Abstract
Cold-pressed pumpkin and safflower seeds oils were stored at 2 ± 1 °C, 20 ± 1 °C and 40 ± 1 °C for 8 weeks. Measurements of lipid quality parameters were performed every 2 weeks. Throughout storage the emission and synchronous fluorimetric spectra of investigated oils were registered and colour L*, a*, b* parameters were determined. It was found that pumpkin oil, regardless of storage temperature, maintains high quality over the entire examined period. Safflower oil demonstrated relatively low oxidative stability, especially when stored at 20 and 40 °C. Evaluated activation energy of oxidation was 82.9 and 36.5 kJ/mol for pumpkin seed and safflower oil, respectively. Emission spectra determined the nature of changes occurring in investigated oils at all storage temperatures. Registered synchronous spectra connected with the principal component analysis (PCA) allowed the crucial factors influencing the oil quality to be identified. In addition, regression models were used to determine the parameters causing the observed colour changes of the examined products.
Similar content being viewed by others
Introduction
Due to increasing interest of consumers in cold-pressed oils, there is a need for in-depth extensive studies on quality and stability of such products as well as the nature of changes occurring during their storage. Cold pressing is a simple and low-energy process, which protects most of bioactive compounds. However, its disadvantage is low yield and difficulties in obtaining a product with a consistent quality. Genetic factors, cultivation, climate, production and storage conditions of oils are critical factors for the content of bioactive compounds [1]. Storage conditions strongly affect the extent of the hydrolytic and oxidative changes and activity of pro- and antioxidants. Lipid oxidation is the main process leading to the quality deterioration of oils [2].
Pumpkin (Cucurbita pepo L.) seed oil shows preventive effects against hypertension and carcinogenic diseases and it is characterized by specific sensory properties. It also contains compounds such as tocopherols and polyphenols, those having important biological and physiological properties [3, 4]. Safflower (Carthamus tinctorius L.) oil is nutritionally similar to the sunflower oil [5]. There are two groups of safflower cultivars: one with high content of linoleic acid and the second with high content of oleic acid [6]. The latter makes a great frying oil, while the first reduces blood cholesterol levels. It is also effective in the prevention and treatment of cardiovascular diseases, male sterility and female infertility [5].
Fluorescence spectroscopy is one of the methods proved to support the traditional chemical analyses on the oxidative status of lipid-containing products. Its main advantages are sensitivity and selectivity. Besides the recording of emission or excitation spectra at a particular excitation/emission wavelength, there is also a possibility of scanning both types of spectra simultaneously, which is called synchronous fluorescence spectroscopy. This results in narrowing of spectral band, simplification of the spectra and contraction of spectral range. The analytical information contained in the spectra can be extracted using various chemometric methods, like principal component analysis (PCA) [7].
The aim of the studies was to determine the influence of storage time and temperature on quality parameters of the cold-pressed pumpkin and safflower seeds oils. The oxidative and hydrolytic processes occuring in stored oils are connected with changes in colour, which are important for the quality of products. Therefore, using standard analytical and fluorimetric methods an attempt was made to objectively correlate the measured colour parameters with chemical and spectral quality indicators.
Materials and methods
Commercially available cold-pressed pumpkin and safflower seeds oils were stored at 2 ± 1 °C, 20 ± 1 °C and 40 ± 1 °C for 8 weeks. They were kept in the original 250 ml dark glass bottles in darkness. Every 2 weeks analysis of samples from these bottles was carried out.
Oil quality indicators
The acid (AV), peroxide (PV) and p-anisidine (pAV) values were determined in accordance with standard methods, namely: Cd 3d-63, Cd 8b-90 and Cd 18-90, respectively [8]. Content of thiobarbituric acid reactive substances (TBARS) was determined using an extraction method [9]. The results, expressed as mg malondialdehyde (MDA) per kg of oil, were calculated from the standard curve (y = 0.1818x, R2 = 0.9138) of the 1,1,3,3-tetraethoxypropane standard (Sigma-Aldrich Chemie GmbH). The total oxidation value (TOTOX) was calculated according to the following equation (Eq. 1):
Activation energy of oxidation processes in the investigated oils was evaluated assuming the first order reaction of oxidative changes at all used temperatures of storage (Eq. 2), and next applying the Arrhenius equation (Eq. 3):
where: t—time (s), k—reaction rate constant (s−1), TOTOX—total oxidation value, Ea—activation energy of oxidation (kJ mol−1), T—storage temperature (K), A—pre-exponential factor.
