for nuts, seeds and fruits of wild edible plants [56, 59]. However, differences in the fatty acid composition between the blanched and control samples were negligible, suggesting that the blanching process did not have any significant effect on the fatty acid composition.
The ratio of PUFA/SFA ≥ 0.4 is commonly used as an “health index” for food or diet rich in fatty acids [60, 61] since PUFA can help to protect type 2 diabetes [62], stroke [63], and cardiovascular diseases [64]. The PUFA/SFA ratio of each analysed KP fruits sample is ~ 2 (Table 4). This is similar to the value reported for G. dura (1.89), C. tetragonoloba (1.71) and L. albus (1.97) [65], suggesting the potential use of KP fruit as alternative source of PUFA.
TBARS
Table 5 shows the change in TBARS of the samples during storage. In the process of lipid oxidation, the quantity of MDA is expected to increase, followed by the reaction with two molecules of TBA via acid catalysed reaction [29], which in turn produces TBARS. However, the results shown in Table 6 indicate a significant (p˂0.05) reduction (~ 50%) in TBARS in the samples collected at day 45 vs. day 0. This further substantiated that the antioxidants present in KP fruit participated in preventing lipid oxidation because the degradation of ETs and vitamin C has been strongly correlated with decrease in TBARS on the 45th day (TEA Pearson R2 = 0.809, p˂0.01; TVC Pearson R2 = 0.897, p˂0.01). Similarly, Maqsood and Benjakul [66] reported preventive effects of tannic acid on lipid oxidation using the TBARS assay. Natural (poly) phenolic extracts [67] and fat diet containing EA [68] also reduced MDA production evidenced by a decrease in TBARS value.
Given the fact that MDA can be harmful for many physiological processes [69], the present results are important regarding the potential use of KP fruit (blanched or untreated) to prevent lipid oxidation.
Conclusion
This study, for the first time, revealed that blanching significantly reduced the vitamin C content of KP fruits stored at 40oC for 45 days. However, the blanching treatment did not have a significant effect on the antioxidant capacity. Neither the storage duration nor blanching has showed any effect on the FEA and fatty acid contents. These results suggest that KP fruits, stored as whole dried fruit, by Indigenous communities at a relatively high temperature of up to 40 °C in remote areas of Australia, retained a high content of EA, fatty acids and vitamin C as well as a strong antioxidant capacity.
Data Availability
The data are available from the corresponding author on reasonable request.
References
M. Chaliha, Y. Sultanbawa, Terminalia ferdinandiana, a traditional medicinal plant of Australia, alleviates hydrogen peroxide induced oxidative stress and inflammation, in vitro. J. Complement. Integr. Med. 17(1), 1–8 (2019)
I. Konczak, F. Maillot, A. Dalar, Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 151, 248–256 (2014)
J. Shalom, I.E. Cock, Terminalia ferdinandiana Exell. Fruit and Leaf Extracts Inhibit Proliferation and Induce Apoptosis in Selected Human Cancer Cell Lines. Nutr. Cancer 70(4), 579–593 (2018)
M.J. Cheesman et al., Terminalia ferdinandiana Fruit and Leaf Extracts Inhibit Methicillin-Resistant Staphylococcus aureus Growth. Planta Med. 85(16), 1253–1262 (2019)
S. Akter et al., Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods 8(8), 281 (2019)
D.J. Williams et al., Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 66, 100–106 (2014)
S. Akter et al., Antimicrobial Activity and Ellagitannins from Terminalia Ferdinandiana. Proceedings, 2019. 36(1)
S. Akter et al., Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods 7(4), 60 (2018)
I. Konczak, P. Roulle, Nutritional properties of commercially grown native Australian fruits: Lipophilic antioxidants and minerals. Food Res. Int. 44(7), 2339–2344 (2011)
Y. Sultanbawa et al., Monitoring quality and bioactivity of Kakadu plum in the Northern Territory. AgriFutures Australia Publication, 2018. No. 18/024
J.C. Brand et al., An outstanding food source of vitamin C. The Lancet 320(8303), 873 (1982)
C. Severini et al., Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J. Food Sci. Technol. 53(1), 501–510 (2016)
L.Y.W. Chua et al., Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: a Review. Food Bioprocess Technol. 12(3), 450–476 (2019)
A. Abedinia et al., Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods, 2021. 10(8)
U. Kidmose, H.J. Martens, Changes in texture, microstructure and nutritional quality of carrot slices during blanching and freezing. J. Sci. Food. Agric. 79(12), 1747–1753 (1999)
E. Garcia, D.M. Barrett, Preservative treatments for fresh-cut fruits and vegetables., Fresh-cut Fruits and Vegetables Science Technology and Market, O. Lamikanra, Editor. 2002, CRC Press: Boca Raton. pp. 267–304
