Abstract
Cold-pressed oils are highly prone to the peroxidation process, which causes a rapid decline in quality. Thus, there is a need to develop instrumental methods instead of conventional chemical analysis consuming large quantities of harmful chemicals. Differential scanning calorimetry (DSC) is a valuable analytical tool for assessing the oxidative stability of oils. Cold-pressed flaxseed, camelina and hemp seed oils from different cultivars, which had been stored for six months in room conditions under natural light exposure, were tested. Chemical methods for measuring changes in oxidative stability during storage of oils included determination of peroxide value (PV), p-Anisidine value (p-AV), total oxidation value (TOTOX) value and acid value (AV). Parameters like oxidation induction time (OIT) in isothermal mode (120, 140 °C) and onset temperature (Ton) in non-isothermal mode (heating rate 2, 5 °C/min) were established from DSC curves. Data for OIT and Ton plotted against time showed a strong, significant (p ≤ 0.05) descending trend for all oils. However, flaxseed and hempseed oils revealed a more rapid deterioration during storage compared to camelina seed oils. All DSC results showed promising repeatability of the oxidative characteristics for three types of cold-pressed oils, regardless of their origins in different cultivars. However, the most suitable for monitoring the deteriorative changes in oils during storage was the isothermal test carried out at a temperature of 120 °C, for which the correlations with chemical indicators (PV, p-AV, TOTOX) were highly significant (p ≤ 0.0001). Linear discriminant analysis (LDA) based on the DSC results revealed, that the first discriminating function significantly separated the fresh oils from stored oils. The study showed that, based on a starting point defined for fresh oils, the DSC technique can be used to effectively and ecologically monitor the deterioration of oils by oxidation, instead of harmful chemical analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold-pressed oils from plant sources have recently become popular on the market due to their high nutritional value [1] and their application in different sectors e.g., the food and pharmaceutical [2], cosmetic [3] and biodiesel industries [4]. In 2021, the worldwide market for cold-pressed oils had a valuation of USD 27.05 billion, and it is anticipated to experience a compound annual growth rate (CAGR) of 5.7% from 2022 to 2028 [5]. Consumers are now becoming very diligent about new sources of foods like cold-pressed oils from plant sources to meet the requirements of essential nutrition. This approach to meeting market requirements is sometimes more based on marketing practices rather than care for product quality. Considering the importance of cold-pressed oil production, it has become extremely important to maintain the quality of products from these oils during their shelf life in the supermarkets. Primarily, the presence of unsaturated carbon bonds (C = C) makes these oils highly prone to oxidation. This oxidation while storing and processing edible oils is caused by distinct chemical mechanisms - autoxidation and photosensitized oxidation - which are dependent on the type of oxygen involved (3O2- atmospheric triplet oxygen; or 1O2- singlet oxygen). While autooxidation is caused by the reaction of 3O2 to lipid free radicals, photosensitized oxidation is the result of oils being exposed to light, sensitizers and atmospheric oxygens, which leads to the production of 1O2 [6]. An oxidation test for oils relates to measuring the degree of oxidation in the oil, which ultimately leads to the formation of off-flavors, odors, and potentially harmful compounds [7]. Different analytical and chemical methods provide different measures of oxidative stability [8, 9]; hence, the choice of method depends on the specific application and the type of oil being analyzed. During storage time analysis, it is important to consider the shelf life condition of the oils [10] and also seeds [11, 12], as well as to consider fast and feasible methods for detecting deterioration stages of the oils. The most popular chemical methods used in the food industry for measuring oxidative stability are peroxide value determination (PV), p-Anisidine value (p-AV), total oxidation value (TOTOX) value and acid value (AV), which generally provide quantitative information about chemical changes caused by the oxidation process [13,14,15]. For example, these methods were used to investigate how temperature and heating time affect the deterioration of virgin olive oil, [16] and sunflower oil [17], both for food and for biodiesel production. Other studies have used these methods to explore how antioxidants increase the oxidative stability of the sunflower oil [18] or rapeseed oil [19]. However, despite of their popularity as conventional chemical measurements, these methods are subject to the technician’s precision, and the quality of both the chemicals and the tools. Moreover, large amounts of harmful chemicals are used in these methods, which produces toxic chemical wastes. Following the green chemistry trend, instrumental methods should be developed and applied to assess the quality of oils. Meanwhile, thermal analysis methods e.g. DSC can generate information on both the physical and chemical changes to explain the oil samples’ comprehensive character [20, 21]. Determining the phase transition [22], crystallization and melting data [23, 24], assessment for oxidation profiles [8, 25] can provide a full spectrum dataset to understand the physicochemical characteristics of fats and oils.
The DSC technique offers versatile tools for assessing the oxidative stability of oils through both isothermal and non-isothermal modes [26]. The isothermal method involves subjecting the oil to a constant temperature, allowing the determination of its oxidation induction time (OIT), making it particularly useful for measuring the oil resistance to oxidation at specific temperature [8]. On the other hand, the non-isothermal method, subjects the oil to an increasing temperature with a defined heating rate that allows the onset temperature of oxidation (Ton) to be measured. The DSC isothermal or non-isothermal oxidation tests were used to study, for instance the effect of seeds roasting [27], oils blending [28], oils extraction method [29], addition of plant extracts to fats [30] or addition of antioxidants to oils [31]. To the best of our knowledge, there are no studies on the applicability of DSC isothermal and non-isothermal oxidation tests to monitor changes in oils caused by quality deterioration during storage at conditions similar to the supermarket shelf. So far the approach taken to evaluate the oxidation state of plant oils using characteristic chemical oxidation indicators like PV or TOTOX has been proposed [32]. Since the DSC technique does not require toxic chemicals for chemical analysis, it is worth testing its usability as a method for monitoring the quality changes in oils during storage. Thus, the aim of this study was to assess the oxidative stability changes in three different cold-pressed oils during six months’ storage by use of the DSC isothermal and non-isothermal tests in comparison with the conventional chemical indexes (PV, p-AV, TOTOX, AV). For this research, three popular cold-pressed oils i.e., flaxseed oil obtained from Linum usitatissimum (L.) crop, camelina seed oil obtained from Camelina sativa (L.) crop, and hemp seed oil obtained from Cannabis sativa (L.) crop, originated from different cultivars from Poland were studied. The most common feature of these three oils is the very high amount of polyunsaturated fatty acids (PUFA), e.g., flaxseed and hempseed oils have a PUFA level of over 70% [11, 21], while in camelina oils PUFAs account for more than 54% of their total fatty acid content [26].