Fatty acid composition
The esterification was performed using the AOCS Official Method Ce 2-66 [8]. The fatty acid composition, in the form of methyl esters, was determined using a Trace GC Ultra gas chromatograph (Thermo Electron Corp., Waltham, USA) equipped with a flame-ionization detector (FID). Separations were carried out on a SUPELCOWAX 10 column (30.0 m × 0.25 mm × 0.25 μm) with helium 5.0 (as a carrier gas) at a flow rate of 1 ml/min. The column was heated using the following temperature settings: 160 °C for 3 min, then increasing to 210 °C with a rate of 3 °C/min thus maintaining this temperature for 35 min. Injector and detector temperatures were 220 and 250 °C, respectively. Individual fatty acid methyl esters were identified by comparison with the standard mixture of Supelco 37 component FAME Mix (Sigma-Aldrich Co.) and of CLA isomers (Sigma-Aldrich Co., St. Louis, MO). The percentages of particular fatty acids were calculated on the basis of their surface. Each sample was analyzed three times.
The resistance of oils to oxidation was expressed as the calculated oxidizability value (COX), obtained from Eq. 4 [10]:
Fluorescence spectra
The fluorescence spectra were registered in front-face mode, using a quartz cuvette with 1 cm optical length in a Varian Cary Eclipse spectrofluorimeter. A xenon lamp was used for excitation. Excitation and emission slits widths were 5 nm. The emissions spectra (λem 410–750 nm) were recorded with the excitation wavelength of 382 nm, based on the investigations of model systems related to lipid oxidation [11]. Synchronous fluorescence spectra were collected by simultaneously scanning the excitation and emission in the range of 250–750 nm with the constant ∆λ between them. The spectra were recorded for ∆λ in the range 10–60 nm every 10 nm.
CIE L*a*b* coordinates
In order to directly measure the colour, CIE L*a*b* coordinates were read using a spectrophotometer (CM-5, Konica Minolta Sensing Inc, Japan). The measurements were performed in transmittance mode, using D65 illuminant with a 10° observation angle. The samples of undiluted oils were poured into glass cuvettes with a 10 mm optical path for safflower oil and 1 mm for oil of pumpkin seed. In CIE system, the L* value is a measure of lightness ranging from 0 (black) to 100 (white), the a* value represents the red/green axis and varies from − 120 (greenness) to + 120 (redness), and the b* value represents the yellow/blue axis and varies from − 120 (blueness) to + 120 (yellowness). The differences between the colours of two samples (ΔE), were calculated, according to the Eq. 5 [12]:
Spectrophotometric determination of carotenoid and chlorophyll contents
Contents of carotenoids were determined according to the procedure described by Hashemi et al. [13], whereas of chlorophyll was determined based on the AOCS method Cc 13i-96 [8], using a Super Aquarius UV/Visible spectrophotometer (Cecil Instruments, UK).
Statistical analysis
All measurements were replicated three times. Differences between mean values were determined by the Tukey-HSD multiple comparison test. In order to evaluate the effect of storage time and temperature on the band intensities in fluorescence spectra, principal component analysis (PCA) was applied. Regression analysis of the obtained results was also carried out. Differences were considered statistically significant at p < 0.05. All statistical tests were implemented using CSS Statistica v. 12.5 (Stat Soft Inc.) software.