K. Liu, Y. Liu, F. Chen, Effect of storage temperature on lipid oxidation and changes in nutrient contents in peanuts. Food science & nutrition, 2019. 7(7): pp. 2280–2290
R. Zhang et al., Influence of modified atmosphere treatment on post-harvest reactive oxygen metabolism of pomegranate peels. Nat. Prod. Res. 34(5), 740–744 (2020)
E. Xanthakis et al., Effect of microwave assisted blanching on the ascorbic acid oxidase inactivation and vitamin C degradation in frozen mangoes, 48 (Innovative Food Science & Emerging Technologies, 2018), pp. 248–257
E. Xanthakis, V.P. Valdramidis, Impact of heating operations on the microbial ecology of foods, in Quantitative Microbiology in Food Processing, A.S. Sant’Ana, Editor. 2017. p. 117–141
H.-W. Xiao et al., Recent developments and trends in thermal blanching – A comprehensive review. Inform. Process. Agric. 4(2), 101–127 (2017)
S. Lin, M.S. Brewer, EFFECTS OF BLANCHING METHOD ON THE QUALITY CHARACTERISTICS OF FROZEN PEAS. J. Food Qual. 28(4), 350–360 (2005)
A.N. Kim et al., Effect of water blanching on phenolic compounds, antioxidant activities, enzyme inactivation, microbial reduction, and surface structure of samnamul (Aruncus dioicus var kamtschaticus). Int. J. Food Sci. Technol. 55(4), 1754–1762 (2020)
A.S. Menon et al. Effects of water blanching on polyphenol reaction kinetics and quality of cocoa beans. in AIP Conference Proceedings. 2015
R. Domínguez et al., A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants, 2019. 8(10)
J. Moore, L. Yu, Methods for Antioxidant Capacity Estimation of Wheat and Wheat-Based Food Products, in Wheat Antioxidants, L. Yu, Editor. 2007, John Wiley & Sons, Inc., Hoboken, New Jersey. p. 118–172
E.M. Bobasa et al., Hydrolysable tannins in Terminalia ferdinandiana Exell fruit powder and comparison of their functional properties from different solvent extracts. Food Chem. 358, 129833 (2021)
Z. Du, W.J. Bramlage, Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 40(9), 1566–1570 (1992)
D.M. Hodges et al., Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207(4), 604–611 (1999)
J.M. DeLong et al., Using a Modified Ferrous Oxidation – Xylenol Orange (FOX) Assay for Detection of Lipid Hydroperoxides in Plant Tissue. J. Agric. Food Chem. 50(2), 248–254 (2002)
M. Landi, Commentary to: “Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds” by Hodges et al., Planta (1999) 207:604–611. Planta, 2017. 245(6): p. 1067–1067
S. Srivarathan et al., Tecticornia sp. (Samphire)—A Promising Underutilized Australian Indigenous Edible Halophyte. Frontiers in Nutrition, 2021. 8(11)
S. Sommano et al., The impact of thermal processing on bioactive compounds in Australian native food products (bush tomato and Kakadu plum). Food Res. Int. 50(2), 557–561 (2013)
F.R. Najwa, A. Azrina, Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods. Int. Food Res. J. 24(2), 726–733 (2017)
S. Ullah et al., Effects of temperature and storage on the antioxidant potential, polyphenols and Vitamin-C contents of azadirachta indica leave aqueous extract. Pak J. Pharm. Sci. 30(5), 1665–1669 (2017)
M.A. Murtaza et al., Studies on Stability of Strawberry Drink Stored at Different Temperatures INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY, 2004. 6(1)
D.J. Williams et al., Organic acids in Kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Res. Int. 89(Pt 1), 237–244 (2016)
S. Muthukumaran et al., Ellagic acid in strawberry (Fragaria spp.): Biological, technological, stability, and human health aspects. Food Qual. Saf. 1(4), 227–252 (2017)
K. Villalba et al., Food Ellagitannins: Structure, Metabolomic Fate, and Biological Properties. In Aires A. (ED.), Tannins - Structural Properties, Biological Properties and Current Knowledge. IntechOpen, 2020
J.M. Landete, Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 44(5), 1150–1160 (2011)
M. Yu, I. Gouvinhas, A. Barros, Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants, 2021. 10(8)
H. Brown, J. Williams, M. Kirwan, Packaged Product Quality and Shelf Life, in Food and Beverage Packaging Technology. 2011. p. 59–83
K. Galić, M. Ščetar, M. Kurek, The benefits of processing and packaging. Trends Food Sci. Technol. 22(2), 127–137 (2011)
Y. Sultanbawa et al., Changes in Quality and Bioactivity of Native Foods during Storage. Vol. Publication No. 15/010 (Rural Industries Research and Development Corporation, Australian Government, Australia, 2015)
S. Akter et al., Antioxidant-Rich Extracts of Terminalia ferdinandiana Interfere with Estimation of Cell Viability. Antioxidants, 2019. 8(6)
D.J. Williams et al., Measuring free ellagic acid: influence of extraction conditions on recovery by studying solubility and UV-Visible spectra. Chem. Pap. 70(8), 1078–1086 (2016)
P.E. Cortbaoui, M.O. Ngadi, Influence of fluctuating environmental factors on phytochemical changes in eggplant and cucumber during postharvest storage. J. Postharvest Technol. 6(3), 018–030 (2018)