Materials and methods
Chemicals
All materials utilized in this study were of the utmost quality and met analytical grade standards. Glacial acetic acid (purity of 100%) and p-Anisidine reagent (purity of 99%) were procured from Sigma Aldrich. The acquisition of potassium hydroxide, starch, and sodium thiosulfate was made from firma Chempur (Poland). Toluene (purity of 99.5%), Isopropanol (purity of 99.7%), Isooctane (pure), and phenolphthalein were acquired from POCH (Gliwice, Poland). Potassium Iodide (purity of 99%) was sourced from ALFA CHEM (Łódź, Poland). Also, distilled water used from Laboratory facility. Spectrophotometric analyses were conducted utilizing the Cary 1E spectrophotometer (Varian, Belrose, Australia). Titration analyses were executed using the Titration unit Solarus (Hirschmann Laborgeräte GmbH & Co. KG, Eberstadt, Germany.
Materials
For this study, the primary objective was to acquire 15 kg of seeds for each specific cultivar or batch of flax, camelina, and hemp from various sources. For cold-pressed flaxseed oils, seeds of three cultivars of flax (Linum usitatissimum L.), i.e., Bukoz from the Polish Institute of Natural Fibers and Medicinal Plants (Poznań, Poland), Dolguniec from SEMCO manufactory (Śmiłowo, Poland), Szafir (SEMCO manufactory (Śmiłowo, Poland) and Hodowla Roślin Strzelce Sp. z o.o. (Strzelce, Poland) were collected along with one sample of unknown cultivar from VitaCorn (Poznan, Poland). All hemp seeds were obtained from Henola cultivar of Cannabis sativa L., which were gathered from five different suppliers, and their collection was done at the Polish Institute of Natural Fibers and Medicinal Plants. Camelina seeds of three cultivars of Camelina sativa L. were obtained from five suppliers. Seeds of the spring Omega cultivar were procured from Poznań University of Life Sciences (Agriculture Research Station Dłoń, Miejska Górka), while two other camelina cultivars, Luna and Śmiłowska, were collected from SEMCO manufactory. This manufactory sourced Luna, a winter cultivar, from two different suppliers and Śmiłowska, a spring cultivar, from two different suppliers as well. All the seeds were cold-pressed under uniform conditions at the SEMCO manufactory, with the temperature maintained below 50 °C. The oils obtained through pressing were then allowed to settle for 24 h and were subsequently stored in brown glass bottles. These containers were chosen for their ability to shield the oils from light exposure as well as to replicate conditions commonly found in the market. A comprehensive storage analysis was conducted, starting from the oils’ initial state and extending up to the sixth month of their shelf life. At each storage interval (0, 2nd, 4th, and 6th month), newly unsealed bottles containing samples of oil were utilized for all analytical procedures. Throughout the duration of the shelf life assessment, the samples were maintained in airtight conditions at room temperature (23–25 °C) beside a window, where they were subjected to natural sunlight exposure. The intention behind this approach was to recreate the real-life conditions that oils may encounter during processes such as transportation, distribution or household end-use.
Determination of oxidative stability by DSC
Oxidative stability was determined by following the ISO 11357-1 [33], and also implementing the ASTM D3895-14 [34]. The oil samples were subjected to analysis using a DSC 7 Perkin Elmer device, which was equipped with an Intracooler II and operated through Pyris software. Prior to testing, the instrument was calibrated using indium (with a melting point of 156.6 °C and a ΔHf of 28.45 J/g) and n-dodecane (with a melting point of -9.65 °C and a ΔHf of 216.73 J/g), while 99.99% pure nitrogen gas was used as the purge gas. To conduct the analysis, approximately 6–7 mg of oil samples were weighed into 50 µl open aluminium pans (Perkin Elmer, No. 02190041). An open and empty aluminium pan was used as the reference. To investigate the oxidation process, a constant oxygen flow of 20 ml/min (with a purity of 99.995%) was maintained during the analysis. Both isothermal and non-isothermal protocols were employed to determine the oxidative stability characteristics of the oils. For the isothermal program, temperatures of 120 and 140 °C were set and specific parameters such as oxidation induction time (OIT), oxidation end time (OET), length of oxidation (∆t = OET - OIT), and the rate of oxidation were determined from the DSC curves. The index of oxidation induction time (OIT) was determined based on the curves obtained after normalization of the oxidation DSC curve, by calculating the intersection of the extrapolated baseline and the tangent line to the descending exotherm. To determine the OET value at the point where the heat flow of the exotherm reached its minimum level, calculations were made to identify the end of the propagation stage and the start of the termination stage of oxidation. The oxidation rate was computed using the equation provided below
Where, Y1 stands for heat flow at OIT point (W/g), Y2 represents heat flow at OET (W/g), and ∆t is the length of oxidation (minutes).
Non-isothermal analysis was conducted using a heating rate of 2 °C/min and 5 °C/min. The onset temperature (Ton) was determined from the oxidation curves by calculating the intersection of the extrapolated baseline and the tangent line to the descending curve of the recorded exotherm. On the other hand, the temperature at which the heat flow reached its minimum value was measured and recorded as Tend, signifying the transition from the propagation stage to the termination stage of the oxidation process. All DSC experiments were carried out in duplicate for each oil sample.