Results and discussion
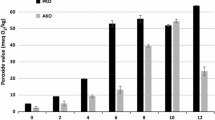
Values of quality indicators obtained for both oils were shown in Table 1. Generally, the peroxide value of safflower oil increased faster than of pumpkin seed oil, whereas the changes in the pAV and TBARS were similar. TOTOX is the most informative indicator describing oils’ susceptibility to oxidation. As for both oils, only the samples stored at 2 °C were characterized by similar values of this indicator. Safflower oil stored at 20 and 40 °C had higher values than pumpkin oil, which is the result of lower oxidation stability of this oil. A high rate of the first oxidation step of safflower oil, even at 20 °C, was also observed by other authors [14]. Evaluated values of the activation energy for both oils are 82.9 and 36.5 kJ mol−1 for pumpkin and safflower oils, respectively, which is further evidence for higher resistance of pumpkin oil to oxidation in comparison to safflower one.
Pumpkin oil practically regardless of the storage temperature showed high oxidative stability. The effect of temperature on the range of oxidative changes in safflower oil was more significant. Only the samples stored at 2 °C retained relatively good quality. The observed difference is connected with the various content of antioxidants in analysed oils. The initial level of carotenoids and chlorophylls in pumpkin oil was 7.44 mg/kg and 3.81 mg/kg, respectively, which is similar to the values obtained by Hashemi et al. [13] and Górnaś et al. [15]. Safflower oil contains of relatively smaller amounts of chlorophylls and carotenoids, which in the present study were 0.31 mg/kg and 2.13 mg/kg respectively. According to Franke et al. [16], carotenoids in this oil amounted to 2.3 mg/kg. The level of carotenoids approximately did not change during storage at 2 °C. Storage at 40 °C caused its decrease to 5.81 mg/kg for pumpkin oil and to 1.20 mg/kg for safflower oil.
The fatty acid composition of both oils follows the values obtained by other authors [6]. Comparison of the obtained values before and after storage (Table 2) showed differences in the proportions of certain fatty acids in the samples stored at 20 and 40 °C. They concerned linoleic (C18:2), palmitic (C16:0), stearic (C18:0) and oleic (C18:1) acids. The reason for the observed changes is the lipid oxidation.
The COX value in pumpkin oil was 5.56 and did not change during storage. In the case of safflower oil it was 8.65 and decreased with time to 8.47 (20 °C) and 8.23 (40 °C), because of lowering content of linoleic acid. These values indicate higher susceptibility to oxidation of safflower than pumpkin oil. For comparison COX in olive oil is equal to 1.54, coconut oil 0.19, rapeseed oil 4.56 [17], and in apricot kernels oil 3.3 [10].
Bands at λem of 410–500 nm present in Figs. 1 and 2 can be ascribed to fluorescence of the secondary products of lipid oxidation [10] and at 500–550 nm as well as at 725 nm to fluorescence of carotenoids [18]. Bands of the chlorophylls’ and pheophytines’ fluorescence are seen at 660–700 nm [19]. Intense bands present at 630 nm (Fig. 1) can be ascribed to protochlorophylls and protopheophytines [20, 21]. Changes of all bands’ intensities are particularly seen in the case of samples stored at 40 °C, especially in comparison with the spectra of samples stored at 2 °C. Main changes on the spectra of pumpkin oil (Fig. 1) are observed in the intensities of the protochlorophyll and protopheophytine fluorescence bands. Samples stored at 40 °C (Fig. 1b) were characterized by decreasing intensities of these bands. A similar decrease caused by time and temperature of storage was also seen by Sikorska et al. [19] and Tena et al. [22] during the research of extra virgin olive oil. Intensities of the bands ascribed to carotenoids also decreased, but these changes are not very distinct due to low quantum yield and because of much lower fluorescence intensity in comparison to chlorophylls, protochlorophylls and protopheophytins ones. Results of the oil quality indicators’ determinations showed that safflower oil is more susceptible on the oxidation than pumpkin one. It was also seen on the spectra in Fig. 2, where the essential changes in intensities of the bands at 410–500 nm took place. Moreover, during storage increased intensity of the band at 650–750 nm was observed. It can be explained by formation of pheophytins from the chlorophylls along with primary and secondary products of lipid oxidation and connected with the change in colour of samples stored at 40 °C. Such changes probably occur also in the samples stored at 2 °C and 20 °C, but after a longer time than used in the present studies. This statement can be based on the Van’t Hoff’s rule, according to which an increase in temperature of 10 °C will cause an increase in the rate of the reaction by 2–4 times. This rule is also used in ASLT (Accelerated Shelf Life Test) methodology, in which it is possible to determine the food quality and shelf life during storage at a lower temperature by measuring the extent of changes taking place in such food stored at an elevated temperature [23].