I. Ali et al., Processing and storage influence on scavenging activity of fruit juices. Marmara Pharm. J. 21(2), 298–304 (2017)
H. Li et al., Evaluating and Predicting the Oxidative Stability of Vegetable Oils with Different Fatty Acid Compositions. J. Food Sci. 78(4), H633–H641 (2013)
J. Ayton, R.J. Mailer, K. Graham, The Effect of Storage Conditions on Extra Virgin Olive Oil Quality (Rural Industries Research and Development Corporation, Australia, 2012)
O.S. Toker et al., Change in major fatty acid composition of vegetable oil depending on phenolic incorporation and storage period. Quality Assurance and Safety of Crops & Foods, 2015. 8 (2): pp. 179–188
A.B. Amaral, M.V. da Silva, S.C.D.S. Lannes, Lipid oxidation in meat: mechanisms and protective factors – a review, 38 (Food Science and Technology, 2018)
A. Selahvarzi et al., Investigation of antimicrobial activity of orange and pomegranate peels extracts and their use as a natural preservative in a functional beverage. J. Food Meas. Charact. 15(6), 5683–5694 (2021)
A. Selahvarzi et al., Evaluation of physicochemical, functional, and antimicrobial properties of a functional energy drink produced from agricultural wastes of melon seed powder and tea stalk caffeine. J. Food Process. Preserv. 45(9), e15726 (2021)
Z. Azarashkan et al., Co-encapsulation of broccoli sprout extract nanoliposomes into basil seed gum: effects on in vitro antioxidant, antibacterial and anti-Listeria activities in ricotta cheese. Int. J. Food Microbiol. 376, 109761 (2022)
B. Ladele et al., Chemical composition and nutritional properties of Terminalia catappa L. oil and kernels from Benin. C. R. Chim. 19(7), 876–883 (2016)
C. Rukmini, P.U. Rao, Chemical and nutritional studies onTerminalia bellirica Roxb. Kernel and its oil. J. Am. Oil Chem. Soc. 63(3), 360–363 (1986)
E. Chivandi, B.C. Davidson, K.H. Erlwanger, Proximate, mineral, fibre, phytate–phosphate, vitamin E, amino acid and fatty acid composition of Terminalia sericea. South Afr. J. Bot. 88, 96–100 (2013)
P. Onial, M.S.M. Rawat, R. Dayal, Chemical Studies of Fatty Oil of Terminalia chebula Seeds Kernels. Anal. Chem. Lett. 4(5–6), 359–363 (2014)
M. Kumar et al., Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 23(5), 797–810 (2011)
K.C. Hayes, Dietary fat and heart health: in search of the ideal fat. Asia Pac. J. Clin. Nutr. 11 Suppl 7, S394–S400 (2002)
M.A. Belury et al., Linoleic acid, glycemic control and Type 2 diabetes. Prostaglandins, Leukotrienes and Essential Fatty Acids, 2018. 132: p. 30–33
M. Hadjighassem et al., Oral consumption of α-linolenic acid increases serum BDNF levels in healthy adult humans. Nutr. J. 14(1), 20 (2015)
S. Maqsood et al., Phenolic Compounds and Plant Phenolic Extracts as Natural Antioxidants in Prevention of Lipid Oxidation in Seafood: A Detailed Review. Compr. Rev. Food Sci. Food Saf. 13(6), 1125–1140 (2014)
J. Chen, H. Liu, Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 21(16), 5695 (2020)
S. Maqsood, S. Benjakul, Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 119(1), 123–132 (2010)
S. Maqsood, S. Benjakul, Effect of Kiam (Cotylelobium lanceolatum Craib) Wood Extract on the Haemoglobin-Mediated Lipid Oxidation of Washed Asian Sea Bass Mince. Food Bioprocess Technol. 6(1), 61–72 (2013)
Y.M. Yu et al., Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J. Nutr. Biochem. 16(11), 675–681 (2005)
A. Reitznerová et al., Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography. Molecules (Basel, Switzerland), 2017. 22(11): p. 1988
Acknowledgements
The authors would like to acknowledge the Traditional Owners of the lands on which the Terminalia ferdinandiana was harvested and respect the knowledge and experience the Traditional Owners hold regarding the care, harvest and use of these plants.
Funding
This research was funded by the Cooperative Research Centre for Developing Northern Australia (CRCNA) and the support of its investment partners the Western Australian, Northern Territory and Queensland Governments and the Australian Research Council (ARC) Industrial Transformation Training Centre for Uniquely Australian Foods (Grant number: IC180100045).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Investigation, and data analysis E.M.B., S.S, A.D.T.P; methodology and supervision, M.E.N., D.C., Y.S.; writing-original draft preparation, E.M.B.; Reviewing the manuscript S.S, A.D.T.P, M.E.N., D.C., Y.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bobasa, E.M., Srivarathan, S., Phan, A. et al. Influence of blanching on the bioactive compounds of Terminalia ferdinandiana Exell fruit during storage. Food Measure 17, 244–252 (2023). https://doi.org/10.1007/s11694-022-01581-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01581-1