Chemical determination of oxidative stability
To evaluate the amount of secondary oxidative products (such as aldehydes, carbonyls, trienes, and ketones) present in the samples, the p-anisidine value (p-AV) was measured according to ISO 6885:2016 [35]. The peroxide value (PV) was determined to quantify the peroxides produced in the samples as an expression of the milliequivalents of excess active oxygen [mEqO2] content as a result of the oxidation reaction by following the ISO 3960:2010 procedure [36]. The total oxidation value (TOTOX) parameter was calculated based on the pAV and PV values by means of the following formula TOTOX = pAV + 2PV, expressing the overall rate of oil oxidation [36]. Acid value (AV), as an indicator of the degree of hydrolytic changes, expressed in milligrams of potassium hydroxide (KOH) per gram of the sample was measured according to the official AOCS method [37]. All chemical analyses were performed in three replications.
Statistics
Statistical analysis of the recorded data was conducted using Statistica 13.3 software (TIBCO Software Inc. USA), employing a robust suite of techniques to evaluate various aspects of oil storage. The significance level was set at α = 0.05 to ensure that the results were statistically significant. Linear Regression Analysis, utilizing the method of least squares estimation was performed. Linear regression analysis was employed to evaluate the effects of oil storage on selected chemical and DSC parameters. This approach allowed modelling the relationship between the dependent and independent variables, providing information on how the storage time of oil affects these parameters. This analysis was also used to assess the significance of the relationship between DSC parameters (predictors) and chemical indicators of oil oxidation. Pearson’s Linear Correlation Coefficients were calculated to express the direction and strength of the linear relationship between the variables, offering a concise summary of how the variables are correlated. Additionally, Principal Component Analysis (PCA) was conducted to identify patterns and relationships between variables and objects. By transforming the data into a new coordinate system, PCA reduced the dimensionality of the dataset while retaining most of the original variance. This allowed for the identification of the principal components that best represent the underlying structure of the data, facilitating a more nuanced understanding of the complex relationships between variables. Linear Discriminant Analysis (LDA) was used to build a classification model to categorize the oils by storage time. In this study, LDA aimed to find the linear combination of features that best separates oils into three distinct classes (0, 4- and 6-month storage time). By maximizing the between-class variance and minimizing the within-class variance, LDA created a decision boundary that effectively classified the oils based on their storage time. This method provided a powerful tool for understanding how storage time influences the characteristics of the oils, enabling precise categorization. The combination of these statistical methods provided a comprehensive analysis of the data, enabling a detailed examination of the effects of oil storage on selected chemical and DSC parameters. The simultaneous use of linear regression, correlation analysis, PCA and LDA provided a multi-faceted approach to understand the complex relationships in the dataset, contributing to the robustness and validity of the findings.
Results
Oxidative stability of cold-pressed oils during storage measured by conventional chemical methods
Conventional chemical analyses were used in food analysis to determine the changes and status of the oxidative stability of fats and oils from ages [38]. The most popular conventional methods for measuring oil quality are by determining peroxide value (PV), acid value (AV) and p-anisidine value (p-AV). The PV expresses the primary oxidation products present in the sample, whereas the p-AV value represents the secondary oxidation products. Based on the PV and p-AV, the TOTOX value (total oxidation rate) is calculated, which represents the overall oxidation status or rancidity status of the oil. The acid value, in turn, denotes the presence of free fatty acids in the oil samples.
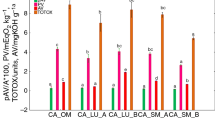
In Fig. 1, scatterplots are shown for the aforementioned chemical indicators obtained during storage of cold-pressed flaxseed, camelina and hempseed oils of various varieties. From the results plotted for PV presented in Fig. 1 it can be seen that for all three oils the values increased until the end of the storage period. The mean values for the starting point of PV measured for fresh oils were 3.2, 3.1, 15.45 mEqO2/kg for flaxseed, camelina and hempseed oils, respectively, while at the end of storage, after six months, they were significantly higher than for fresh oils i.e. 12.4, 9.8, 32.85 mEqO2/kg (p ≤ 0.05). Standards developed by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO), known as Codex Alimentarius, can be considered as a reference for food standards and regulations. According to Codex Alimentarius, with the standard limit for the PV value established as less than 15 mEqO2/kg oil [39], cold-pressed flaxseed and camelina seed oil did not exceed the limit after six months of light exposure at room temperature, while the hempseed oils certainly did. The ascending trend in quality changes can be more precisely expressed by comparing the slope of the straight line, which was lowest for camelina oil (1.07) and highest for hemp oil (2.84), indicating the most pronounced oxidative changes in this oil. As shown in Fig. 1, correlations of PV with time were very strong and significant (p ≤ 0.05) for all oils, as the correlation coefficients (r) were between 0.87 and 0.90.
The second indicator of the oxidative deterioration of the oils was the p-anisidine value (p-AV), for which a similar ascending trend during storage was observed (Fig. 1, scale on the right). This measure accounts for the quantification of secondary oxidation products like aldehydes, carbonyls, trienes and ketones formed in the oil. For the three oils tested, the starting point of p-AV was 0.79, 0.24, 1.21, while after six months it had increased to significantly higher values of 1.87, 1.06, 3.78 for flaxseed, camelina, and hempseed oils, respectively (p ≤ 0.05). The slope value of the straight line plotted for the p-AV data showed that for hempseed oils it was four times higher (slope 0.44) than for flaxseed and camelina oils (slope 0.18 and 0.14, respectively). The correlation found between p-AV and storage time was significant (p ≤ 0.05) for all three oils, for which the r values were between 0.85 and 0.93 (Fig. 1). Based on the PV and p-AV results, the TOTOX values were calculated, which cover the overall quality changes at all stages of shelf life. The slopes of straight lines plotted from TOTOX values (Fig. 1) show that the rate of total quality deterioration of oil during storage time was two times lower for flaxseed oil (slope 3.18), and three times lower for camelina oil (slope 2.29) than for hempseed oil (slope 6.13). After six months of storage, the values of TOTOX for all oils were significantly higher than for fresh oils (p ≤ 0.05). As all TOTOX data were generated from PV and p-AV values, the correlation coefficients versus time were also high and significant, like was the case for PV and p-AV (p ≤ 0.05).