In order to evaluate the effect of time and storage conditions on the band intensities on the fluorescence spectra, principal component analysis (PCA) was applied to the synchronous spectra obtained with the three displacement values Δλ = 10, 30, 60 nm. The PCA approach transforms such variables as wavelengths and intensities of fluorescence bands into new ones called principal components (PCs). They are mutually orthogonal, and each successive PC contains less of the total variability of the initial data set. The results of PCA are shown in Fig. 3. With regard to the samples of pumpkin seed oil two components, PC1 and PC2, explain the whole of the variation caused by temperature and storage time (Fig. 3a, c, e). The largest increase of PC1 (as well as PC2) was observed for samples stored at 40 °C. In the case of those stored at both lower temperatures (2 and 20 °C), the increase of PC2 was relatively small and was accompanied by a simultaneous decrease of the PC1 component, with time of storage.
Principal component analysis similarity maps determined by principal component 1 (PC1) and PC2 for the fluorescence spectra with displacements Δλ = 10 nm (a, b), 30 nm (c, d), 60 nm (e, f) of pumpkin (a, c, e) and safflower (b, d, f) seed oils, respectively. Numbers at tags identify the investigated samples. The first digit denotes the storage time in weeks while the second one denotes the storage temperature (0, 1, 2 for 2 °C, 20 °C, and 40 °C respectively). Percentages shown on the axis indicate how large a fraction of the total variance is explained by the given principal component
Principal component analysis performed for the safflower oil samples gave, for ∆λ = 10 nm, three components, PC1, PC2 and PC3 (Fig. 3b), which explained the variation. Analysing the changes between subsequent points, an increase of the PC3 component at all storage temperatures was observed, with quite small changes of PC1. As for the whole variation, for samples stored at 40 °C it is explained by three components, whereas for the remaining ones by two of them only. This indicates that in safflower oil stored at 40 °C there is an additional factor responsible for different transformations, compared to the oils kept at both lower temperatures. These different mechanisms of transformations could also be seen in the results of other analyses, discussed earlier (Table 1, Fig. 2). Results of PCA obtained for ∆λ = 30 and 60 nm showed that two components, PC1 and PC2, participate in the explanation of variation (Fig. 3d, f). The component PC1 explained ca. 87% of variation; in the case of pumpkin oil the same component explained more than 97% of whole the variation. A considerable increase of PC1 was observed for the samples stored at 40 °C, whereas in the remaining samples there were practically no changes due to different storage temperatures. However, the values of principal components fluctuated with the storage time. Moreover, the creation of a separate group by the points obtained from the analysis of samples stored in 40 °C is also seen (Fig. 3b, d, f).
Colour, an important factor determining the visual oil acceptance, is usually attributed to the presence of such components as chlorophylls and/or carotenoids. The initial colour parameters for investigated oils together with their changes during storage and ∆E calculated according to Eq. 4 are presented in Table 3. Initially, pumpkin seed oil was more green (negative a*) and during storage the a* value gradually shifted towards red. This is in agreement with the results of analysis of fluorescence spectra (Fig. 1), which showed lowering fluorescence intensities of protochlorophylls and protopheophytins. Changes in L*, a* and b* values obtained for pumpkin oil stored at different temperatures showed a similar trend towards modifications in fluorescence band intensities.