Another important chemical indicator of oil quality is the acid value (AV), indicating a hydrolysis process occurring in the oil, which can result in the production of free fatty acids (FFA). Different factors such as high temperature [17], prolonged storage [40], or microbial contamination [41] can cause higher AV values. For flaxseed and camelina seed oil, AV values were established for the ascending trend with a slope of 0.22 and 0.21, respectively, while for hempseed oil the slope of the increase was almost four times higher (0.92) than for flaxseed and camelina oils. At the beginning of storage, the mean values of AV were 0.98, 0.99, 14.13 for flaxseed, camelina and hempseed oils, respectively, while after six months of storage, significantly higher values were observed for all oils, i.e. 2.31, 2.27 and 19.69, respectively. However, oils which exceed the limit of 4.0 mg KOH/g for AV, according to Codex Alimentarius recommendations [39], should not be considered as acceptable for consumption. In this study, flaxseed and camelina oils did not exceed the limit after storage time, but for hempseed oil the AV value was over the limit. Presumably, the quality of the seeds of hemp oil played an important role in the oxidation character of the samples [11]. Significant correlations between AV and storage time were also noted for all oils (p ≤ 0.05), although the correlation coefficients were lower than for PV and p-AV, since the r values were between 0.44 and 0.67.
The results obtained for chemical indicators of the deterioration of three oils are in agreement with the previous studies in this area. Increasing values of PV and p-AV during storage were also reported for camelina seed oils, although the storage conditions (darkness under 8 °C for 3 weeks) were different than those used in this study [42]. There were also studies showing a significant increase in chemical indexes (i.e. AV, PV, p-AV) for stored flaxseed oil, which are comparable to this study [43, 44]. In another study on hempseed oils oxidized under accelerated test (60 °C), it was established that cold-pressed hempseed oil exceeded the Codex Alimentarius limit for PV after 18 days of storage [45].
Oxidative stability of cold-pressed oils during storage measured by DSC technique
Differential scanning calorimetry is a technique that enables resistance of oils to thermal oxidation to be measured by determining isothermally the oxidation induction time (OIT) or non-isothermally induction temperature (Ton). Deterioration in oil quality during storage can cause a lowering of resistance to thermal oxidation. However, there are no studies showing whether these parameters change during shelf life. Resistance to thermal oxidation is a specific feature of each oil, and is influenced by the fatty acid composition, especially the content of polyunsaturated fatty acids, as well as antioxidants like tocopherols, polyphenols or prooxidants, for instance, chlorophylls and phospholipids. Determining oxidative stability by means of isothermal (OIT) or non-isothermal (Ton) process implemented using the DSC technique involves exposing the oil or fat sample to elevated temperatures in the presence of oxygen to accelerate the oxidation process.
Data acquired using these experimental methods can be used successfully to optimize processing and storage conditions to extend the shelf life of the oil. In Fig. 2, the DSC curves for one selected cold-pressed flaxseed oil (Szafir cultivar) are shown for both measurements i.e., isothermal at two different temperatures (120 and 140 °C) and non-isothermal at two different scanning rates (2 and 5 °C/min). To measure the changes by the isothermal mode, the oxidation induction time (OIT) parameter was calculated from the curves as a function of heating time at a constant temperature. The OIT value is equal to the time of initiation of the exothermic oxidation reaction. The oxidation profiles show the changes occurring during six months of storage. In Fig. 2a, changes in the oxidation profiles manifested by shifting the initiation time to lower values for every storage period can be seen. This suggests that storing oil samples for six months reduces the OIT, and thus resistance to thermal oxidation, as a consequence of the deterioration of the oil. Another option for measuring thermal oxidation stability is the non-isothermal analysis by DSC, where an oil sample is subjected to an increasing temperature at a constant heating rate. In this study, two different heating rates 2 and 5 °C/min were applied to observe any changes in the heat flow, manifested as exothermic phenomena during oxidation. The parameter calculated as the onset temperature (Ton) is equal to the temperature at which the oxidation process starts. As was the case with the isothermal measurement, the non-isothermal analysis showed a similar trend of decreasing oil stability during storage for all the oils tested. As shown in Fig. 2b, for a heating rate of 2 and 5 °C/min the oxidation process was initiated in fresh flaxseed oil samples at higher temperatures than after six months.
Figure 3 presents the changes in OIT values during storage of the three oils measured at 120 and 140 °C. Generally, comparing the values obtained for temperatures of 120 and 140 °C it can be seen that for a higher temperature (140 °C) lower OIT values were obtained for all oils tested. This observation is in agreement with other studies [26, 46, 47]. The points presented on each straight line for each period represent the various cultivars of flaxseed 5a, camelina 5b and hempseed oil 5c, thus the differences between the values for the same time and the same oil can be observed, which are due to differences in the composition of various cultivars.
Generally, it can be seen that for all cultivars and all types of oils a decreasing trend was observed during the whole storage period, so for all three oils, the time required for initiation of oxidation (OIT) decreased till the end of storage. During the whole storage period of six months, OIT values determined at 120 °C were significantly reduced for flaxseed, camelina, and hempseed oils from values 43, 72, 51 min to 33, 65, 41 min, respectively. A similar trend was observed for the 140 °C program, where OIT values changed significantly for flaxseed oils from 10 to 7 min. and for camelina oil from 20 to 17 min., while for hempseed oil changes were not significant (p > 0.05) i.e., from 11 to 9 min. The highest OIT results for camelina oil were also observed by other authors, where among ten various oils flaxseed and hempseed oil was also analysed [48]. The rate of OIT decline can be compared for three oils by the slope of the straight line, shown in the equation in Fig. 3, which indicates how steep the line is. For the temperature program of 120 °C, the slope for camelina oil was the lowest, while for the isothermal program of 140 °C slope value was the lowest for hempseed oil. Other parameters calculated from isothermal curves i.e., oxidation end time (OET), length of oxidation (∆t = OET - OIT), and the rate of oxidation were also plotted against the storage time, as presented in Fig. S1 (Supplementary materials). Just as OIT expresses the starting point of the propagation, the parameter of OET, measured at the minimum value of the heat flow of the oxidation exotherm, indicates the end of the propagation stage and the start of the termination of oxidation. In Fig. S1, it can be seen that the values of OET decreased within the storage time for all oils, similarly to the OIT parameter. However, the changes were only significant for flaxseed oil (p ≤ 0.05). Slope values calculated for the relationship OET vs. time shown in the equations were higher for the analysis at 120 °C for all oils than for the isothermal mode at 140 °C. On the other hand, the changes in oxidation length (∆t) and oxidation rate within the storage time were not significant (p > 0.05), since they did not show any trend.
Figure 4 shows changes in Ton obtained from non-isothermal measurement by DSC for the three cold-pressed oils. It can be seen that for a higher heating rate (5 °C/min), Ton values were higher than for 2 °C/min, which is the result of longer exposure to oxygen at a lower scanning rate. This is in agreement with another study conducted to describe the flaxseed oils by means of non-isothermal DSC, where the authors reported a Ton value of 186 °C at a heating rate of 20 °C/min for fresh flaxseed oil [46]. Following the oxidation curves presented in Fig. 4 confirm that for all three cold-pressed oils, the Ton values decreased during the storage of oils for both heating rates (2 and 5 °C/min). The mean values of onset temperatures (Ton) determined at a heating rate of 2 °C/min were reduced after six months for flaxseed, camelina, and hempseed oils from values 147, 154, 147 °C to 142, 152, 144 °C, respectively.
For a heating rate of 5 °C/min, the mean value of Ton decreased after six months from 160, 168, 161 °C to 155, 166, 157 °C for flaxseed, camelina, and hempseed oils, respectively. Correspondingly, for non-isothermal measurement, the highest slope was observed for flaxseed oil for both heating rates (i.e., 0.84 for 2 °C/min and 0.70 for 5 °C/min). Implementing the DSC non-isothermal technique to show differences in the oxidative stability of various vegetable oils was presented by Qi et al. [49], using various heating rates i.e., 5, 7.5, 10, 12.5, and 15 °C/min. In addition to the Ton parameter, Tend was also determined as the parameter indicating the end of the propagation process. Fig. S2 (supplementary materials) presents the values of Tend calculated for heating rates 2 and 5 °C/min and plotted against storage time for flaxseed oil (Fig. S2a), camelina seed oil (Fig. S2b) and hempseed oil (Fig. S2c). It can be seen that a significant decrease in the Tend values within storage time were observed for all oils only for a heating rate of 5 °C/min (p ≤ 0.05).
The data analysed for DSC parameters show promising repeatability of oxidative characteristics for three types of cold-pressed oils, regardless of their originating from different cultivars. In the supplementary material in Table S1 data concerning the repeatability of OIT and Ton measurement was shown. It can be seen that the for OIT the coefficient of variation (CV) was lower in the case of OIT measured at 120 °C (0.68–2.56) than for OIT at 140 °C (1.17 and 5.85). For the non-isothermal determination there were no differences in CV for the determination of Ton at 2 °C/min (0.20–0.38) and Ton at 5 °C/min (0.14–0.42). Thus, the stability trends presented in this research work can be considered as a basis for further statistical analysis for predicting the oxidative stability of the samples for a prolonged storage time.
The relationship between DSC parameters and chemical indicators of the oxidative stability of cold-pressed oils during storage
In order to establish the usability of DSC analysis for assessing the oxidative state of oils, the DSC parameters of OIT and Ton were compared with chemical indicators. Table 1 presents the statistical parameters (slope, correlation coefficient r, p-value) of the relationship between DSC parameters (OIT and Ton) obtained from isothermal and non-isothermal analysis and chemical indicators (PV, p-AV, AV, TOTOX). For all three oils, both OIT and Ton parameters appeared to have a descending trend within the time of storage, in contrast to the results of the chemical methods, for which all indexes increased during six months. Thus, more deteriorated oil stipulates less temperature or time for the initiation of the oxidation process by means of DSC parameters. The correlation coefficient values indicate that the highest significant (p ≤ 0.05) correlations were observed between DSC parameters and PV, TOTOX values for flaxseed oils, while for camelina seed oils they were lowest.
For camelina seed oils, in particular, the correlations between AV values and all DSC parameters were not significant (p > 0.05). However, the chemical indicators for hempseed oil were significantly correlated with all the DSC parameters. For the data presented here, it can be stated that the DSC parameters can be considered to detect any oxidative degradation of the oil samples from the fresh condition to the end of shelf life, since they demonstrated parallel, and in most cases, significant correlations with chemical indicators. A similar approach to comparing analytical thermal methods with chemical indicators was presented by other authors, where flaxseed, camelina and hempseed oils were also analysed among ten various types of fresh cold-pressed oils. [48]. The isothermal pressure differential scanning calorimetry (PDSC) technique at a temperature of 120 °C was used in this study. PDSC results were also analysed for correlations with chemical methods (i.e., PV, p-AV and TOTOX), where PV and TOTOX values were reported to have significant correlations (-0.44 and 0.40, respectively), while for p-AV the correlation was not significant (r = 0.12) [48].
Chemometric analysis for the discrimination of fresh and stored oils based on chemical and DSC parameters
Principal component analysis (PCA) was used as an exploratory data analysis to gain insights into the underlying structure of results obtained from chemical and DSC analysis. This unsupervised technique shows the natural grouping of the samples studied, as well as the variables in a multidimensional space. First, two principal components with eigenvalues exceeding one were extracted, explaining 94% of the total variance. Figure 5a and b shows the results obtained in the space formed by the two first principal components.