With regard to safflower oil stored at 2 °C and 20 °C, the initial values of colour parameters only slightly changed. Measurements of the a* value showed insignificant differences in the case of samples stored at 2 °C and an increasing proportion of the green component in the colour of oil stored at 20 °C. Colour analysis of oil stored at 40 °C showed a decrease in the lightness parameter and increases in yellow and red components when compared with the oil at the beginning of the experiment (Table 3). Changes in the amount and type of colouring seen on the fluorescence spectra (Fig. 2) are also present in the results of colour measurements. Colour difference (∆E), calculated according to Eq. 4, only in the case of safflower oil stored at 2 ± 1 °C and at 20 ± 1 °C in the initial period, did not exceed the value of 2, related to the limit of the colour difference perception by an untrained observer. However, this oil from the beginning of storage at 40 ± 1 °C had totally changed colour in comparison to the initial one. Pumpkin oil stored even at the lowest of investigated temperatures changed its colour after 2 weeks, which is probably caused by the transformation of protochlorophylls and protopheophytins [21].
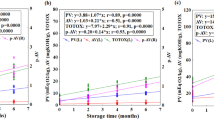
In order to find the best linear models for the observed ΔE changes and to explain the influence of storage-induced processes on colour of the investigated oils regression analysis (Table 4) was conducted. According to the obtained regression model, changes in colour of pumpkin seed oil result from storage time and temperature influence as well as the rate of hydrolytic and oxidative processes, which lead to the formation of secondary products reacting with p-anisidine. When analysing the values of the following regression parameters, it can be stated that an increase of all of them resulted in greater change in colour, with the strongest influence of storage time and TOTOX value.
On the other hand, the regression model obtained for safflower oil showed the influence of both hydrolytic changes and the pathway of the secondary oxidation process determined by TOTOX and TBARS. The strongest positive influence was observed for the acid value, whereas the coefficient b3 associated with TBARS had a negative value, indicating that an increase of this factor should not cause a distinct change in colour. Similar absolute standardized values of coefficients b2 and b3 suggest the competing nature of primary oxidation products’ reactions towards formation of different types of secondary products, seen in the results of oil quality indicators’ analyses. They interact with carotenoids, tocopherols and chlorophylls present in the investigated oils, causing the change of colour and influencing the different extent of oxidative stability.
Conclusion
Cold-pressed oils are considered relatively resistant to oxidative processes, particularly because of the presence of bioactive compounds. However, these oils differ in their storage stability. Pumpkin oil, in contrast to safflower one, maintained high quality over the whole investigated period of time. Differences in stability of both oils were seen in their activation energy of oxidation as well as in calculated oxidizability value (COX) strongly related to fatty acid composition. Moreover, the presence of bioactive substances, like chlorophyls and carotenoids influenced on the rates of oxidative processes occurring during storage of the investigated oils.
Analysis of the fluorescence spectra of pumpkin and safflower oils, stored at three different temperatures, enabled determination of the extent of changes in pigments present in these oils and claimed the direction and range of the oxidative changes of lipids. Results of spectrofluorometric analysis agree with those of the measurements of CIE L*a*b* coordinates. In addition, the obtained regression models and results of PCA analysis made it possible to determine the distinct factors affecting oil quality, such as time and temperature of storage, which accelerated oxidative changes causing transformation of carotenoids and chlorophylls.