PCA analysis of (a) loading plots PC1 and PC2 with an illustration of DSC parameters (OIT at 120 and 140 °C, Ton at 2 °C/min and 5 °C/min) and chemical indexes of oxidative stability (PV, p-AV, AV, TOTOX), (b) distribution of three plant oils based on a projection of cases and (c) discrimination analysis by LDA based on observed values (DSC and chemical) during shelf life for all three oils
The result given in Fig. 5b showed a tendency of grouping between the samples of each oil. This figure shows the separation of oil samples in two groups according to PC1. The first group consisted of camelina seed oil with the highest DSC indexes and the second group of flaxseed and hempseed oil. This distribution can be interpreted from the loading plot Fig. 5a, which indicates all chemical indexes are higher for hempseed and flaxseed oil. A strong negative correlation between DSC indexes and the first component can be observed (-0.82, -0.88. -0.88, -0.88 for OIT at 120 °C, OIT at 140 °C, Ton at 2 °C/min, Ton at 5 °C/min).
In contrast, there is a strong positive correlation between chemical indexes and PC1 (0.87, 0.83, 0.73, 0.84 for PV, p-AV, AV, and TOTOX, respectively). Additionally, it can be noted that samples stored for 4 and 6 months for each oil type are located more to the right side of the two-dimensional space, which correlates with high chemical oxidation indexes. The trend supports an attempt to build a model for classifying oils in terms of storage time. For this purpose, Linear Discriminant Analysis (LDA) was used, which is one of the supervised learning pattern recognition methods. LDA is based on determining linear discriminant functions, which maximize the ratio of between-class variance and minimize the ratio of within-class variance. LDA was successfully applied to describe the freshness of olive oils using fluorescence spectroscopy, where the authors obtained 100% predictive power for the model [50]. Moreover, Kalua et al. [51] made successful use of the method for discriminating the storage conditions and freshness of virgin olive oil. Once all data had been standardized, the LDA analysis was carried out for the entire dataset i.e., for chemical oxidation indexes and DSC parameters of all types of oils and three periods of storage (0, 4, 6 months). Two discriminant functions were obtained, based on Wilk’s Lambda statistics, with p < 0.05. The first classification function explains 92% of the variance, which means that 92% of all discriminatory power is explained by this function. As Fig. 5c shows, the first discriminant function distinguishes between fresh oils and those stored for 4 and 6 months. The second classification function appears to distinguish between oils stored for 4 and 6 months, but its discriminating power is low. More than 95% of the fresh oils were correctly classified, while for those stored for 4 and 6 months, 69% and 60% correct classifications were obtained, respectively.
Considerably better classification models were obtained for individual oil types, as is shown in Fig. 6. This is probably associated with the homogeneity of the oil sample composition among one type, which allows for better detection of changes due to storage time and their use for discrimination. The discriminant analysis resulted in statistically significant functions (Wilk’s Lambda statistic with p < 0.05) for all the oil types. In all models, the first discriminant function distinguished fresh oils from other oils, while the second discriminant function distinguished oils stored for four months from those stored for six months. This classification model shows high discrimination performance for the three classes, according to their membership. For flaxseed oil, all samples were correctly classified into the appropriate class. In the case of camelina seed and hemp seed oil, a misclassification was noted for 1 sample, which was classified as being stored for 6 months while it had been stored for 4 months. The accuracy of the models obtained indicates the potential use of DSC parameters combined with chemical indexes to build discriminant models to detect non-fresh oils within a certain type. Obviously, to build predictive models it would be required to conduct research on a significantly larger number of oil samples stored under different temperature and time conditions. As Fig. 6 shows, the LDA composed of both DSC results and chemical analysis results, the next step was to investigate whether it is possible to discriminate between fresh and stored oils based only on all DSC parameters (i.e., OIT, OET, Δt, oxidation rate, Ton, Tend).
Figure 7 shows LDA analysis conducted for three oils based on only DSC results from isothermal mode at 120 and 140 °C (OIT, OET, Δt, oxidation rate) and from non-isothermal program at 2 °C/min and 5 °C/min (Ton and Tend). The discriminant analysis resulted in statistically significant functions (Wilk’s Lambda statistic with p < 0.05) for flaxseed and hempseed oil, while for camelina oil it was not significant. However, all “0” month camelina oils samples were properly classified as fresh oils. For flaxseed oils, all fresh and stored samples were correctly classified into the appropriate classes, and a misclassification was only observed between the 4th and 6th -month groups, for which one 6th -month sample was identified as a 4th -month sample. In the case of hempseed seed oils, two samples were classified as being stored for 4 months, while they had actually been stored for 6 months. As can be seen in Fig. 7, for flaxseed and hempseed oils the distinction between fresh and stored oils was very distinct, in contrast to camelina oils, for which the discrimination was not so clear, since these oils were the most resistant to the oxidation process.
Conclusion
In this study, deteriorative changes in three cold-pressed oils (flaxseed, camelina, hempseed) during six months’ storage in conditions similar to the supermarket shelf were researched. The changes were measured by conventional chemical methods like PV, p-AV, TOTOX, and AV, as well as by means of the instrumental method of differential scanning calorimetry (DSC). The novelty of this study was its finding that DSC parameters obtained from isothermal (OIT) and non-isothermal (Ton) measurements changed corresponding to the chemical indicators during the shelf life of oils. All these parameters gradually decreased until the end of the storage period, while for chemical indicators a linear growth was observed. The oil most prone to oxidation was flaxseed oil, for which the most significant correlations between DSC parameters and PV and TOTOX were found. Of the three oils studied, the most thermally resistant to oxidation was camelina oil, for which the lowest values of the slopes of straight line plotted from DSC and chemical analysis data versus storage time were obtained. Summing up, all DSC results from the isothermal and non-isothermal experiment show promising repeatability of measuring the oxidative state for three types of cold-pressed oils, regardless of their origins in different cultivars. However, the most suitable test for monitoring the deteriorative changes in oils during storage was the isothermal test carried out at a temperature of 120 °C, since for this measurement the slope values of the straight lines plotted versus time for all three oils were the highest among all the DSC parameters tested (OIT at 120, 140 °C, Ton at 2, 5 °C/min). Moreover, for the parameters of OIT determined at 120 °C the correlation coefficients with chemical indicators were also highly significant (p ≤ 0.0001). Thus, the approach presented here can be used to monitor oxidative deterioration of oils based on the starting point determined for fresh oils. However, these findings can also contribute to the fortification, as well as modification practices, to enhance the shelf-life stability of cold-pressed oils. One practical aspect of this study is the possibility to use this approach for predicting the shelf life for consumer safety, and also improving the stability of the oils. Linear discriminant analysis (LDA) revealed that the first discriminating function significantly separated the fresh oils from stored oils.
Supplementary Information The supplementary material is available at https://data.mendeley.com/datasets/bmcs4kwnw9/1.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
A. Durazzo, M. Fawzy Ramadan, M. Lucarini, Front. Nutr. 8, 2021 (2022). https://doi.org/10.3389/fnut.2021.836651
M.F. Ramadan, Introduction to Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications (Elsevier Inc., 2020), pp. 1–5. https://doi.org/10.1016/B978-0-12-818188-1.00001-3
M. Ligĕza, D. Wyglădacz, A. Tobiasz, K. Jaworecka, A. Reich, Fam Med. Prim. Care Rev. 18, 443 (2016). https://doi.org/10.5114/fmpcr.2016.63699
M. Zulqarnain, M.H.M. Ayoub, M.H. Yusoff, I. Nazir, M. Zahid, F. Ameen, D. Sher, Floresyona, And E. Budi Nursanto, Sustain. 13, 1 (2021). https://doi.org/10.3390/su13020788
G.V. Reasearch, Cold Pressed Oil Market Size, Share & Trends Analysis Report By Product (Coconut Oil, Palm Oil, Ground Nut Oil, Rapeseed Oil, Soybean Oil, Sunflower Oil), By Distribution Channel, By Region, And Segment Forecasts, 2022–2028. https://www.grandviewresearch.com/industry-analysis/cold-pressed-oil-market-report. (Accessed 24 April 2023)
E. Choe, D.B. Min, Compr. Rev. Food Sci. Food Saf. 5, 169 (2006). https://doi.org/10.1111/j.1541-4337.2006.00009.x
M.H. Gordon, The Development of Oxidative Rancidity in Foods (Woodhead Publishing Ltd, 2001). https://doi.org/10.1016/9781855736160.1.5
M.D.A. Saldana, S.I. Martinez-Monteagudo, in Appl. Calorim. a Wide Context - Differ. Scanning Calorimetry, Isothermal Titration Calorim. Microcalorim, edited by A. A. Elkarody (2013), pp. 445–474
F. Shahidi, U.N. Wanasundara, Food Lipids (CRC Press, 2002), pp. 484–507
S. Pirsa, S. Asadi, Food Addit. Contam. - part A Chem. Anal. Control Expo Risk Assess. 38, 856 (2021). https://doi.org/10.1080/19440049.2021.1891299
M. Islam, Y.V. Rajagukguk, A. Siger, J. Tomaszewska-Gras, Foods 12, (2023). https://doi.org/10.3390/foods12010135
Y.V. Rajagukguk, M. Islam, J. Tomaszewska-Gras, Foods. 12, 358 (2023). https://doi.org/10.3390/foods12020358
H. Abramovič, V. Abram, Food Technol. Biotechnol. 43, 63 (2005)
A. Asdagh, S. Pirsa, Int. J. Biol. Macromol. 165, 156 (2020). https://doi.org/10.1016/j.ijbiomac.2020.09.192
M. Momot, B. Stawicka, A. Szydłowska-Czerniak, Appl. Sci. 13 (2023). https://doi.org/10.3390/app13031924
A.M. Giuffrè, C. Zappia, M. Capocasale, JAOCS J. Am. Oil Chem. Soc. 94, 819 (2017). https://doi.org/10.1007/s11746-017-2988-9
A.M. Giuffrè, M. Capocasale, C. Zappia, M. Poiana, J. Oleo Sci. 66, 1193 (2017). https://doi.org/10.5650/jos.ess17109
F. Giuffrida, F. Destaillats, M.H. Egart, B. Hug, P.A. Golay, L.H. Skibsted, F. Dionisi, Food Chem. 101, 1108 (2007). https://doi.org/10.1016/j.foodchem.2006.03.010