References
A. Nawirska-Olszańska, A. Kita, A. Biesiada, A.Z. Sokół-Łętowska, Kucharska. Food Chem. (2013). https://doi.org/10.1016/j.foodchem.2013.02.009
T. Tsiaka, D.C. Christodouleas, A.C. Calokerinos, Food Res. Int. (2013). https://doi.org/10.1016/j.foodres.2013.08.036
L. Rezig, M. Chouaibi, K. Msaada, S. Hamdi, Ind. Crops Prod. (2012). https://doi.org/10.1016/j.indcrop.2011.12.004
S. Nederal, M. Petrović, D. Vincek, D. Pukec, D. Škevin, K. Kraljić, M. Obranović, Ind. Crops Prod. (2014). https://doi.org/10.1016/j.indcrop.2014.05.044
V. Emongor, Asian J. Plant Sci. (2010). https://doi.org/10.3923/ajps.2010.299.306
F. Salaberría, D. Constenla, A.A. Carelli, M.E. Carrín, J. Am. Oil Chem. Soc. (2016). https://doi.org/10.1007/s11746-016-2886-6
J. Sádecká, J. Tóthová, Czech J. Food Sci. (2007). https://doi.org/10.17221/687-CJFS
Official methods and recommended practices of the AOCS. (AOCS Press, Champaign, 2004)
J. Pikul, D.E. Leszczynski, F.A. Kummerow, J. Agric. Food Chem. (1989). https://doi.org/10.1021/jf00089a022
A. Siger, K. Dwiecki, W. Borzyszkowski, M. Turski, M. Rudzińska, M. Nogala-Kałucka, Food Chem. (2017). https://doi.org/10.1016/j.foodchem.2017.01.022
A. Veberg, G. Vogt, J.P. Wold, LWT Food Sci. Technol. (2006). https://doi.org/10.1016/j.lwt.2005.03.009
H.M.G. Stokman, T. Gevers, J.J. Koenderink, Comput. Vis. Image Underst. (2000). https://doi.org/10.1006/cviu.2000.0860
S.M.B. Hashemi, A.M. Khaneghah, M. Koubaa, J. Lopez-Cervantes, S.H.A. Yousefabad, S.F. Hosseini, M. Karimi, A. Motazedian, S. Asadifard, Food Res. Int. (2017). https://doi.org/10.1016/j.foodres.2016.11.033
A.A. Mariod, S.Y. Ahmed, S.I. Abdelwahab, S.F. Cheng, A.M. Eltom, S.O. Yagoub, S.W. Gouk, Int. J. Food Sci. Technol. (2012). https://doi.org/10.1111/j.1365-2621.2012.03028.x
P. Górnaś, A. Siger, K. Juhneviča, G. Lacis, E. Šne, D. Seglina, Eur. J. Lipid Sci. Technol. (2014). https://doi.org/10.1002/ejlt.201300425
S. Franke, K. Fröhlich, S. Werner, V. Böhm, F. Schöne, Eur. J. Lipid Sci. Technol. (2010). https://doi.org/10.1002/ejlt.200900251
X. Liu, N. Hoshino, S. Wang, E. Masui, J. Chen, H. Zhang, Eur. J. Lipid Sci. Technol. (2018). https://doi.org/10.1002/ejlt.201700528
A. Lai, E. Santangelo, G.P. Soressi, R. Fantoni, Postharvest Biol. Technol. (2007). https://doi.org/10.1016/j.postharvbio.2006.09.016
E. Sikorska, I.V. Khmelinskii, M. Sikorski, F. Caponio, M.T. Bilancia, A. Pasqualone, T. Gomes, Int. J. Food Sci. Technol. (2008). https://doi.org/10.1111/j.1365-2621.2006.01384.x
N. Mukaida, N. Kawai, Y. Onoue, Y. Nishikawa, Anal. Sci. (1993). https://doi.org/10.2116/analsci.9.625
E. Naziri, M.N. Mitic, M.Z. Tsimidou, Eur. J. Lipid Sci. Technol. (2016). https://doi.org/10.1002/ejlt.201500261
N. Tena, D.L. García-González, R. Aparicio, J. Agric. Food Chem. (2009). https://doi.org/10.1021/jf902009b
T.P. Labuza, An integrated approach to food chemistry: illustrative cases, in Food Chemistry, Second edn., ed. by O.R. Fennema (Marcel Dekker, Inc., New York, 1985), pp. 913–938
Acknowledgments
The authors would like to thank Stanisław Zięba for statistical analysis and valuable discussion of results.
Funding
This research was financed by the Ministry of Science and Higher Education of the Republic of Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banaś, J., Maciejaszek, I., Surówka, K. et al. Temperature-induced storage quality changes in pumpkin and safflower cold-pressed oils. Food Measure 14, 1213–1222 (2020). https://doi.org/10.1007/s11694-019-00370-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00370-7