Q. Zhou, X. Jia, Q. Deng, H. Chen, H. Tang, F. Huang, Food Sci. Nutr. 7, 3731 (2019). https://doi.org/10.1002/fsn3.1232
M. Islam, L. Bełkowska, P. Konieczny, E. Fornal, J. Tomaszewska-Gras, J. Food Drug Anal. 30 (2022). https://doi.org/10.38212/2224-6614.3402
J. Tomaszewska-Gras, M. Islam, L. Grzeca, A. Kaczmarek, E. Fornal, Molecules. 26, 1 (2021). https://doi.org/10.3390/molecules26071958
J. Tomaszewska-Gras, Grasas y Aceites. 67 (2016). https://doi.org/10.3989/gya.0453161
J. Tomaszewska-Gras, J. Therm, Anal. Calorim. 113, 199 (2013). https://doi.org/10.1007/s10973-013-3087-2
M. Islam, A. Kaczmarek, M. Rudzińska, J. Tomaszewska-gras, Appl. Sci. 13 (2023). https://doi.org/10.3390/app13063975
R.M. Maggio, L. Cerretani, C. Barnaba, E. Chiavaro, Food Biophys. 7, 114 (2012). https://doi.org/10.1007/s11483-012-9248-1
M. Islam, M. Muzolf-Panek, E. Fornal, J. Tomaszewska-Gras, J. Therm. Anal. Calorim. (2022). https://doi.org/10.1007/s10973-022-11367-8
M.B. Różańska, P.Å. Kowalczewski, J. Tomaszewska-Gras, K. Dwiecki, S. Mildner-Szkudlarz, Antioxidants. 8, 313 (2019). https://doi.org/10.3390/antiox8080313
J. Thurgood, R. Ward, S. Martini, Food Res. Int. 40, 1030 (2007). https://doi.org/10.1016/j.foodres.2007.05.004
H.D. Belayneh, R.L. Wehling, E.B. Cahoon, O.N. Ciftci, J. Food Sci. 82, 632 (2017). https://doi.org/10.1111/1750-3841.13652
M. Muzolf-Panek, A. Kaczmarek, J. Tomaszewska-Gras, R. Cegielska-Radziejewska, T. Szablewski, M. Majcher, And K. Stuper-Szablewska, Antioxidants. 9, 1 (2020). https://doi.org/10.3390/antiox9090903
C.P. Tan, Y.B. Che Man, Trends Food Sci. Technol. 13, 312 (2002). https://doi.org/10.1016/S0924-2244(02)00165-6
J. Cao, L. Deng, X.M. Zhu, Y. Fan, J.N. Hu, J. Li, Z.Y. Deng, J. Agric. Food Chem. 62, 12545 (2014). https://doi.org/10.1021/jf5047656
ISO, EN ISO 11357-6:2013 Plastics - Differential Scanning Calorimetry (DSC) - Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT) (ISO 11357-6:2008) (2013)
A.S.T.M. International, ASTM D3895-19. Test Method for Oxidative-Induction Time of Polyolefins by Differential Scanning Calorimetry (ASTM Publisher, West Conshohocken, PA, USA, 2019)
ISO 6885, Animal and Vegetable Fats and Oils: Determination of Anisidine Value. ISO: Geneva, Switzerland 2016, (2016)
ISO 3960, Animal and Vegetable Fats and Oils—Determination—Iodometric (Visual) Endpoint; International Organization for Standardization: Geneva, Switzerland, 2017, (2010)
AOCS, AOCS Official Method Acid Value. Cd 3d-63, (Urbana, IL, USA, 2009)
S.S.O. Hung, S.J. Slinger, J. Am. Oil Chem. Soc. 58 (1981). https://doi.org/10.1007/BF02887323
Codex, Alimentarius, Standard for Named Vegetable Oils Codex Stan 210–1999 (1999)
M. Islam, A. Kaczmarek, A. Grygier, J. Tomaszewska-Gras, Foods 12, 2954 (2023). https://doi.org/10.3390/foods12152954
R. KRAKOWSKI, Combust. Engines. 186, 59 (2021). https://doi.org/10.19206/CE-140730
H. Abramovič, B. Butinar, V. Nikolič, Food Chem. 104, 903 (2007). https://doi.org/10.1016/j.foodchem.2006.12.044
M. Tańska, B. Roszkowska, M. Skrajda, D. Grzegorz, J. Oleo Sci. (2016). https://doi.org/10.5650/jos.ess15243
K. Hasiewicz-Derkacz, A. Kulma, T. Czuj, A. Prescha, M. Zuk, M. Grajzer, M. Łukaszewicz, J. Szopa, BMC Biotechnol. 15, 1 (2015). https://doi.org/10.1186/s12896-015-0178-0
M. Tura, D. Ansorena, I. Astiasarán, M. Mandrioli, T.G. Toschi, Antioxidants 11, (2022). https://doi.org/10.3390/antiox11030490
E. Rudnik, A. Szczucinska, H. Gwardiak, A. Szulc, A. Winiarska, Thermochim. Acta. 370, 135 (2001). https://doi.org/10.1016/S0040-6031(00)00781-4
K. Ratusz, E. Popis, H. Ciemniewska-Żytkiewicz, M. Wroniak, J. Therm. Anal. Calorim. 126, 343 (2016). https://doi.org/10.1007/s10973-016-5642-0
E. Symoniuk, K. Ratusz, E. Ostrowska-Ligęza, K. Krygier, Food Anal. Methods. 11, 1095 (2018). https://doi.org/10.1007/s12161-017-1081-1
B. Qi, Q. Zhang, X. Sui, Z. Wang, Y. Li, L. Jiang, Food Chem. 194, 601 (2016). https://doi.org/10.1016/j.foodchem.2015.07.148
A. El Orche, M. Bouatia, M. Mbarki, J. Anal. Methods Chem. 2020, 1 (2020). https://doi.org/10.1155/2020/8860161
C.M. Kalua, D.R. Bedgood, A.G. Bishop, P.D. Prenzler, E.H. Graham, (2006). https://doi.org/10.1021/jf061038j
Funding
This research was funded by the NATIONAL SCIENCE CENTRE, POLAND, grant number: 2018/31/B/NZ9/02762.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Mahbuba Islam and Jolanta Tomaszewska-Gras]; Methodology: [Mahbuba Islam and Jolanta Tomaszewska-Gras]; Formal analysis and investigation: [Mahbuba Islam, Anna Kaczmarek and Jolanta Tomaszewska-Gras]; Writing - original draft preparation: [Mahbuba Islam]; Writing - review and editing: [Mahbuba Islam, Anna Kaczmarek and Jolanta Tomaszewska-Gras]; Funding acquisition: [Jolanta Tomaszewska-Gras]; Resources: [Jolanta Tomaszewska-Gras]; Supervision: [Jolanta Tomaszewska-Gras]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Informed consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, M., Kaczmarek, A. & Tomaszewska-Gras, J. Differential scanning calorimetry as a tool to assess the oxidation state of cold-pressed oils during shelf-life. Food Measure 17, 6639–6651 (2023). https://doi.org/10.1007/s11694-023-02152-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02152-8